Abstract
Free full text

Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer
Abstract
Previously, clinical approaches to using the immune system against cancer focused on vaccines that intended to specifically initiate or amplify a host response against evolving tumours. Although vaccine approaches have had some clinical success, most cancer vaccines fail to induce objective tumour shrinkage in patients. More-recent approaches have centred on a series of molecules known as immune checkpoints—whose natural function is to restrain or dampen a potentially over-exuberant response. Blocking immune checkpoint molecules with monoclonal antibodies has emerged as a viable clinical strategy that mediates tumour shrinkage in several cancer types. In addition to being part of the current treatment armamentarium for metastatic melanoma, immune checkpoint blockade is currently undergoing phase III testing in several cancer types.
Introduction
Cancer immunotherapy refers to a number of approaches intended to activate the immune system to induce objective responses and disease stabilization.1 Kidney cancer and melanoma are natural targets for such approaches,2–4 because both tumour types are frequently infiltrated with CD8+ lymphocytes,5 and occasionally undergo spon taneous regressions.6 Indeed, interleukin-2 (IL-2), a cytokine that supports T-cell proliferation, is a standard-of-care treatment for young, healthy patients with kidney cancer and melanoma, and in rare instances benefits from this treatment have lasted more than 10 years.7 By c ontrast, non-small-cell lung cancer (NSCLC) has been considered to be insensitive to immuno logical approaches8 because immunotherapy with cancer vaccines had not demonstrated clinical benefit and spontaneous regressions had not been observed. Now, clinical data suggest that this is not the case; objective responses in NSCLC have been reported in trials involving agents that block immune checkpoint molecules.9,10 Indeed, the largest interventional clinical trial ever initiated for NSCLC, involving over 2,200 patients, is testing a vaccine directed against the protein MAGE-A3, a cancer-associated protein that belongs to a class of molecules known as cancer-testis antigens,11 expressed only in tumours and in germ cells. What melanoma, lung and kidney cancers have in common are new and exciting data that show a significant rate of objective clinical response to anti bodies that block immune checkpoints—a treatment that has rapidly been advanced into randomized phase III clinical trials. In this article, we will first briefly review the basic immunology underlying an anti-tumour immune response. We will then review and discuss clinical trial results in each of the three tumour types, focusing on both cancer vaccines and on agents that block immune checkpoints, in a manner that allows the reader to compare and contrast the approaches to immunotherapy in kidney cancer, lung cancer and melanoma.
Basic immunology
Although a comprehensive discussion of the basic immuno logy underlying an anti-tumour immune response is beyond the scope of this Review, a few introductory points are worth noting. Cancer vaccines are used in approaches that seek to raise a specific T-cell or B-cell response against cancer (Figure 1). When a vaccine is injected into the skin, components of the vaccine known as pathogen-associated molecular patterns12 activate resting dendritic cells (DC) and programme them to migrate to a local lymph node. Thus, a vaccine generally includes components intended to activate DCs and the precise agents used vary widely between different vaccines. Another common term for these activating components is ‘adjuvant’, as they ‘add’ immunogenicity to the protein or peptide components of a vaccine. The other key component of a vaccine is the target protein or peptide that is expected to be over-expressed in tumours compared with normal tissue. The choice of vaccine antigen(s) is somewhat empiric and, similar to adjuvant selection, varies widely between cancer vaccines.13 Once a resting DC has been loaded with antigen, activated, and has migrated to a lymph node, it then displays fragments of proteins in the form of small peptides. Cellular recognition of these small peptide fragments (antigens) is complex; peptides are not presented alone, but instead are bound within a geneti-cally diverse set of host molecules collectively encoded by a set of genes within the major histo compatibility complex (MHC). Specific receptors on CD4+ and CD8+ T cells recognize a structure composed of both MHC molecules and a specific peptide.14 Simple recognition (a good fit) is insufficient for full T-cell activation; T cells must also receive additional activation signals provided by functionally mature DCs to proliferate and acquire effector function. In the case of CD8+ T cells, the desiredeffector function is the ability to lyse target cells that express the same MHC–peptide complex that served to activate them, that is, their target antigen. Once fully activated, CD8+ T cells leave the lymph node, and traffic widely through the body in search of their targets.15
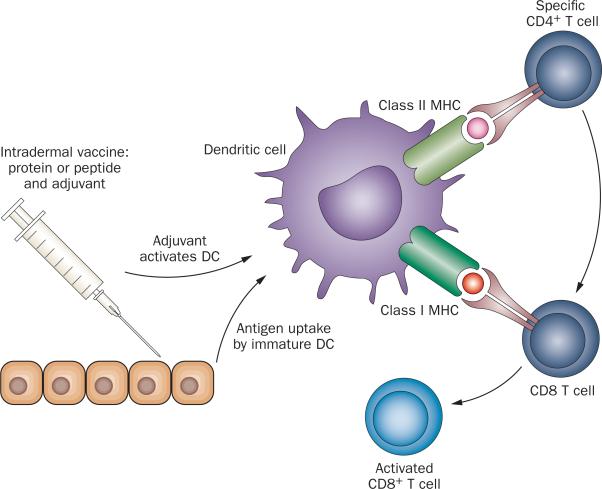
Mechanism of action of cancer vaccines. Cancer vaccines work by providing a target antigen or antigens to a specialized cell known as the dendritic cell (DC). These cells reside at the site of antigen injection (usually intradermal), where they take up and process antigen. Immunostimulatory molecules in the vaccine preparation (adjuvants) activate DCs, which respond by upregulating the molecules they need to interact with (T cells), and migrating to a lymph node. Once in a lymph node, activated DCs present antigen to T cells; if the T cell recognizes its cognate antigen in the proper context, it is then activated. Upon activation, CD4+ T cells produce cytokines that help CD8 T cells to fully mature. Upon full maturation, CD8+ T cells in turn proliferate and then leave the lymph node to traffic widely throughout the body. When an activated T cell senses a cell bearing its target antigen (tumour antigen) in the periphery, it can then lyse that cell, potentially mediating an antitumour response.
Unfortunately, most tumours have evolved multiple mechanisms to evade immune-mediated destruction.16 One of these mechanisms involves cell-surface expression of one or more of a series of molecules that effectively limit T cell proliferation and killing capacity.17 Collectively, such molecules are referred to as immune checkpoints,18,19 perhaps the best known of which is CTLA-4 (cytotoxic T-lymphocyte antigen-4) (Figure 2).20 Early preclinical studies using transplant-able murine colon carcinoma and fibrosarcoma lines showed that blocking CTLA-4 permits anti-tumour T cells to acquire effector function,21 a finding that has recently been borne out in randomized phase III studies in patients with metastatic melanoma. In these two large, randomized phase III trials, involving a total of 1,178 patients, blocking CTLA-4 with the monoclonal antibody ipilimumab resulted in a significant survival benefit. In the first of these studies, which enrolled previously treated patients, the median overall sur-vival with single-agent ipilimumab was 10.1 months versus 6.4 months for patients treated with a gp100 vaccine on the control arm (HR 0.66; P = 0.003).22 In the second of these studies, which enrolled treatment naïve patients and randomly assigned them to receive either ipilimumab plus chemotherapy with dacarbazine, versus dacarbazine alone, median overall survival was 11.2 months versus 9.1 months (HR 0.72, P <0.001).23 Long-term follow-up from the first trial showed that approximately 15% of treated patients were alive 5 years after enrollment. CTLA-4 blockade is moving forward in lung cancer, because this immune checkpoint molecule likely evolved to protect self-tissues from autoimmunity. Thus, it is not surprising that clinical trials of anti-CTLA-4 (including the pivotal phase III trials) were associated with an approximate 20% incidence of grade 3 and 4 immunerelated adverse events (IRAEs), including colitis and dermatitis.24–27

Immune checkpoint blockade. This approach to immunotherapy is exemplified by antibodies directed against CTLA-4 (ipilimumab, tremilimumab), which block the immunosupression mediated by the interaction between B7 family members (on antigen-presenting cells) and CTLA-4 (on CD8+ and CD4+ T cells). A second major checkpoint, mediated by the interaction between PD-1 on T cells and its ligand PD-L1 on either antigen-presenting cells or tumour cells, has been the subject of several recent clinical trials, and has shown evidence of efficacy in both non-small-cell lung cancer and renal cell carcinoma.
A second immune checkpoint, programmed death-1 (PD-1), has garnered significant interest as the blockade of PD-1 with a singl-eagent was associated with objective responses in melanoma, kidney cancer, and perhaps somewhat surprisingly, lung cancer (Figure 2).9,28,29 Toxicity rates for both agents are difficult to compare given that PD-1 blocking antibodies have only recently entered phase III development. Nonetheless, the rate of grade 3 and 4 adverse events seems to be lower with PD-1 blockade than with CTLA-4 blockade,10,29 possibly because the PD-1/PD-ligand (PD-L1) pathway acts more peripherally than the CTLA-4/B7-1 pathway, which may operate in the lymph nodes. In contrast to cancer vaccines, objective tumour regressions and longterm complete responses,30 have been routinely observed with both PD-1 and CTLA-4 blockade, driving enthusiasm for ongoing phase III and combination trials. At the current time, it remains unclear why cancer vaccines rarely generate objective tumour shrinkage, but accumulating clinical data suggest that current vaccines may be unable to circum vent effectively the multiple immunosuppressive mechanisms operative in the tumour microenvironment.31
Peptide vaccines
MAGE-A3 vaccine in lung cancer
MAGE-A3 is a cancer testis-antigen11 that is expressed at significant levels only in the testes, where it remains inaccessible to T cells because MHC molecules are not expressed there. Endogenous immune tolerance to MAGE-A3 is generally absent. In lung cancer (and several other tumour types), MAGE-A3 expression increases with tumour stage; overall the antigen is expressed in approximately 35% of lung tumours.32 To target MAGE-A3 with a vaccine, a series of adjuvants were developed by GlaxoSmithKline.33 The initial adjuvant system, AS02, was an oil–water emulsion that included peptide and two stimulatory molecules. The first of these, monophosphoryl lipid A (MPL),34 activates DCs through Toll-like receptor 4 (TLR4). The second component of AS02, QS21, enhances protein antigen uptake by DCs. In a phase II study of the MAGE-A3 vaccine, 182 patients with surgically resected, MAGE-A3-positive NSCLC tumours (stages IB and II) were random ly assigned (2:1) to receive MAGE-A3 vaccine (n = 122) or placebo (n = 60).35 In this trial, patients did not receive adjuvant chemotherapy, according to the standard of care at that time. The primary end point of the trial, disease-free interval, was not significantly different between the two groups (HR 0.74, 95% CI 0.44–1.2, P = 0.107). In general, the vaccine was well tolerated with only three treatment-related adverse events. A tumour gene-expression profile was investigated, and revealed a 43% relative risk reduction for recurrence in the vaccine treated group in patients with a favourable gene-signature profile (HR 0.57, 95% CI 0.25–1.34, P = 0.99).36 These data supported the design and initiation of a large phase III trial, MAGRIT (Table 1).37 On the basis of data from a random ized phase II study in melanoma, the immunological adjuvant for this trial was changed; instead of AS02, the adjuvant used in the phase III study also included CpG 7909, a synthetic 24-mer oligonucleotide designed to target selectively an additional Toll-like receptor, TLR9. The primary end point was disease-free survival, and 2,270 patients with completely resected tumours expressing MAGE-A3 were randomly assigned to receive either vaccine or placebo. Enrollment is now completed. In keeping with current clinical practice, patients were permitted to receive adjuvant chemotherapy before trial randomization. Notably, this trial is the largest ever interventional study in NSCLC, reflecting an increased interest in immunotherapy for lung cancer.
Table 1
Selected phase II and III vaccine trials in lung, RCC and melanoma
| Agent and trial | Phase | Design and description | n | Results and comments |
|---|---|---|---|---|
| MAGE-A3 vaccine with AS02 adjuvant37 | II | Randomized trial of vaccine or placebo post resection of stage IB and II MAGE-A3+ NSCLC | 182 | Gene expression profile revealed a 43% reduction of recurrence in vaccine treated group (HR 0.57, 95% CI 0.36–1.2, P = 0.99). Primary end point of disease-free interval was not significantly different between the two groups (HR 0.74, P = 0.107) |
| Liposomal MUC-1 peptide vaccine (L-BLP-25)64 | II | Randomized trial of L-BLP-25 vs BSC in patients with stage IIIB or IV NSCLC with stable or responsive disease post chemotherapy or chemoradiation | 171 | Primary end point of median OS 17.2 months L-BLP-25 vs 13 months BSC (P = 0.066); subgroup analysis: stage IIIB patients OS 30.6 months vs 13.3 months in BSC arm |
| Vacccinia/MUC-1 vaccine (TG4010)69 | II | Randomized trial of cisplatin and vinorlbine with TG4010 vs TG4010 as a single agent until disease progression followed by addition of vinorelbine and cisplatin in patients with MUC-1-positive advanced NSCLC | 65 | Primary end point of response was met only for the concurrent TG4010 and chemotherapy arm; response rate 29.5% |
| Vacccinia/MUC-1 vaccine (TG4010)70 | IIB | Randomized trial of gemcitabine and cisplatin vs the same combination with TG4010 in patients with stage 4 NSCLC | 108 | Patients with normal level of activated NK cells at baseline had an improvement in 6-month PFS and OS. Patients with high levels of active NK cells had increased toxic effects. Primary end point of 6-month PFS met only for the concurrent TG4010 arm (43%), but not significantly different from chemotherapy alone (35%) |
| Allogeneic whole cell NSCLC line vaccine with anti-sense TGF-β (Belagenpumatucel-L)49 | II | Randomized multi-dose trial in NSCLC with low volume stage II, IIIA, IIIB, IV disease | 75 | Response rate 15%; OS, 441 days in advanced-stage disease setting |
| MAGE-A3 NCT00480025 | III | Randomized phase III trial of patients with resected stage IB–IIIA MAGE-A3+ NSCLC post resection or adjuvant chemotherapy | 2,289 | Primary end point: DFS |
| L-BLP-25 NCT00409188 | III | Randomized trial comparing vaccine vs placebo in patients with unresectable stage III with stable or responding disease after chemoradiotherapy | 1,464 | Primary end point: OS not met |
| L-BLP-25 NCT01015443* | III | Randomized trial comparing vaccine vs placebo in patients with unresectable stage III with stable or responding disease after chemoradiotherapy | 420 | Primary end point: OS |
| L-BLP-25 NCT00828009‡ | II | BLP25 vaccine and bevacizumab after chemoradiotherapy for patients with unresectable stage IIIA/B NSCLC | 55 | Primary end point: safety |
| TG4010 NCT01383148 | IIB/III | Randomized trial comparing platinum combination chemotherapy with or without vaccine in patients with stage IV NSCLC | 1,000 | Primary end point: OS |
| Belagenpumatucel-L NCT00676507 | III | Randomized trial of vaccine or placebo in patients with stage IIIA, IIIB or IV NSCLC with stable or responding disease after initial chemotherapy | 506 | Primary endpoint: OS |
| Multipeptide vaccine with GM-CSF adjuvant (IMA901)38 | I | Phase I study of multi-peptide vaccine in advanced RCC, patients must be HLA-A*02+ | 28 | Well tolerated, one partial response |
| Multipeptide vaccine with GM-CSF adjuvant (IMA901)38 | II | Randomized phase II study, patients received vaccine ± immunomodulatory cyclophosphamide. HLA-A*02+ patients | 68 | TREG depletion noted in cyclophosphamide arm. Well tolerated, one PR. Trend toward improved OS in vaccine + low-dose cyclophosphamide arm (HR = 0.57, P = 0.090) |
| Tumour RNA pulsed autologous DCs (AGS-003)55 | II | Single arm phase II study in patients with newly diagnosed clear cell RCC undergoing resection. Vaccine administered + sunitinib | 25 | Well-tolerated. Median PFS from registration for patients receiving at least one dose of AGS-003 was 11.9 months. Median OS from registration not yet reached |
| IMA901 with GM-CSF adjuvant NCT01265901‡ | III | Randomized, controlled study in the first-line setting in combination with sunitinib. Vaccine administered and low-dose cyclophosphamide. Patients must be HLA-A*02+ | 330 | Primary end point: OS secondary end point: OS in patients with favourable gene signature. Enrolment completed |
| RNA-loaded autologous DC vaccine (AGS-003) NCT01582672 | III | Randomized phase III trial of standard of care sunitinib ± vaccine in advanced RCC | 450 | Primary end point: OS |
| Melanoma | ||||
| gp100111 | III | gp100 peptide in montanide + high-dose IL-2 vs IL-2 alone | 185 | Clinical response rate and PFS significantly higher in combination group |
| Allovectin-7 NCT00395070§ | III | Allovectin 7 vs DTIC or temozolomide in unresectable stage III/IV melanoma | ~375 | Primary outcome measures: ORR at ≥24 weeks; secondary outcome measures: safety/tolerability of Allovectin-7; OS |
| MAGE-A3 NCT00796445§ | III | MAGE-A3 vaccine vs placebo in patients with surgically resected stage IIIB/C cutaneous melanoma with macroscopic lymph-node involvement | 1,349 | Secondary outcome measures include Anti-MAGE-A3 and anti-protein D seropositivity status. Primary outcome measure: DFS |
| GVAX melanoma NCT01435499‡ | I | Allogeneic, GM-CSF secreting, whole melanoma cell vaccine administered with or without low-dose cyclophosphamide to patients with resected stage IIB–IV melanoma | 19 | Secondary outcome measures include in vitro correlates of anti-melanoma immunization (serological and cellular immune responses). Primary outcome measures: safety, tolerability; secondary outcome measures: in vitro correlates of anti-melanoma immunization |
| T-Vec74§ | III | T-Vec (attenuated herpes simplex virus) vs GM-CSF | 436 | Durable response rate 16.3% (T-Vec) vs 2.1% (GM-CSF) |
Abbreviations: BSC, best supportive care; CD, dendritic cells; DFS, disease-free survival; DTIC, dacarbazine; GM-CFS, granulocyte-macrophage colony stimulating factor; HR, hazard ratio; NK, natural killer; NSCLC, non-small-cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; RCC, renal cell carcinoma; RR, relative risk; vs, versus.
Multi-targeted peptide vaccine in RCC
In contrast to optimizing adjuvant with a single antigen, the leading vaccine approach in kidney cancer focuses on targeting multiple carefully selected antigens with a less complex adjuvant. To select relevant antigens, kidney tumours from a series of 32 patients who expressed the common Class I MHC molecule HLA-A*02 were isolated, and the cell surface peptides residing in Class I MHC molecules were eluted and subjected to mass spectrometry analysis.38 This approach identified a set of nine tumour-associated peptides (TUMAPs), which were incorporated into a vaccine using granulocytemacrophage colony stimulating factor (GM-CSF) as an adjuvant. GM-CSF is a strong inducer of DC migration, but perhaps less robust than several of the Toll-like receptor agonists in terms of inducing DC activation. Phase I and phase II data on this agent were recently reported in a single publication.38 The phase I study enrolled a total of 28 patients with advanced renal cell carcinoma (RCC); patients were required to be HLA-A*02 positive. These patients received up to eight IMA901 multi-peptide vaccinations, each preceded by GM-CSF as an adjuvant. The vaccine was well tolerated, with no grade 3 or 4 adverse events noted. At a 3-month follow-up point, a single patient (out of the 28) showed a partial response, 16 patients had disease progression and 11 patients had stable disease. Immune responses to the targeted peptides were detected in several of the treated patients. To improve clinical activity, investigators made use of well-established data that showed that low doses of cyclophosphamide have vaccine-potentiating immune effects,39 which are at least partially mediated by the depletion of regulatory T cells (TREG) that turn off an immune response.40,41 The phase II study of IMA901 was a randomized trial, in which 68-HLA-A*02 positive patients with RCC were randomly assigned to receive either IMA901, or IMA901 preceded by a single immuno modulatory dose of intravenous cyclophosphamide (300 mg/m2). As noted previously,42 objective tumour regressions were relatively rare, with a single partial response among 64-patients confirmed on central review. Careful immunological analyses showed an increased T-cell response to the targeted peptides, and confirmed that low-dose cyclophosphamide depletes regulatory T cells in humans. There was a trend towards improved overall survival in the arm receiving vaccine plus low-dose cyclophosphamide (HR 0.57, P = 0.090), but this was not statistically significant. Despite these phase II results, a randomized phase III trial was initiated,43 in which IMA901 was added to first-line sunitinib in patients with metastatic RCC (Table 1). Enrollment of 330 patients to this trial was completed in 2012.
MAGE-A3 vaccine in melanoma
Similar to NSCLC, MAGE-A3 is expressed in approximately 65% of melanomas.32 In early studies, a fusion protein—composed of MAGE-A3 plus influenza protein D—was administered in a phase I–II trial to 32 patients with metastatic melanoma whose tumours expressed MAGE-A3.44 The vaccine was injected every 3 weeks without an additional adjuvant. Surprisingly, among the 26 patients who received ≥four vaccinations, a single partial response and four mixed responses, in which some lesions respond while others remain stable, were observed. These data are noteworthy as objective responses are relatively rare in vaccine trials. A subsequent randomized phase II trial investigated the administration of the MAGE-A3 vaccine with two immunological adjuvants—AS02B or AS15—which included the TLR-9 agonist CpG mentioned above.45 AS15 demonstrated more robust MAGE-A3-specific T cell and antibody responses, as well as objective tumour regressions, and was subsequently used in phase III trials in both NSCLC and melanoma. Analysis of pretreatment tumour biopsies revealed a gene-expression signature that correlated with clinical response.46 On the basis of these findings, a multi-institutional, randomized, placebo-controlled trial is being conducted for patients with resected stage IIIB and IIIC melanoma.47
Cell-based vaccines
Cell-based vaccine for lung cancer
The belagenpumatucel-L vaccine for lung cancer (Lucanix®, NovaTx Corporation, San Diego) is an allogeneic whole-cell vaccine, composed of several lung cancer cell lines.48,49 The theoretical advantage to a cell- based vaccine like this is that a variety of tumour antigens may be processed and cross-presented by a patient's endogenous DCs. This vaccine includes two adjuvants; allogeneic MHC (on the cancer cell lines), which strongly activates a large fraction of host lympho cytes, and an antisense molecule targeting TGF-β2. Uptake and expression of the TGF-β antisense molecule theoretically counter- acts the immunosuppressive effects of TGF-β in the tumour microenvironment, potentiating DC activation and preventing the induction of regulatory T cells. The first phase II study of this vaccine evaluated several dose levels in a group of patients with NSCLC with either low-volume early stage disease or with late-stage disease (stages II, IIIA, IIIB and IV).49 A total of 75 patients were randomly assigned to one of three doses: (1.25, 2.5, or 5.0 × 107 cells/injection) of b elagenpumatucel-L on a monthly or every other month schedule. In the group of patients with advanced disease, a response rate of 15% was noted along with an overall survival of 441 days. There was no difference in response or progressionfree survival (PFS) across the three dose groups. As expected, the vaccine was well-tolerated. On the basis of these results, a randomized phase III trial of vaccine ‘main tenance therapy’ has been initiated in patients with stage IIA, IIIB or IV NSCLC that is either stable or responding after initial chemotherapy (Table 1).50
Autologous cell-based vaccine for RCC
Autologous cancer vaccines, manufactured from lysate or whole cells from tumours from individual patients, have been tested in RCC and lung cancer.51,52 As expected, such approaches are complicated by the variability and complexity in generating a vaccine from variable amounts of tissue from patients. To overcome these challenges, a novel approach was developed, whereby a vaccine is generated using RNA derived from tumour tissue, rather than tumour lysate or cells (AGS-003, Argos Therapeutic Inc., Durham).53 This means that substantial quantities of vaccine can be manufactured using a relatively small amount of resected tumour. Rather than relying on the patient's endogenous DCs (which are often defective or dysfunctional), the AGS-003 vaccine used autologous DCs generated ex-vivo, through maturation of immature monocytes in the presence of the cytokines IL-4 and GM-CSF.54 To manufacture AGS-003, patients undergo leukopheresis, and DCs are generated. Simultaneously, tumour RNA is prepared and used to transfect those autologous DCs to generate a mature, cell-based vaccine, which is then frozen and stored for repeated intra-nodal injections.53 In the phase II trial involving the AGS-003 vaccine,55 the incorporation of sunitinib, a standard therapy for RCC, was shown to possess pro immunogenic properties. A phase III trial of AGS-003 is currently in progress (Table 1, NCT01582672);56 this trial will randomly assign 600 patients with metastatic high-risk RCC to receive either sunitinib alone or one cycle (6 weeks) of sunitinb followed by AGS-003 coadministered along with sunitinib. The primary end point of the study is PFS.
Cell-based vaccines for melanoma
One vaccination approach for melanoma was known as Canvaxin™ (CancerVax Corp., USA), an agent com- posed of three irradiated, allogeneic melanoma cell lines.57 After non-randomized phase II trials had suggested promising results as compared with historical controls (5-year overall survival 39% for vaccine-treated patients versus 19% for nonvaccine),58 a phase III trial of Canvaxin™ in the adjuvant setting was conducted. The study enrolled 1,656 patients; 496 and 1,166 of whom had resected stage III or IV melanoma, respectively. In the phase III trial an adjuvant was used; patients were randomly assigned to receive Bacillus Calmette-Guerin (BCG) plus Canvaxin™ or BCG plus placebo. After an interim analysis, the trial was closed by an independent Data and Safety Monitoring Board, on the basis of a low likelihood that a significant survival advantage would be demonstrated in the vaccine arm.59 Correlative immunological studies identified two factors that correlate with improved survival: an elevated IgM response against a glycoprotein expressed by the vaccine called TA90, and a strong delayed-type hypersensitivity response to the vaccine. These data were interesting, implying that a B-cell (antibody) response might be involved, but d evelopment on this agent has been discontinued.
Another adjuvant commonly incorporated in wholecell vaccines is GM-CSF, an important DC attractant. By recognizing that an optimal source of tumour antigens is the patient's own tumour, several trials have demonstrated both clinical and immunological responses to autologous, lethally irradiated, GM-CSF-secreting tumour vaccines in patients with advanced metastatic melanoma. For example, a 1998 trial by Soiffer and colleagues60 enrolled 33 patients with stage IV melanoma. Autologous GM-CSF secreting melanoma vaccines were successfully produced and administered to 21 patients, using retroviral transduction of the GM-CSF gene into short-term cultured tumour cells. One patient experienced a partial response, and evidence of induced cellular and serological immunological activity was observed in biopsies of tumours and vaccine sites. A similar study by Luiten and colleagues61 enrolled 64 patients with stage IV melanoma. Owing to the time required for autologous melanoma vaccine production with retro viral GM-CSF gene transduction, only 28 patients received the full treatment regimen. Among them, however, six patients (21%) experienced long term survival of more than 5 years. A phase I study of an allogeneic GM-CSF-secreting whole melanoma cell vaccine (GVAX®, Aduro Bio Tech, Berkeley, CA) administered alone or in combination with immunomodulatory cyclophosphamide is currently underway for patients with surgically resected at-risk melanoma.62 As in the IMA901 trial for RCC, low-dose cyclophosphamide is used to decrease the number and functionality of TREG.39–41
Additional vaccine approaches
A liposomal vaccine targeting MUC-1
The glycoprotein mucin-1 (MUC-1) is a glycosylated transmembrane protein expressed in normal tissue, especial ly epithelial cells.63 However, MUC-1 can be overexpressed or aberrantly glycosylated in cancer, including NSCLC; approximately 60–70% of NSCLC express the MUC-1 antigen. To target MUC-1 in NSCLC, Merck KGaA (Darmstadt, Germany) engineered a liposomal delivery system to deliver the MUC-1 lipopeptide. This lyophilized liposomal product is a combination of a 25-amino-acid lipopeptide (BLP-25), monophosphoryl lipid A (MPL) and three lipids. To evalu ate L-BLP-25 in NSCLC, a randomized trial was carried out in 171 patients, comparing the vaccine to best supportive care in those patients whose disease had stabilized after treatment with chemotherapy (with or without radiotherapy).64 Patients in the vaccine arm received a single low-dose cyclophosphamide infusion (300 mg/m2) 3 days before initial vaccination. Median overall survival (primary end point), was not significantly different between arms: 17.2 months in the L-BLP-25 group versus 13 months in the best supportive care arm (P = 0.066). However, patients with loco-regional disease (stage IIIB) seemed to show some clinical benefit, with a median survival time of 30.6 months versus 13.3 months in those receiving best supportive care (HR 0.548, 95% CI 0.301– 0.999). Unsurprisingly, the vaccine was well tolerated with few treatment-related adverse events. On the basis of these results, a phase III trial was designed to include only patients with potentially curable stage IIIB disease. In this trial, a total of 1,464 patients with unresect able stage III disease and stable or responding disease after definitive concurrent chemotherapy and radiation were randomized in a 2:1 manner to either L-BLP-25 vaccine or observation (Table 1). Overall survival was not significantly improved in the whole population with a median survival of 25.6 months for the L-BLP-25 group compared to 22.3 months in the observation group (HR 0.88 95% CI 0.75–1.03, P = 0.123). The subset of patients who received concurrent chemotherapy with radiation benefit ed from the vaccine whereas the patients who received sequential chemotherapy followed by radiation did not.65 An identical trial in an Asian patient population is ongoing.66
Viral-based vaccines in NSCLC and RCC
An additional method to generate a cancer vaccine is to incorporate the target antigen into a viral backbone. Poxviruses are particu larly well-suited for such approaches, as they are well-tolerated, can carry a fairly large DNA payload, incorporate several endo genous TLR agonists, and have been administered safely to millions of patients in the worldwide campaign that eliminated smallpox.67 To target MUC-1 in this manner, a Modified Vaccinia Ankara (MVA) strain known as TG4010 was developed (Transgene, Strasbourg, France). As some evidence of response was noted in NSCLC patients in the initial phase I trial of TG4010,68 a phase II trial was designed to evaluate its efficacy in combination with chemo therapy in patients with NSCLC. Patients were random ly assigned to either receive vaccine in combination with standard cisplatin and vinorelbine chemotherapy (Arm A) or to a delayed arm (Arm B) in which chemotherapy was administered only after patients had disease progression following vaccine treatment alone. A total of 65 patients with stage IIIB and IV NSCLC were enrolled. This trial used a Simon 2 stage design, and only Arm A (combination treatment) moved onto stage 2. Of the 44 patients treated on this arm, 37 were evaluable for response, and a response rate of 29.5% was reported.69 On the basis of the relative success of the parallel treatment arm, a followon randomized trial phase IIb that compared chemotherapy alone (gemcitabine and cisplatin) to chemotherapy plus concurrent vaccination was initiated in 148 patients. The 6 month PFS (primary end point) did not differ significantly between arms (43% in the vaccinated group versus 35% in the unvaccinate d group; P = 0.13).70 However, a significantly higher response rate was seen in the vaccinated group overall (42% versus 28% respectively, P = 0.03). Median overall survival was not different between the two arms either; 10.7 months in the TG4010 plus chemotherapy arm versus 10.3 months in the chemotherapy alone arm (HR 0.9, 95% CI 0.6–1.3). This trial led to an ongoing randomized phase IIb/III trial comparing platinum combination chemotherapy for 4–6 cycles with or without vaccine until disease progression (Table 1). The primary end point is overall survival, and the target enrolment is 1,000 advanced stage patients with MUC-1 positive tumours.71 A randomized phase II trial of TG4010 was also carried out in patients with RCC.72 In this trial, patients were treated with cytokine therapy or cytokine therapy plus TG4010. Although the vaccine was well-tolerated, no significant overall survival differences were noted between the two treatment arms, likely dampening enthusiasm for further development of TG4010 in RCC.
Vector-based vaccines in melanoma
One interesting approach to vaccination that has been studied primarily in melanoma, involves intra-lesional injection with an attenuated herpes simplex virus, the goal being lysis of tumour cells (providing an antigen source), along with virally encoded GM-CSF to attract DCs.73 This vaccine is known as talimogenela herparepvec (T Vec) and is being developed by Amgen (Thousand Oaks, CA). In a phase III trial, 436 patients with injectable, unresectable AJCC stage IIIB/C or IV melanoma were randomly assigned to receive either intralesional injection of T Vec, or GM-CSF administered subcutaneously.74 The primary end point of the study was durable response rate (DRR), defined as an objective partial or complete response per modified WHO criteria, beginning within 12 months of treatment initiation and lasting ≥6 months. DRR was 16.3% for patients receiving vaccine compared with 2.1% for those in the GM-CSF group (unadjusted OR 8.9; 95% CI 2.7–29.2; P <0.0001).74 The overall objective response rate for the T-Vec arm was 26.4% and 5.7% for the GM-CSF arm. Of note, among patients who received T-Vec, tumour regressions occurred both in injected and non-injected lesions. These data support the notion that local intra-tumoural vaccination can result in systemic immune responses. Analysis of overall survival in this trial population is ongoing.
Blocking CTLA-4
Lung cancer and kidney cancer
The CTLA-4 blocking antibody ipilimumab (BMS, Princeton, NJ) has been approved by the FDA for the treatment of patients with metastatic melanoma. On the basis of those data and the notion that lung cancer infiltrating T cells might be partially responsive to CTLA- 4 blockade, ipilimumab is currently being evaluated in a randomized phase III trial for the treatment of advanced NSCLC (Table 2). That phase III study is based on the results of an innovative phase II trial that compared two different schedules of ipilimumab combined with chemotherapy versus chemotherapy alone.75 In terms of an immunological rationale, one might postu late that taxane-based chemotherapy could potentially release tumour-specific antigens to help prime an anti-tumour response. Furthermore, several preclinical studies showed that the relative sequence of chemotherapy with immuno therapy can affect outcome.40,41 In this randomized phase II trial, therefore, patients with advanced and previously untreated NSCLC were randomly assigned to receive standard chemotherapy (paclitaxel and carboplatin) or standard chemotherapy plus ipilimumab (10 mg/kg) given according to two different schedules. In one arm (a ‘phased’ schedule), patients received two cycles of chemotherapy followed by four cycles of ipilimumab plus chemotherapy. In a second (concurrent) arm, patients received all three drugs concurrently for four cycles followed by two cycles of chemotherapy alone. If patients had stable or responding disease, they were permitted to continue on maintenance therapy with ipilimumab (once every 12 weeks) until disease progression. The primary end point of this randomized phase II study was PFS as assessed by immuneresponse criteria (irPFS).76 This end point takes into account the type of responses that can occur in patients receiving immunotherapy; that is, tumours might increase in size before significant regression. The precise mechanisms underlying tumour progression prior to regression are unknown, but it has been speculated that this phenomena might reflect an inflammatory infiltrate that later mediates response.76 A total of 204 patients were enrolled, and the study met its primary end point of improved irPFS for the phased versus the control arm (HR 0.72; P = 0.05). Phased chemotherapy and ipilimumab also improved PFS as assessed by modified WHO criteria (HR, 0.69; P = 0.02). In addition, median overall survivals of 12.2 months, 9.7 months, and 8.3 months were reported in the phased, concurrent and control arms, respectively, but these were not significantly differ ent. In a preplanned subset analysis, patients with squamous-cell histology showed a significantly improved irPFS as well as overall survival with the phased schedule versus control (chemotherapy alone), although patient numbers were small. In patients with squamous-cell carci noma (SCC) treated with the phased schedule versus control, the HR ratio for irPFS and overall survival were 0.55 (95% CI 0.27–1.12) and 0.48 (95% CI 0.22– 1.03), respectively. The combination of ipilimumab with paclitaxel and carboplatin was not significantly more toxic than chemotherapy alone, except in the concurrent arm in which treatment discontinuation was greater due to toxi city. Taken together, these data suggest that a phased treatment schedule could potentially provide clinical benefit in patients with lung cancer compared with chemo therapy alone. A phase III trial testing that hypothesis was initiated in 2011.77 The trial compares the phased schedule of ipilimumab with paclitaxel and carbo platin versus paclitaxel and carbo platin alone in patients with stage IV SCC. The primary end point of the trial is overall survival and the target enrollment is 920 patients. Ipilimumab is also being evaluated in combination with platinum and etoposide in patients with extensive-stage small-cell lung carcinoma.78
Table 2
Selected phase II and III trials of immune checkpoint blockade in lung, RCC and melanoma
| Agent and study | Phase | Cancer type | Design and description | n | Results and comments |
|---|---|---|---|---|---|
| Nivolumab (anti-PD-1)10 Phase I multi-dose trial of BMS-936558 | Ib | NSCLC, RCC, MEL | NSCLC expansion cohorts at 1, 3, 10 mg/kg RCC expansion cohorts at 1 mg/kg MEL expansion cohorts randomized to 0.1, 0.3 and 1.0 mg/kg | 122 with NSCLC 34 with RCC 107 with MEL | NSCLC RR 17%, median OS ~10 months RCC RR 33%, median OS >22 months MEL RR 31%, median OS ~17 months |
| BMS-936559 (anti-PD-L1) Phase I multi-dose trial of BMS-9365599 | I | NSCLC, RCC, MEL | NSCLC expansion cohorts at 1, 3, 10 mg/kg dose levels RCC cohort | 49 with NSCLC 17 with RCC | RR 10% (duration of response 9.8–16.6 months); 24 week SD rate 12% RR 12%; 24 week SD rate 41% |
| Ipilimumab (anti-CTLA-4)75 | II | NSCLC | Randomized trial of paclitaxel and carboplatin or delayed schedule of paclitaxel, carboplatin and ipilumumab, or concurrent schedule of the three-drug combination | 204 | Primary end point (ir-PFS) improved in the delayed arm vs chemotherapy. Patients with squamous cell histology had a significantly improved irPFS and OS on the delayed schedule. Nonsquamous histology did not experience an improvement |
| Nivolumab NCT01642004 | III | NSCLC | Randomized trial of nivolumab vs docetaxel in the second-line treatment setting in patients with stage IV squamous cell NSCLC | 264 | Primary end point: RR and OS |
| Nivolumab NCT01673867 | III | NSCLC | Randomized trial of nivolumab vs docetaxel in the second or third-line treatment setting in patients with stage IV nonsquamous cell NSCLC | 574 | Primary end point: OS |
| Nivolumab NCT01721759 | II | NSCLC | Trial of single agent nivolumab for treatment of advanced squamous cell NSCLC status post at least two prior systemic regimens | 100 | Primary end point: RR |
| Nivolumab NCT01354431 Enrollment completed | II | RCC | Randomized, blinded, dose-ranging study of patients with progressive or advanced RCC who have received prior antiangiogenic therapy | 150 | Primary end point = PFS compared between three dose levels (0.3, 2.0, 10 mg/kg every 3 weeks) |
| Nivolumab NCT01358721 | I | RCC | Exploratory study evaluating the immune effects of various dose levels of anti-PD-1 in patients with metastatic RCC | 80 | Companion biomarker study to study above, includes a treatment naïve cohort |
| Nivolumab NCT0166878 | III | RCC | Randomized, open-label, study of anti-PD-1 vs everolimus in patients with advanced or metastatic RCC. Patients have received prior antiangiogenic therapy | 822 | Primary end point: OS |
| Nivolumab NCT01844505 | III | MEL | Randomized, double-blind study of nivolumab monotherapy or nivolumab combined with ipilimumab vs ipilimumab monotherapy | 915 | Primary end point: OS |
| Lambrolizumab NCT01704287 | III | MEL | Randomized study of two different doses of lambrolizumab vs chemotherapy of choice in patients with advanced (progressed) MEL | 510 | Co-primary end point: PFS and OS |
| Lambrolizumab NCT01704287 | III | MEL | Randomized, double-blind study of two schedules (every 2 weeks or once every 3 weeks) of lambrolizumab vs ipilimumab in patients with advanced (progressed) MEL | 645 | Co-primary end point: PFS and OS |
| Ipilimumab + chemotherapy NCT01285609 | III | NSCLC | Randomized paclitaxel/carboplatin alone or plus ipilimumab in patients with stage IV squamous cell NSCLC | 920 | Primary end point: OS |
| Nivolumab + chemotherapy NCT01454102 | I | NSCLC | Open-label, randomized multi-arm trial of platinum combination chemotherapy, ipilimumab, bevacizumab as maintenance, and erlotinib in patients with NSCLC in the first-line treatment or maintenance treatment setting | 108+ | Dose de-escalation phase I study with primary end point of safety |
| Nivolumab + TKI NCT01472081 | I | RCC | Anti-PD-1 in combination with sunitinib, pazopanib or ipilimumab in patients with metastatic RCC | 72 | Primary end point: safety and tolerability |
| Nivolumab + anti-KIR NCT01714739 | I | All solid tumours, followed by cohort expansions for RCC and NSCLC | Study of the safety, tolerability and efficacy of KIR antibody (BMS-986015) administered in combination with anti-PD-1 in advanced refractory solid tumours | 150 | Primary end point: safety and tolerability |
| Nivolumab + anti-IL-21 NCT01629758 | I | All solid tumours, followed by cohort expansions for RCC and NSCLC | Dose-escalation study of BMS-982470 (recombinant interleukin-21, rIL-21) in combination with BMS-936558 (anti-PD-1) | 165 | Primary end point: safety |
| MPDL3280A (anti-PD-Ll) + vemurafenib NCT01656642 | Ib | MEL | Open-label, multicentre, dose-escalation and cohort-expansion study in combination with vemurafenib in patients with V600E BRAF mutation | 44 | Another study of safety and tolerability of ipilimumab plus vemurafenib was closed to further accrual after high levels of toxicity were observed among the first 12 patients enrolled |
Abbreviations: KIR, anti-killer inhibitory receptor; MEL, melanoma; NSCLC, non-small-cell lung cancer; OS, overall survival; PD-1, programmed death-1; RCC, renal cell carcinoma; RR, response rate; SD, stable disease; vs, versus.
CTLA-4 blockade has also been evaluated in patients with metastatic RCC; a phase- II trial conducted primarily at the National Cancer Institute (NCI) treated 61 patients with 3 mg/kg doses of ipilimumab every 3 weeks, or with a single 3 mg/kg ‘loading dose’ followed by 1 mg/kg doses every 3 weeks. In this trial, sequential cohorts, with no planned comparative analyses were assessed.79 Partial responses were observed in five out of 40 patients receiving the higher dose. Grade 3 or 4 IRAEs were observed in 33% of patients, potentially a higher rate than that observed in melanoma patients, and likely reflecting the continuous 3 week dosing regimen used here. Interestingly, a clear association between immunerelated toxicity and responses was observed in this trial as well. At this time, single agent CTLA-4 blockade is not under study in RCC, most likely owing to competition from the relative plethora of targeted agents, both FDA-approved and in clinical trials.
Blocking CTLA-4 in melanoma
The preclinical data and the clinical trial results that resulted in FDA approval of ipilimumab for metastatic melanoma have been reviewed previously.24 Recently, updated survival and toxicity data from a phase III study of ipilimumab plus dacarbazine versus dacarbazine plus placebo in treatment-naive patients with melanoma were presented.80 The 3 year and 4 year survival rates for patients treated with combination therapy were 21.2% and 19.0%, respectively, compared with 12.1% and 9.6% for patients treated with dacarbazine plus placebo. More recently, Hodi and colleagues81 presented data from a phase II trial comparing ipilimumab plus GM-CSF versus ipilimumab alone. The study included 245 patients with unresectable stage III or metastatic stage IV melanoma. Although rates of response and PFS were similar between the two arms, with a median follow up of 13.3 months, 1 year overall survival was significantly higher in patients who received combination therapy: (68.9% versus 52.9%, stratified log rank p1 = 0.016, p2 = 0.033, HR 0.65). The adverse event rate for patients who received the GM-CSF-containing regimen was also improved compared with ipilimumab alone. Several combinator ial studies involving ipilimumab and stereotactic radiation, chemotherapy (for example, abraxane), interferon, IL-21, oncogenic pathway inhibitors, anti-PD-1 or T cell therapy are currently underway for patients with melanoma (Table 2). Not all combinations will be well tolerated; a recent trial of ipilimumab plus vemurafenib was halted prematurely after demonstrating high levels of hepatotoxicity.82 Ipilimumab is also being evaluated in combination with T-Vec for patients with unresectable melanoma.83
Blocking PD-1
PD-1 blockade in lung cancer
A monoclonal antibody that blocks the interaction between the immune checkpoint molecule PD-1 and its ligand PD-L184 entered clinical trials in patients with cancer in late 2007.85 Preclinical data available at that time showed modest efficacy for single-agent PD-1 blockade in established mouse tumour models;17 thus, clinical expectations were fairly low. Surprisingly, evidence of clinical activity in multiple tumour types was noted even in the initial dose-escalation study, in which the drug was administered in an intermittent schedule, with a first dose followed by two additional doses given at 3 and 4 month time points.85 Objective responses were noted in patients with melanoma and RCC, which were not unexpected. More unexpected, though, were responses in a patient with colorectal cancer, and a mixed response in a patient with NSCLC; both of these tumour types are generally considered to be non-immunogenic.85 Toxicity in this trial proved surprisingly mild, with no grade 3 or 4 adverse events reported. However, the intermittent dosing schedule should certainly be taken into account when consider ing toxicity in this early trial. This initial phase I trial was followed by a multi-dose phase Ib trial that enrolled 129 patients with NSCLC.10 On the basis of pharmaco kinetic data from the first phase I trial that showed a serum halflife of 12–20 days, dosing was markedly accelerated; the PD-1 agent was given at 2-week intervals, with tumour evaluations conducted every 8 weeks. The once every 2-week dosing schedule proved to be tolerable at all dose levels tested with no maximum tolerated dose reached. Activity was seen in patients with melanoma, RCC, and NSCLC, even during the lead-in dose-escalation portion of the trial. On the basis of these encouraging results, three cohorts of 32 NSCLC patients each were enrolled at 1 mg/kg, 3 mg/kg, or 10 mg/kg dose levels, as part of a study amendment. Equal numbers of squamous versus non-squamous histologies were enrolled at each dose level. The most common treatment-related adverse effects rated grade 2 or higher included fatigue, anaemia, and diarrhoea, which were similar to the treatment group as a whole. The most worrisome toxi city in this trial was lung inflammation (pneumonitis). Grade 1 and 2 pneumo nitis was reported in 2% of the overall population and in 3% of the NSCLC group. Grade 3 or 4 pneumonitis was seen in only three patients in the entire study with two of those being patients with NSCLC. However, two patients with NSCLC died owing to pneumonitis-related complications. The response rate in the NSCLC population was 17% (22/129 patients), remarkable for this population who had been heavily pretreated.86 In addition to the patients who had a standard RECIST response (tumour shrinkage without progression), six additional patients had significant response on protocol-specified imaging studies carried out 8 weeks after studies that had shown initial tumour progression. Some of these responses were durable with a median duration of 74 weeks. Several NSCLC patients remained on treatment for a total of 2 years, the time allowed on treatment as dictated by the clinical protocol.86 The median overall survival in these patients (half of whom had received three to five previous therapies) was 9.6 months. On the basis of the preliminary activity seen in this large phase Ib trial, two phase III trials of BMS 936558/MDX-1106 (now designated nivolumab), were recently initiated in patients with NSCLC (Table 2). Both trials will compare nivolumab with standard docetaxel-based chemotherapy.
Another trial is enrolling patients in a second-line treatment setting (Table 2).87 In this trial, the co-primary end points are response rate and overall survival. In a second study, patients with non-squamous-cell lung cancer who have received one previous platinum-based chemotherapy (not including maintenance therapy), or one previous platinum based-regimen and tyrosine kinase inhibitor if they have EML4-ALK translocation or EGFR mutation positive disease, are being treated with either docetaxel or nivolumab. The primary end point of this phase III study is overall survival (Table 2).88 Several early phase trials of nivolumab in lung cancer are underway. For lung cancer patients with SCC previously treated with at least two chemotherapy regimens, a single-arm trial of nivolumab as a single agent is accruing patients.89 In the first-line treatment setting for metastatic NSCLC, there is a fairly complex ongoing phase I trial combining nivolumab with several standard platinum chemotherapy combinations, erlotinib as first-line therapy, or with bevacizumab as maintenance therapy (NCT01454102).90 This phase I study will also evaluate the safety and efficacy of administering nivolumab as a single agent in the first-line setting, as maintenance therapy or as monotherapy in patients with untreated, asymptomatic brain metastases. The trial also includes an arm with combined checkpoint blockade, in which nivolumab is administered in parallel with ipilimumab. Preliminary data from the combination of nivolumab with platinum-based chemotherapy was presented in 2013.91 In general, the combinations were well tolerated with no observed dose-limiting toxicities. In total, 20% of patients stopped treatment due to treatment-related adverse events. Response rates to the chemotherapy plus nivolumab combinations ranged from 33% to 50%. No difference was seen between 5 mg/kg and 10 mg/kg doses of nivolumab in combination with chemotherapy. Given the small numbers of patients involved, it is not yet clear whether the response rate to the combination of chemo therapy and PD-1 blockade is significantly different from the response to anti-PD-1 monotherapy. Nor is it clear whether this combination regimen will be further pursued in larger randomized trials.
PD-1 blockade in RCC
In contrast to NSCLC, the observation of objective responses to a single-agent in RCC patients treated with anti-PD-1 in phase I trials was not completely unexpect ed. Indeed, a patient with advanced RCC showed a stable partial response for over 2 years in the first-in-man dose-escalation study.85 Perhaps more noteworthy, this sustained partial response eventually evolved into a documented complete response, and the patient has remained off-treatment for over 5 years at last followup.30 This indication of clinical activity for PD-1 blockade in RCC was supported by data from the more dose-intense phase Ib trial discussed above.92 In that study, the objective response rate was 30–35%, with an additional 10% of patients showing prolonged stable disease.92 On the basis of the activity seen in phase I trials, three phase I and II studies of nivolumab in RCC have been initiated, and a phase III trial is now open to accrual. One critical study in the development of this agent was a dose-ranging study.93 This trial enrolled a total of 150 patients, randomized into treatment cohorts at doses of 0.3 mg/kg, 2 mg/kg and 10 mg/kg, treated every 3 weeks (in contrast to the once every 2 week dosing in the phase Ib study). Accrual to that trial has been completed, but final data have not yet been reported. A second, perhaps more-interesting trial (NCT01358721)94 mirrors that design, but incorporates carefully collected pretreatment and post-treatment biopsies in an effort to define biomarkers predictive of response. The ‘biomarker trial’ also includes a group of patients with previously untreated disease, which has not been carried out before for anti-PD-1 in RCC. On the basis of unpublished data from our lab and others that showed that tyrosine kinase inhibitors such as sunitinib and pazopanib can prove additive with PD-1 blockade in orthotopic animal models of RCC, a phase I study (NCT01472081) combining PD-1 blockade with the tyrosine kinase inhibitors pazopanib or sunitinib95 was initiated; that study is currently ongoing. Most importantly from a clinical standpoint is a potentially pivotal, randomized phase III study (NCT01668784; Table 2).96 This trial will randomly assign 820 previously treated RCC patients in a 1:1 ratio to receive either nivolumab at a dose of 3 mg/kg every 2 weeks or to standard second-line therapy with the mTOR inhibitor everolimus given at a dose of 10 mg once daily. The primary end point of the study is overall survival.
PD-1 blockade in melanoma
In the first large phase I trial of nivolumab, objective responses were observed in 31% of 107 heavily-pretreated patients with melanoma, whereas an additional 7% of patients experienced stable disease lasting ≥24 weeks.97 Responses tended to be rapid and durable: 45% of responding patients achieved a partial response or complete response within 8 weeks of treatment initiation, and median duration of response was 104 weeks. Similar to results from another trial of nivolumab,30 some responses persisted even after therapy was discontinued. Overall, the drug was well-tolerated demonstrating a 5% rate of grade 3 or 4 adverse events at a follow up ≥1 year. A second anti-PD-1 antibody, lambro lizumab (MK3475, Merck, Geneva) was evaluated in a phase I trial that included pre-treated patients, some of whom had previously received ipilimumab.98 Across all dose levels the confirmed objective response rate according to RECIST 1.1 criteria was 38%, although 52% of patients who received lambro lizumab at the highest dose level (10 mg/ kg every 2 weeks) demonstrated a response. Grade 3 or 4 toxic effects were observed in 12.6% of patients. There were no significant differences in rates of response or toxi city between ipilimumab-naïve patients and those who received prior ipilimumab, suggest ing that the immuno suppressive pathways m ediated by CTLA-4 and PD-1 are me chanistically distinct.
Blocking PD-L1 in multiple cancers
In addition to blocking PD-1, the inhibitory interaction between PD-1 on T-cells and PD-L1 on either tumour cells or other cells in the tumour micro environment can be blocked by antibodies directed against the ligand PD-L1.17–19 Indeed, in animal models of chronic infection99 and self-tolerance,100 blocking PD-L1 can be as efficacious as blocking PD-1. A fully human mono-clonal antibody was initially developed by the Medarex corporation as MDX-1105, then brought to the clinic as BMS-936559 (BMS Princeton, NJ). The first-in-human study of that agent enrolled 207 patients, including 75 patients with metastatic NSCLC and 52 patients with metastatic melanoma.9 The antibody was administered once every 2 weeks in 6-week treatment cycles for up to 16 cycles or until confirmed complete response or disease progression was documented. The maximum toler ated dose was not reached during the dose-escalation portion of the trial and expansion cohorts of several tumours—including NSCLC—were subsequently enrolled. In general, the antibody was well tolerated with a grade 3 and 4 treatment–related toxicity rate of only 9%.9 The most common treatment-related adverse effects were fatigue, infusion reaction, and diarrhoea. A total of 49 NSCLC patients were evaluable for response at the time of publication. Of these 49 patients, five patients had an objective response. Six additional patients had stable disease lasting for more than 6 months. Similarly, nine of 52 patients with melanoma (17%) experienced an objective response, five of which lasted ≥1 year. An additional 14 patients (27%) experienced stable disease that lasted ≥24 weeks. So, in general, BMS 936559 was well-tolerated and demonstrated activity in NSCLC and melanoma.
Overall and disease-specific data from a phase I study that assessed a second anti-PD-L1 antibody, MPDL3280A, were also reported recently.101 In this phase I trial, the anti-PD-L1 antibody was given once every 3 weeks. No maximum tolerated dose was found and the agent was generally well tolerated with grade 3 and 4 treatment-related adverse events occurring in 13% of patients. Only 2% of patients experienced grade 3 or 4 IRAEs, and no grade 3–5 pneumonitis was seen. The response rate in lung cancer patients was 22%, 29% in patients with mela-noma, and 15% in patients with RCC. However, it should be noted that these data encompass several dose levels; for example, patients with RCC were treated at 3 (n = 2), 10 (n = 12), 15 (n = 18) and 20 mg/kg (n = 21).102 As is the case with PD-1 blockade, disease stabilization occurred in approximately 35% of patients with RCC. Similar to anti-PD-1, anti-PD-L1 was generally well-tolerated, with approximately 40% of patients experiencing grade 3 or 4 adverse events including hyphophosphatemia, fatigue and hyperglycaemia. Anti-PD-L1 tumour staining was performed on a subset of patients in the MPDL3280A trial; of the 36 patients with PD-L1-positive tumours, 13 patients responded, giving a response rate of 36% in that group. Conversely, patients with PD-L1 negative tumours also responded, although the response rate was significantly less (13%). Taken together, these clinical trials confirm the concept that blocking the interaction between PD-1 and PD-L1 is a promising approach to treating multiple tumour types; however, it also indicates that blocking PD-L1 might be associated with a lower overall rate of objective responses, at least in these early datasets.
Combined immune checkpoint blockade
Early pre-clinical data showed that combinatorial blockade of PD-1 and CTLA-4 resulted in significantly increased antitumour immunity when compared with blocking either checkpoint alone.103 These observations were recently translated to the clinic, in an important phase I combination trial. Wolchok and colleagues104 tested the concurrent and sequential administration of ipili mumab and nivolumab. Doses of both drugs were varied among seven cohorts of patients. Among 52 patients who received both agents concurrently, 21 (40%) demonstrated a con-firmed objective response according to modified WHO criteria. Many responses were rapid and profound: at 12 weeks, 16 patients demon strated reductions in tumour burden of ≥80%. Grade 3 or 4 treatment-related toxic effects were observed in 53% of patients, although in only six patients were these adverse events dose-limiting. Tumour biopsies were evaluated for PD-L1 expression with a newly developed automated assay that uses a rabbit mono clonal anti-human PD-L1 antibody (clone 28-8).105 Among patients who received a concurrent regimen, six of 13 with PD-L1–positiv etumour samples and nine of 22 with PD-L1–negative tumour samples demon-strated objective responses; that is, PD-L1 expression seemed to have less of a relation ship with response in this combination trial than observed in previous anti-PD-1 monotherapy trials. Studies in patients with RCC106 and NSCLC107 have recently opened combined anti-CTLA-4 and anti-PD-1 arms, and it will be interesting to see if the melanoma results will extend to other cancer types. It also remains to be seen whether this combination will prove tolerable, or whether further dose and schedule optimization will be required. Finally, it should be noted that relevant preclinical work has demon strated synergy combining PD-1 blockade with blockade of other immune checkpoint molecules, including LAG-3108 and TIM-3;109 so translating those combinations into the clinic could provide additional treatment options going forward.
Conclusions
Logically, inducing an anti-tumour immune response through vaccination is appealing, and would seem to be fairly straightforward. In clinical practice, achieving objective anti-tumour responses through vaccination is quite rare,110 although at least one phase III trial has documented improved overall survival with the vaccine Sipuleucel-T in prostate cancer resulting in subsequent FDA approval.42 Cancer vaccines remain valid treatment approaches in lung cancer, RCC and melanoma, with at least six random ized phase III trials in various stages of accrual and completion. It is worth mentioning that one of these trials, the MAGRIT trial of a MAGE-A3 vaccine for NSCLC is the largest interventional trial ever conducted in that disease, reflecting the interest in bringing a lung cancer vaccine to patients. In very sharp contrast, immune checkpoint blockade with CTLA-4, PD-1 and PD-L1 blocking antibodies has demon strated clear evidence of objective responses, driving renewed enthusiasm for cancer immuno therapy in multiple cancer types. This reinvigoration is perhaps most prominent in the case of NSCLC, which was previously thought to be a tumour type insensitive to immunotherapy. Indeed, two agents blocking PD-1 have rapidly moved from phase I to phase III trials in multiple tumour types, setting the stage for a series of results that are eagerly awaited over the next several years. Notably, several of these trials (the phase I study in NSCLC especially) seek to combine conventional therapy with immune checkpoint blockade; such an approach is likely to be required if we want most of our cancer patients to respond to treatment.
Acknowledgements
C. G. Drake is a Damon Runyon-Lilly Clinical Investigator and is supported by National Institutes of Health R01 CA127153, 1P50CA58236-15, the Patrick C. Walsh Fund, the One-in-Six Foundation, the Koch Foundation and the Prostate Cancer Foundation. E.J. Lipson is supported by the Melanoma Research Alliance and the John P. Hussman Foundation. J.R. Brahmer is supported by the Stand Up to Cancer Foundation.
Footnotes
Competing interests C. G. Drake has declared associations with the following companies: BMS, CoStim, Dendreon, Pfizer Inc. J. R. Brahmer has declared associations with the following companies: BMS Inc., Merck Inc. See the article online for full details of the relationships. E. J. Lipson declares no competing interests.
Author contributions All authors researched the data for the article and co-wrote the article. C.G. Drake edited the manuscript prior to submission, and all three authors reviewed and edited the article before submission and after peer review.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/nrclinonc.2013.208
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4086654?pdf=render
Citations & impact
Impact metrics
Article citations
First-Line Nivolumab and Relatlimab Plus Chemotherapy for Gastric or Gastroesophageal Junction Adenocarcinoma: The Phase II RELATIVITY-060 Study.
J Clin Oncol, 42(17):2080-2093, 09 May 2024
Cited by: 3 articles | PMID: 38723227 | PMCID: PMC11191068
Fourteen years of cellular deconvolution: methodology, applications, technical evaluation and outstanding challenges.
Nucleic Acids Res, 52(9):4761-4783, 01 May 2024
Cited by: 4 articles | PMID: 38619038 | PMCID: PMC11109966
Review Free full text in Europe PMC
Informing immunotherapy with multi-omics driven machine learning.
NPJ Digit Med, 7(1):67, 14 Mar 2024
Cited by: 2 articles | PMID: 38486092 | PMCID: PMC10940614
Review Free full text in Europe PMC
Triple-Negative Breast Cancer Intrinsic FTSJ1 Favors Tumor Progression and Attenuates CD8+ T Cell Infiltration.
Cancers (Basel), 16(3):597, 31 Jan 2024
Cited by: 0 articles | PMID: 38339348 | PMCID: PMC10854779
Sirpα on tumor-associated myeloid cells restrains antitumor immunity in colorectal cancer independent of its interaction with CD47.
Nat Cancer, 5(3):500-516, 10 Jan 2024
Cited by: 10 articles | PMID: 38200243
Go to all (232) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (Showing 18 of 18)
- (2 citations) ClinicalTrials.gov - NCT01358721
- (1 citation) ClinicalTrials.gov - NCT01582672
- (1 citation) ClinicalTrials.gov - NCT00480025
- (1 citation) ClinicalTrials.gov - NCT00676507
- (1 citation) ClinicalTrials.gov - NCT01454102
- (1 citation) ClinicalTrials.gov - NCT01472081
- (1 citation) ClinicalTrials.gov - NCT01015443
- (1 citation) ClinicalTrials.gov - NCT00409188
- (1 citation) ClinicalTrials.gov - NCT01714739
- (1 citation) ClinicalTrials.gov - NCT01673867
- (1 citation) ClinicalTrials.gov - NCT01354431
- (1 citation) ClinicalTrials.gov - NCT01721759
- (1 citation) ClinicalTrials.gov - NCT01656642
- (1 citation) ClinicalTrials.gov - NCT00828009
- (1 citation) ClinicalTrials.gov - NCT00796445
- (1 citation) ClinicalTrials.gov - NCT00395070
- (1 citation) ClinicalTrials.gov - NCT01629758
- (1 citation) ClinicalTrials.gov - NCT01383148
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Regulatory T cells-an important target for cancer immunotherapy.
Nat Rev Clin Oncol, 11(6):307, 29 Apr 2014
Cited by: 3 articles | PMID: 24781417
Reply: Regulatory T cells-an important target for cancer immunotherapy.
Nat Rev Clin Oncol, 11(6):307, 29 Apr 2014
Cited by: 3 articles | PMID: 24781414 | PMCID: PMC4732695
Prediction of response to anticancer immunotherapy using gene signatures.
J Clin Oncol, 31(19):2369-2371, 28 May 2013
Cited by: 35 articles | PMID: 23715576
Upcoming innovations in lung cancer immunotherapy: focus on immune checkpoint inhibitors.
Chin Clin Oncol, 4(4):48, 01 Dec 2015
Cited by: 3 articles | PMID: 26730760
Review
Funding
Funders who supported this work.
NCI NIH HHS (6)
Grant ID: U10 CA180802
Grant ID: P50 CA058236
Grant ID: T32 CA009071
Grant ID: R01 CA127153
Grant ID: 1P50CA58236‑15
Grant ID: P30 CA006973





