Abstract
Free full text

Neutralizing activity of monoclonal antibodies to heat-sensitive and heat-resistant epitopes of Rickettsia rickettsii surface proteins.
Abstract
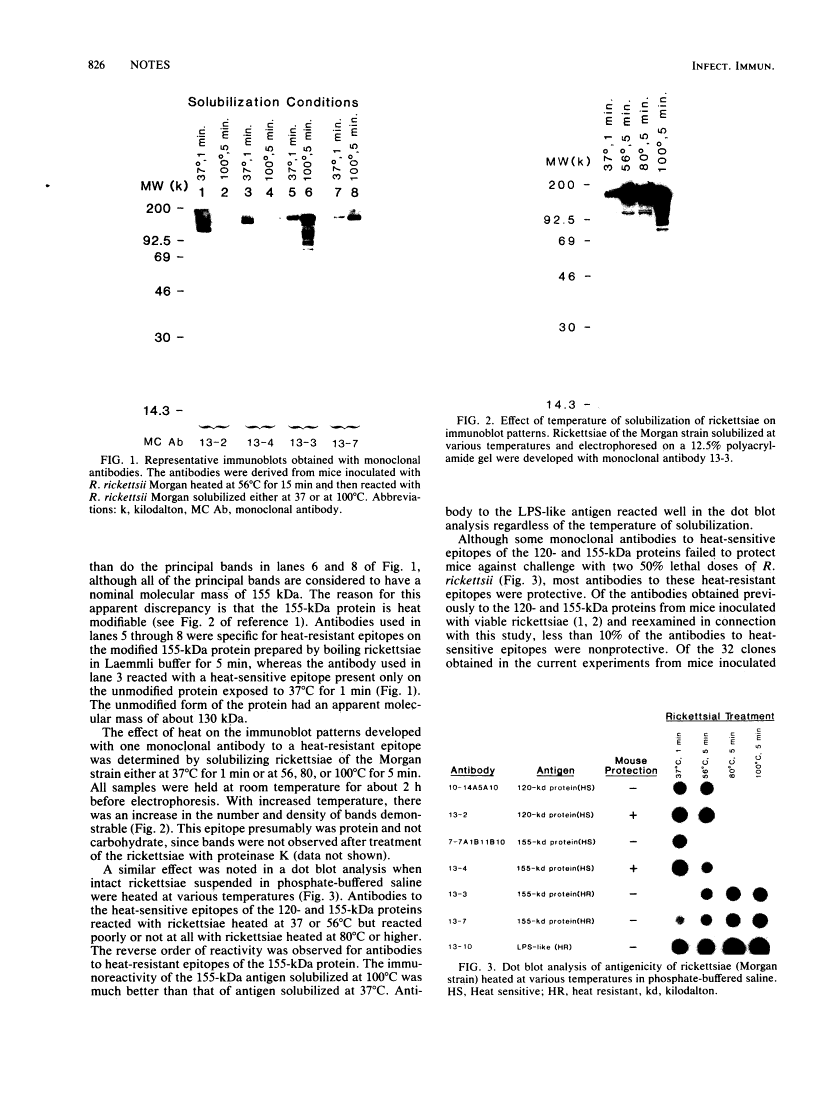
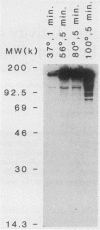
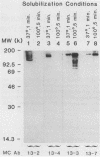
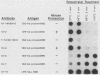
Antiprotein monoclonal antibodies derived from mice inoculated with Rickettsia rickettsii heated at 56 degrees C for 15 min are of two types: one is type specific for epitopes denatured by moderate temperatures, and the other is specific for epitopes resistant to 100 degrees C for 5 min. The heat-resistant epitopes are found by immunoblotting on multiple polypeptides after solubilization of the rickettsiae at temperatures of 56 degrees C or higher. Most, but not all, antibodies to the heat-sensitive epitopes passively protected mice against two 50% lethal doses of R. rickettsii, whereas none of the antibodies to heat-resistant epitopes did.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (876K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker RL, List RH, Mann RE, Hayes SF, Thomas LA. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J Infect Dis. 1985 Jun;151(6):1052–1060. [Abstract] [Google Scholar]
- Anacker RL, List RH, Mann RE, Wiedbrauk DL. Antigenic heterogeneity in high- and low-virulence strains of Rickettsia rickettsii revealed by monoclonal antibodies. Infect Immun. 1986 Feb;51(2):653–660. [Europe PMC free article] [Abstract] [Google Scholar]
- Anacker RL, Philip RN, Williams JC, List RH, Mann RE. Biochemical and immunochemical analysis of Rickettsia rickettsii strains of various degrees of virulence. Infect Immun. 1984 Jun;44(3):559–564. [Europe PMC free article] [Abstract] [Google Scholar]
- Batteiger B, Newhall WJ, 5th, Jones RB. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. [Abstract] [Google Scholar]
- BELL EJ, PICKENS EG. A toxic substance associated with the rickettsias of the spotted fever group. J Immunol. 1953 May;70(5):461–472. [Abstract] [Google Scholar]
- Ferguson M, Minor PD, Magrath DI, Qui YH, Spitz M, Schild GC. Neutralization epitopes on poliovirus type 3 particles: an analysis using monoclonal antibodies. J Gen Virol. 1984 Jan;65(Pt 1):197–201. [Abstract] [Google Scholar]
- Hitchcock PJ, Brown TM. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. [Europe PMC free article] [Abstract] [Google Scholar]
- Kondo E, Kondo Y. Monoclonal antibodies to hog thyroglobulin recognizing disulfide-dependent conformational structures. Mol Immunol. 1984 Jul;21(7):581–588. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- STOENNER HG, LACKMAN DB, BELL EJ. Factors affecting the growth of rickettsias of the spotted fever group in fertile hens' eggs. J Infect Dis. 1962 Mar-Apr;110:121–128. [Abstract] [Google Scholar]
- Weber K, Pringle JR, Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. [Abstract] [Google Scholar]
- Weiss E, Coolbaugh JC, Williams JC. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.55.3.825-827.1987
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/55/3/825.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/55/3/825
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/55/3/825
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/108084723
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/iai.55.3.825-827.1987
Article citations
Vaccine Design and Vaccination Strategies against Rickettsiae.
Vaccines (Basel), 9(8):896, 12 Aug 2021
Cited by: 9 articles | PMID: 34452021 | PMCID: PMC8402588
Review Free full text in Europe PMC
The neglected challenge: Vaccination against rickettsiae.
PLoS Negl Trop Dis, 14(10):e0008704, 22 Oct 2020
Cited by: 10 articles | PMID: 33091016 | PMCID: PMC7581008
Review Free full text in Europe PMC
Targeted knockout of the Rickettsia rickettsii OmpA surface antigen does not diminish virulence in a mammalian model system.
mBio, 6(2):e00323-15, 31 Mar 2015
Cited by: 45 articles | PMID: 25827414 | PMCID: PMC4453529
Pathogenic Rickettsia species acquire vitronectin from human serum to promote resistance to complement-mediated killing.
Cell Microbiol, 16(6):849-861, 13 Dec 2013
Cited by: 18 articles | PMID: 24286496 | PMCID: PMC4028375
Tropism and pathogenicity of rickettsiae.
Front Microbiol, 3:230, 25 Jun 2012
Cited by: 20 articles | PMID: 22737150 | PMCID: PMC3382384
Review Free full text in Europe PMC
Go to all (41) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Production and characterization of monoclonal antibodies to Rickettsia rickettsii.
Infect Immun, 46(2):289-294, 01 Nov 1984
Cited by: 20 articles | PMID: 6209219 | PMCID: PMC261528
Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii.
J Infect Dis, 151(6):1052-1060, 01 Jun 1985
Cited by: 65 articles | PMID: 3923129
Protective monoclonal antibodies recognize heat-labile epitopes on surface proteins of spotted fever group rickettsiae.
Infect Immun, 56(10):2587-2593, 01 Oct 1988
Cited by: 51 articles | PMID: 2458318 | PMCID: PMC259616
Cloned gene of Rickettsia rickettsii surface antigen: candidate vaccine for Rocky Mountain spotted fever.
Science, 235(4784):83-85, 01 Jan 1987
Cited by: 53 articles | PMID: 3099387