Abstract
Free full text

Kinetics and characterization of interferon production by murine spleen cells stimulated with Legionella pneumophila antigens.
Abstract
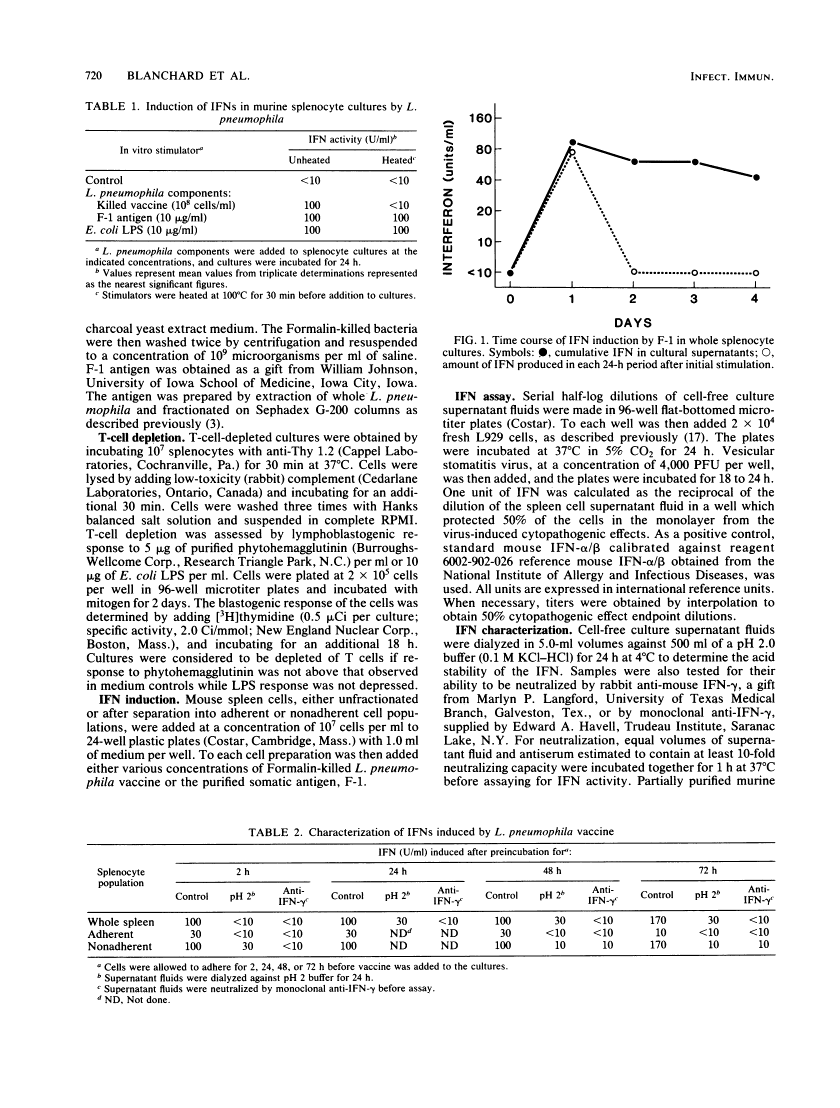
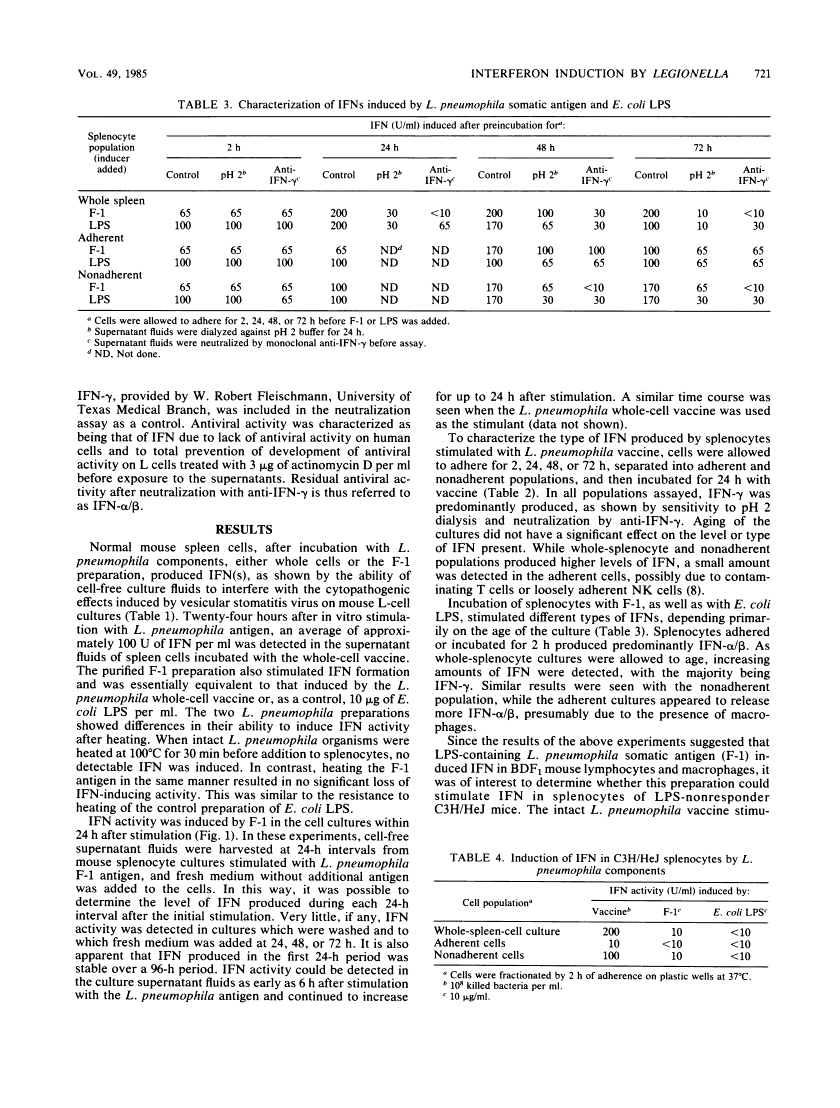
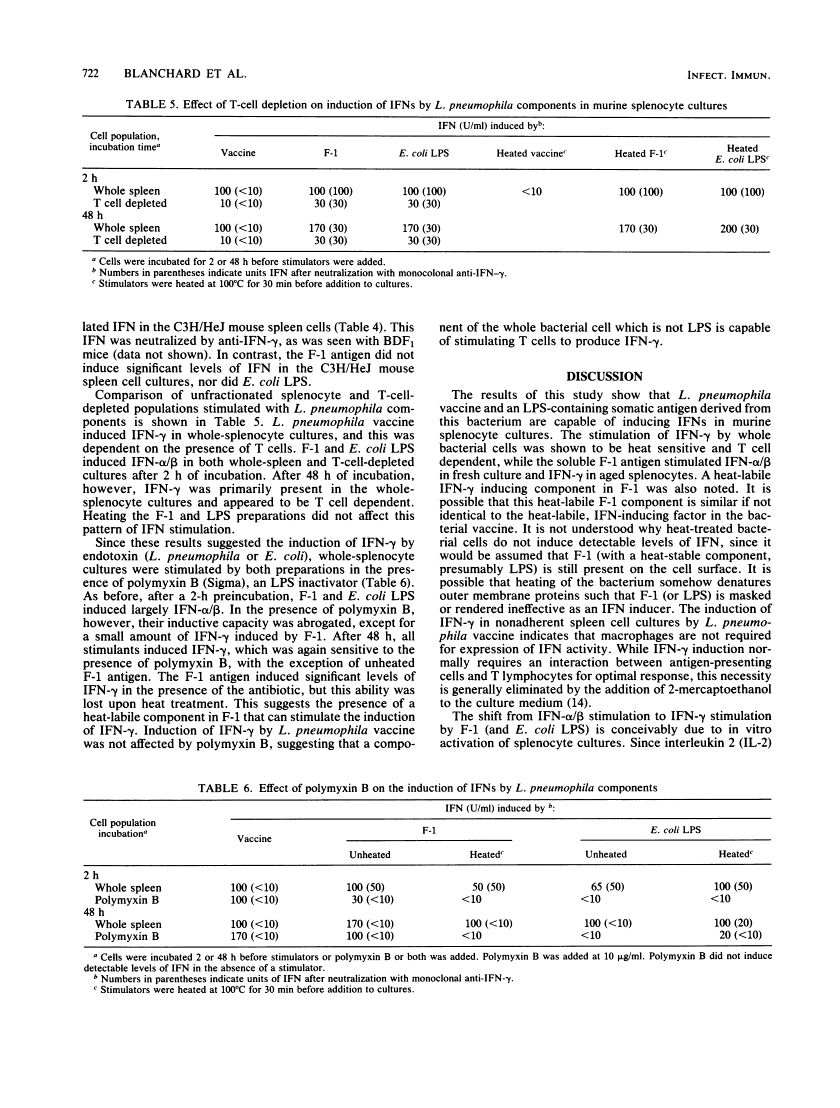
Formalin-killed Legionella pneumophila bacterial cells, as well as a purified cell wall preparation (designated F-1 antigen) containing lipopolysaccharide (LPS), stimulated production of interferons (IFNs) in mouse spleen cell cultures. L. pneumophila whole-cell vaccine induced an IFN that was pH 2 labile and neutralized by anti-IFN-gamma indicating that IFN-gamma was the dominant form present. F-1 antigen induced a mixture of IFNs, depending upon the age of the culture and cell types present. In freshly prepared whole-spleen cultures and in 2-h adherent cultures, F-1 induced predominantly IFN-alpha/beta. In whole-spleen cultures that were allowed to age for 24 to 48 h before stimulation, F-1 was seen to induce mostly IFN-gamma, with low levels of IFN-alpha/beta present. Since only IFN-alpha/beta was produced in T-cell-depleted populations (at 2 h or at 48 h), it is suggested that T cells are responsible for IFN-gamma production in aged cultures. Additionally, heat-treated F-1, Escherichia coli LPS, and heat-treated E. coli LPS all induced similar levels of IFN-gamma in whole-splenocyte or nonadherent cell cultures which were incubated 48 h before stimulation. This suggests that LPS present in F-1 is responsible for IFN-gamma production and that an activated cell population is required. These results show that L. pneumophila antigens can induce the production of various types of IFN in mouse spleen cell cultures through several mechanisms.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (971K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Claeys H, Van Damme J, De Ley M, Vermylen C, Billiau A. Activation of natural cytotoxicity of human peripheral blood mononuclear cells by interferon: a kinetic study and comparison of different interferon types. Br J Haematol. 1982 Jan;50(1):85–94. [Abstract] [Google Scholar]
- Daisy JA, Benson CE, McKitrick J, Friedman HM. Intracellular replication of Legionella pneumophila. J Infect Dis. 1981 Mar;143(3):460–464. [Abstract] [Google Scholar]
- Elliott JA, Johnson W, Helms CM. Ultrastructural localization and protective activity of a high-molecular-weight antigen isolated from Legionella pneumophila. Infect Immun. 1981 Feb;31(2):822–824. [Europe PMC free article] [Abstract] [Google Scholar]
- Farrar WL, Johnson HM, Farrar JJ. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [Abstract] [Google Scholar]
- Friedman F, Widen R, Klein T, Friedman H. Lymphoid cell blastogenesis as an in vitro indicator of cellular immunity to Legionella pneumophila antigens. J Clin Microbiol. 1984 Jun;19(6):834–837. [Europe PMC free article] [Abstract] [Google Scholar]
- Friedman H, Widen R, Klein T, Johnson W. Immunostimulation by Legionella pneumophila antigen preparations in vivo and in vitro. Infect Immun. 1984 Jan;43(1):347–352. [Europe PMC free article] [Abstract] [Google Scholar]
- Fukazawa Y, Kagaya K, Miura H, Shinoda T, Natori K, Yamazaki S. Biological and biochemical characterization of macrophage activating factor (MAF) in murine lymphocytes: physiocochemical similarity of MAF to gamma interferon (IFN-gamma). Microbiol Immunol. 1984;28(6):691–702. [Abstract] [Google Scholar]
- Horwitz DA, Bakke AC, Abo W, Nishiya K. Monocyte and NK cell cytotoxic activity in human adherent cell preparations: discriminating effects of interferon and lactoferrin. J Immunol. 1984 May;132(5):2370–2374. [Abstract] [Google Scholar]
- Horwitz MA. Cell-mediated immunity in Legionnaires' disease. J Clin Invest. 1983 Jun;71(6):1686–1697. [Europe PMC free article] [Abstract] [Google Scholar]
- Horwitz MA, Silverstein SC. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. [Europe PMC free article] [Abstract] [Google Scholar]
- Horwitz MA, Silverstein SC. Interaction of the legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. II. Antibody promotes binding of L. pneumophila to monocytes but does not inhibit intracellular multiplication. J Exp Med. 1981 Feb 1;153(2):398–406. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson HM, Farrar WL. The role of a gamma interferon-like lymphokine in the activation of T cells for expression of interleukin 2 receptors. Cell Immunol. 1983 Jan;75(1):154–159. [Abstract] [Google Scholar]
- Kasahara T, Hooks JJ, Dougherty SF, Oppenheim JJ. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983 Apr;130(4):1784–1789. [Abstract] [Google Scholar]
- Kirchner H, Nicklas W, Giebler D, Keyssner K, Berger R, Storch E. Induction of interferon gamma in mouse spleen cells by culture supernatants of mycoplasma arthritidis. J Interferon Res. 1984 Summer;4(3):389–397. [Abstract] [Google Scholar]
- McMaster PR, Tsang VC, Wong KH, Feeley JC, Gann DS. Comparative adjuvant activities of Legionella pneumophila and Mycobacterium tuberculosis. Int Arch Allergy Appl Immunol. 1984;73(4):357–362. [Abstract] [Google Scholar]
- Scott GM, Wallace J, Tyrrell DA, Cantell K, Secher DS, Stewart WE., 2nd Interim report on studies on "toxic" effects of human leucocyte-derived interferon-alpha (HuIFN-alpha). J Interferon Res. 1982;2(1):127–130. [Abstract] [Google Scholar]
- Vogel SN, Hilfiker ML, Caulfield MJ. Endotoxin-induced T lymphocyte proliferation. J Immunol. 1983 Apr;130(4):1774–1779. [Abstract] [Google Scholar]
- Widen R, Klein T, Friedman H. Enhanced susceptibility of cyclophosphamide-treated mice to infection with Legionella pneumophila. J Infect Dis. 1984 Jun;149(6):1023–1024. [Abstract] [Google Scholar]
- Wong GH, Clark-Lewis I, Harris AW, Schrader JW. Effect of cloned interferon-gamma on expression of H-2 and Ia antigens on cell lines of hemopoietic, lymphoid, epithelial, fibroblastic and neuronal origin. Eur J Immunol. 1984 Jan;14(1):52–56. [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.49.3.719-723.1985
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/49/3/719.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/49/3/719
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/49/3/719
Citations & impact
Impact metrics
Citations of article over time
Article citations
Decreased capacity of aged mice to produce interferon-gamma in Legionella pneumophila infection.
Mech Ageing Dev, 81(2-3):97-106, 01 Jul 1995
Cited by: 6 articles | PMID: 8569284
Antitumor effect of DT-5461a, a synthetic low-toxicity lipid A analog, involves endogenous tumor necrosis factor induction subsequent to macrophage activation.
Int J Immunopharmacol, 16(11):887-893, 01 Nov 1994
Cited by: 10 articles | PMID: 7868293
Role of gamma interferon in induction of natural killer activity by Legionella pneumophila in vitro and in an experimental murine infection model.
Infect Immun, 56(5):1187-1193, 01 May 1988
Cited by: 24 articles | PMID: 3128479 | PMCID: PMC259782
Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice.
Infect Immun, 56(2):370-375, 01 Feb 1988
Cited by: 108 articles | PMID: 3257460 | PMCID: PMC259290
In vitro and in vivo suppressive effects of delta-9-tetrahydrocannabinol on interferon production by murine spleen cells.
Int J Immunopharmacol, 8(7):819-824, 01 Jan 1986
Cited by: 40 articles | PMID: 2430904
Go to all (6) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Interferon-gamma induction by lipopolysaccharide: dependence on interleukin 2 and macrophages.
J Immunol, 136(3):963-970, 01 Feb 1986
Cited by: 68 articles | PMID: 2867114
Differing macrophage and lymphocyte roles in resistance to Legionella pneumophila infection.
J Immunol, 148(2):584-589, 01 Jan 1992
Cited by: 9 articles | PMID: 1729375
Legionella pneumophila-induced blastogenesis of murine lymphoid cells in vitro.
Infect Immun, 43(1):314-319, 01 Jan 1984
Cited by: 20 articles | PMID: 6360903 | PMCID: PMC263428
In vitro and in vivo suppressive effects of delta-9-tetrahydrocannabinol on interferon production by murine spleen cells.
Int J Immunopharmacol, 8(7):819-824, 01 Jan 1986
Cited by: 40 articles | PMID: 2430904