Abstract
Free full text

Role of gut pathogens in development of irritable bowel syndrome
Abstract
Acute infectious gastroenteritis is one of the most commonly identifiable risk factors for the development of irritable bowel syndrome (IBS). A number of bacterial, viral and parasitic pathogens have been found to be associated with the development of IBS and other functional gastrointestinal (GI) disorders. Epidemiological studies have identified demographic and acute enteritis-related risk factors for the development of post-infectious-IBS (PI-IBS). Immune dysregulation, alterations in barrier function, serotonergic and mast cell activation have been identified as potential pathophysiological mechanisms. Additionally, variations in host genes involved in barrier function, antigen presentation and cytokine response have been associated with PI-IBS development. However, it is unknown whether specific pathogens have unique effects on long-term alterations in gut physiology or different pathogens converge to cause common alterations resulting in similar phenotype. The role of microbial virulence and pathogenicity factors in development of PI-IBS is also largely unknown. Additionally, alterations in host gut sensation, motility, secretion, and barrier function in PI-IBS need to be elucidated. Finally, both GI infections and antibiotics used to treat these infections can cause long-term alterations in host commensal microbiota. It is plausible that alteration in the commensal microbiome persists in a subset of patients predisposing them to develop PI-IBS.
Introduction
Gastrointestinal (GI) infections are globally prevalent and cause significant morbidity and mortality. Chronic GI disorders including celiac disease1, inflammatory bowel disease (IBD)2 and irritable bowel syndrome (IBS)3 have been causally associated with infectious gastroenteritis (IGE). Since the first description of post-infectious IBS (PI-IBS) provided by Chaudhary and Truelove in 1962 among patients with proven or presumptive episode of bacterial or amoebic dysentery4, numerous epidemiological studies have confirmed the association between acute IGE with different pathogens and development of IBS and other functional GI disorders (FGIDs)3. In addition to outbreaks in community or hospitalized settings, acute IGE sustained during a travel5 or military recruitment6 has also been associated with development of PI-IBS. Studies in different settings and with different pathogens have yielded a wide range (4-36%) of individuals developing PI-IBS after an episode of IGE3. Bacterial IGE seems to be more closely related to the development of long-term PI-IBS phenotype7 as compared to viral IGE8. Long-term follow up studies suggest that bacterial pathogen related PI-IBS phenotype may persist for up to 8-10 years after the acute IGE is over9,10. Epidemiological studies have identified female gender, age <60 yr, smoking, enteritis severity, and pre-enteritis psychological stress as risk factors for development of PI-IBS11. Existing literature supports the role of enterochromaffin (EC) cell hyperplasia, altered barrier function, immune dysregulation and potentially mast cell activation in the pathophysiology of PI-IBS. However, a number of prior studies have been conducted after epidemics often involving >1 pathogen or in highly selected tertiary referral patients. Thus, there is limited insight into unique pathogen-specific mechanisms from isolated, community-based gastroenteritis cases, and into physiological and clinical sequelae based on the pathogen and host characteristics.
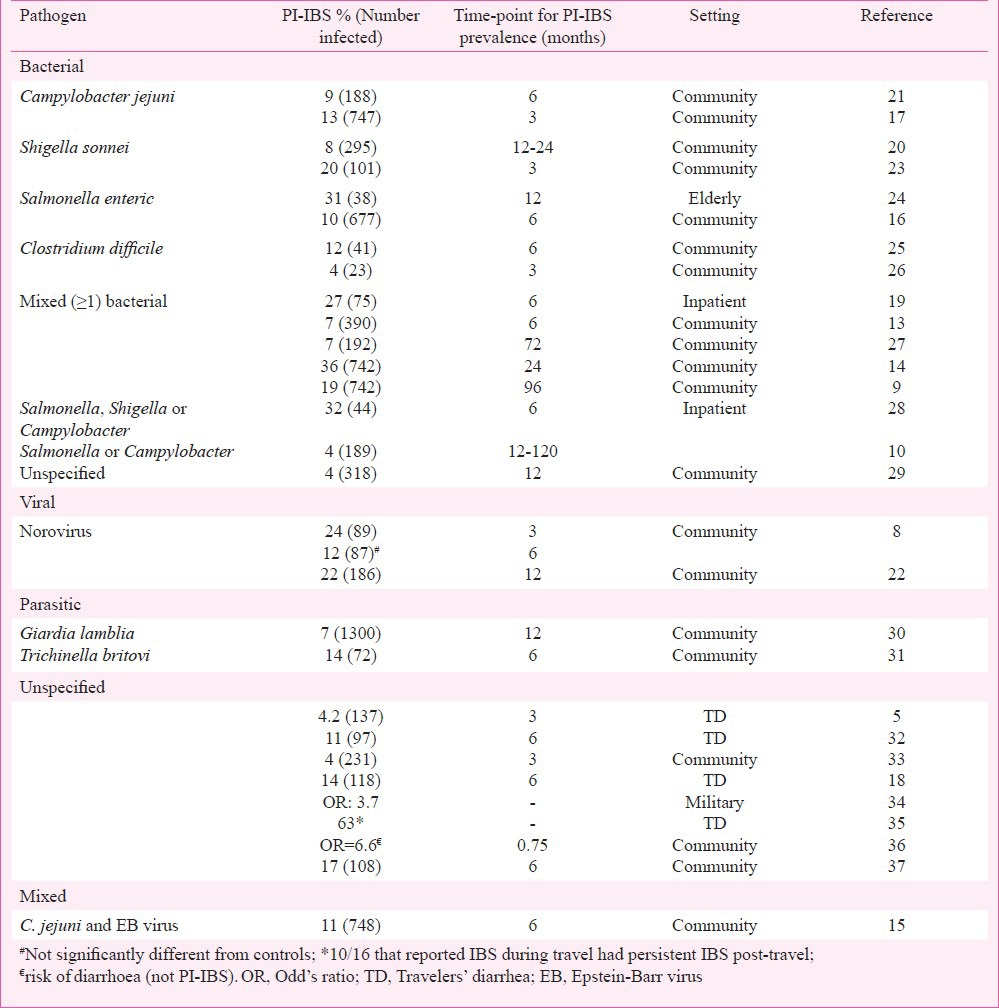
Epidemiology
Gastrointestinal infections with isolated (bacterial, viral), mixed (> 1 bacteria, bacteria and a virus), and unspecified (travelers’ diarrhoea) pathogens have all been associated with the development of PI-IBS. Most of the larger cohorts are from outbreaks involving bacterial pathogens12. However, in several studies involving outbreaks, the information on the pathogen involved is often not available in all subjects and is only assumed. Although, these studies have shown a wide range (4-36%) of individuals developing PI-IBS, a meta-analysis revealed median prevalence of 10 per cent (compared to 1.2% in controls)13. This also showed a relative risk ranging between 2-12 and a pooled odds ratio of 7.3 for development of PI-IBS. The Table provides a comprehensive list of PI-IBS epidemiological studies according to the pathogen involved. An outbreak in Walkerton, Canada in 2000 caused by contamination of municipal water supply from livestock faecal material resulted in acute gastrointestinal illness in over half of the town's 4800 residents has provided significant epidemiological information about PI-IBS12. Although, pathogen information was not available in all cases, mixed bacterial infection with Campylobacter jejuni and Escherichia coli O157:H7 was found be responsible for most of the cases. Two years post-infection, a PI-IBS rate of 36 per cent among patients that had IGE during the outbreak was seen12.
Table
Percentage of individuals developing post-infections - irritable bowel syndrome (PI-IBS) with different enteritides
Risk factors
Age and gender
Age >60 yr was found to be protective for development of PI-IBS in a large community survey (relative risk: 0.36)14. Younger age was found to be an independent risk factor for PI-IBS development in the Walkerton outbreak cohort12. However, other studies did not confirm an effect of age on PI-IBS development15,16. Studies have debated the effect of gender on PI-IBS development when the effect was either not found16 or was lost once psychological variables were controlled for17,38. However, in other larger studies15 including the Walkerton outbreak cohort12, female gender was identified as a risk factor. This goes along with the increased prevalence of IBS and other FGIDs in women.
Smoking
A single study showed smoking to be associated with PI-IBS development (odds ratio: 4.8)39. However, smoking can be a marker for psychological distress, hence associating with PI-IBS, which makes it harder to draw any conclusions based on the limited evidence.
Antibiotic use
Prevalence of PI-IBS following Salmonella enteritidis enteritis was not found be different among groups that did or did not use antibiotics (17.6 vs 9.3%, not significant) in one study16. However, another post-Salmonella study showed an increase in the prevalence of persistent GI symptoms in a group that received antibiotics (9.5 vs 2.9%)40. A travelers’ diarrhoea study showed antibiotic use to be associated with development of PI-IBS (relative risk: 4.1)18. It is quite plausible that antibiotic induced changes in commensal microflora can persist in vulnerable subset of hosts predisposing them to long-term changes in gut physiology resulting in development of PI-IBS.
Psychological factors
There was an increased prevalence of anxiety, depression, somatization, and neurotic traits at the time of IGE in the group that developed PI-IBS19. Patients who develop IBS report more adverse life events in preceding three months and hypochondriasis scores38 and these features predicted PI-IBS development independent of the anxiety and neuroticism scores. A study also showed depression to be a risk factor (relative risk: 3.2)17. Another study showed higher levels of perceived stress, anxiety, somatization, negative illness beliefs at the time of acute enteritis to be associated with PI-IBS. Depression was not found to be a risk factor in this study41. The influence of psychological stress on PI-IBS is interesting and under-studied. Catecholamines and other stress mediators have been shown to play a role in modulating pathogenic infectivity and host epithelial-microbial interactions42 which can play a role in increasing severity of enteritis and subsequent development of post-infectious phenotype.
Enteritis severity
Shigella enteritis lasting >14 days was more significantly associated with PI-IBS development as compared to illness lasting <8 days (relative risk: 4.6)20. Similarly, another mixed bacterial enteritis study showed greater likelihood of PI-IBS development with illness lasting >3 weeks vs <8 days (relative risk: 11.4)14. Diarrhoea >7 days was associated with PI-IBS development in the Walkerton outbreak cohort12. Additionally, presence of bloody stools, abdominal cramps, and > 10lb weight loss was also found to be associated with PI-IBS in this cohort. A single study examining the role of bacterial pathogenicity factors showed Campylobacater jejuni strain producing toxin with elongating effects on the Chinese hamster ovary cells was associated with increased risk of developing persistently deranged bowel habits21. Overall, this suggests that enteritis severity appears to play a role in the development of PI-IBS. However, clinical severity of enteritis might be related to microbial virulence or variations in host response. More needs to be done to elucidate role of microbial virulence factors in development of PI-IBS.
Clinical features
In the absence of a biomarker or a universally accepted definition for PI-IBS, the Rome criteria43 for defining IBS are usually used to define PI-IBS. This requires presence of recurrent abdominal pain or discomfort at least three days/month in the last three months associated with two or more of the following: (i) improvement with defaecation, (ii) onset associated with a change in frequency of stool, and (iii) onset associated with a change in form (appearance) of stool. These historical characteristics must be fulfilled for the last three months with symptom onset at least six months prior to diagnosis. To characterize as PI-IBS, the individual must not meet criterion for IBS prior to the infection and the acute IGE must be characterized either by presence of positive stool cultures or presence of ≥2 of the following: fever, vomiting, or diarrhoea. The stool cultures are often not available since a number of individuals develop acute IGE as travelers’ diarrhoea. The PI-IBS phenotype is most commonly IBS-D44 and IBS subtype phenotypic clusters have been found to remain stable over time45. Previous treatment for anxiety or depression was reported less frequently in PI-IBS than non-PI-IBS (26 vs 54%).
Natural history
The Walkerton outbreak cohort investigators reported the 8 year follow up data and the prevalence of IBS dropped to 15.4 per cent after 8 years, still remaining significantly increased compared with controls without history of acute IGE (OR 3.12; 95% CI 1.99 to 5.04)9. This suggests that PI-IBS phenotype persists for a prolonged duration. Another study showed that symptoms persist for upto 10 years following the initial infection10. A recent analysis of the defense database showed an increased risk of dyspepsia, constipation, and gastroesophageal reflux disease among military recruits who had suffered from IGE from norovirus46. In another food-borne outbreak study of norovirus, 23.6 per cent reported symptoms consistent with PI-IBS at three months vs 3.4 per cent who remained well (odds ratio: 6.9). However, at 6, 12, and 24 months, the prevalence of IBS was similar among exposed versus non-exposed individuals8. Vomiting during the IGE episode independently predicted risk of PI-IBS at three months8. From a recent viral outbreak in Italy, 13 per cent of IGE patients were found to have PI-IBS at 12 months, and most of these were mixed-IBS (IBS-M). This study also showed that the presence and severity of symptoms progressively diminished at 12 month follow up as compared to the 6 month survey22. Both these studies suggest that post-viral IBS may be a more transient phenomenon as compared to bacterial PI-IBS. This might be related to a lesser degree of initial epithelial damage and host inflammatory response caused by viral as compared to bacterial pathogens.
Mechanisms
Mucosal cellular changes
Post-Campylobacter IBS is characterized by an increase in rectal mucosal enterochromaffin (EC) cells, lamina propria T-lymphocytes17,47, CD8 intraepithelial lymphocytes and calprotectin-immunoreactive (ir) cells47. Post-Shigella IBS is characterized by increase in ileal mast cells and nerve fibers immunoreactive for neuron-specific enolase, substance P, and 5-hydroxytryptamine (5-HT, serotonin) seen adjacent to the mast cells20. Another study showed an increase in 5-HT-ir EC cells, peptide YY (PYY)-ir EC cells, intraepithelial lymphocytes, CD3, CD8 lymphocytes, mast cells and CD68 cells48. Colonic mucosal supernatants from PI-IBS patients resulting from unspecified IGE caused stimulation of peritoneal mast cell protease-activated receptor (PAR2) mRNA expression49. Another study looking at mucosal tissue, however, showed decrease in mast cell PAR4 expression but unchanged PAR2 expression50. Mast cell numbers were unchanged but activated mast cells and tryptase concentration were increased in supernatants from PI-IBS patients (unspecified IGE). Similar to post-Campylobacter IBS, increased mean chronic inflammatory cells38, EC cells, lamina propria T lymphocytes without any changes in mast cell numbers were seen44. Overall, EC cell hyperplasia and activation seem to play a significant role in pathophysiology of PI-IBS, however, the role of mast cells is unclear at this time.
Alterations in gut permeability
An increase in 0-6 h lactulose/mannitol (L/M) ratio initially and at 12 wk has been observed after acute IGE with C. jejuni; however, it is unclear if these patients met criteria for IBS47. Another study showed increase in 3-6 h Cr51 EDTA excretion initially after Campylobacter gastroenteritis and at 6 months, however, this cohort is also not confirmed to have IBS phenotype51. Increased L/M excretion after overnight collection has also been reported in PI-IBS patients from the Walkerton outbreak cohort52. Using 51Cr-EDTA excretion, proximal small bowel permeability was found to be altered in PI-IBS (unspecified pathogen); however, in this study non-PI-IBS patients had greater alterations in small bowel permeability as compared to PI-IBS patients53. In vivo permeability alterations or ex vivo changes in mucosal barrier function are unknown in PI-IBS resulting from other bacterial or viral pathogens.
Cytokine expression
No differences were seen in mucosal interleukin (IL)-10, tumour necrosis factor (TNF)α and IL-1β expression in post-Campylobacter IBS51. Peripheral blood mononuclear cell TNFα cytokine expression has been found to be increased in post-Campylobacter IBS, however, no differences were seen in IL-10, or IL-1β expression. This study also showed increased TNFα rs180062951. Another post-Campylobacter study did not show any differences in IL-18 and interferon (INF)γ polymorphisms54. Increased terminal ileal and rectosigmoid IL-1β expression in post-Shigella IBS25 and rectal IL-1β expression in post-mixed infection IBS55 have been seen. Patients with PI-IBS following mixed infection showed an increase in peripheral blood mononuclear cell TNF-α, IL-1β, IL-6, and lipopolysaccharide-stimulated IL-6 levels56.
Gene expression
The Walkerton outbreak cohort showed variations in three genes to be independently associated with PI-IBS. These are cytokine gene IL6 [rs206986; OR 1.509 (1.031-2.209)], tight junction E-cadherin gene CDH1 [rs16260; OR 1.398 (1.069-1.829) and TLR9 [rs 5743836; OR 1.536 (1.080-2.182)] encoding for pattern recognition receptor for bacteria that detects unmethylated CpG dinucleotide57. Together, this reflects a role of host immune activation and barrier function genetics in the development of IBS in that cohort. In another study51, mucosal expression of CCL11 [chemokine (C-C) motif ligand l], CCL13, Calpain 8 and TNFSF15 (TNF superfamily member 15) increased while NR1D1, G-protein coupled receptor 161 (GPR161) and gamma-aminobutyric acid receptor subunit epsilon (GABRE) decreased post-C. jejuni group (not PI-IBS). TNFα rs1800629 minor allele frequency was increased in post-C. jejuni group compared to healthy controls51. Polymorphisms in IL-18 and INF γ were studied in a C. jejuni/coli gastroenteritis cohort but none were found to be linked with the development of PI-IBS at 6-month follow up54.
Treatment
The first step in the management should be to illicit the history of IGE preceding the onset of symptoms. This finding can often be a subtle on history and patients may just endorse acute onset of symptoms during or after a travel without recall of classical symptoms of acute IGE. A stool culture is unlikely to be available in most cases. Although PI-IBS is characterized by acute onset of symptoms after a GI infection, many patients provide a history of respiratory illness preceding the onset of IBS. Although, this cannot be typically characterized as PI-IBS, it can be considered to be in the same spectrum of FGIDs. Once PI-IBS is clinically suspected, it is often helpful to educate the patient about the role of gut infections in development of IBS symptoms and a suspected causal link can be helpful in “legitimizing” the disorder. It is also helpful to reassure since the PI-IBS is likely to improve over time than deteriorate, especially if a viral pathogen is suspected to be involved.
Subsequently, the overall clinical management is driven by nature and severity of the symptoms and is not different from management of IBS, details of which can be found elsewhere58. A few clinical trials specific to PI-IBS have been performed. In a randomized, double-blind, placebo controlled trial of prednisolone at a dose of 30 mg/day for three weeks, lamina propria T-lymphocytes decreased but no differences were seen in EC cell counts or clinical endpoints of abdominal pain or diarrhoea59. An open-labelled trial of 18 patients using mesalamine 800 mg three times a day for 30 days showed improvement in scores for abdominal pain, distension, stool frequency and consistency60. This study also showed similar improvements in 43 non-PI-IBS patients treated with same dose and duration of mesalamine. Another double-blind, placebo controlled study using mesalamine (Asacol®) 1.6 g twice a day in 20 patients with PI-IBS showed improvement in global symptoms, abdominal pain, bloating, stool urgency, frequency, or consistency and quality of life61.
Conclusions
Gastrointestinal infections with pathogenic microbes constitute an important risk factor for development of IBS and other FGIDs. In spite of a significant knowledge of epidemiological risk factors, little is known about pathophysiological mechanisms of PI-IBS. Existing literature supports the role of immune dysregulation, altered barrier function, EC cell activation and host genetics. Much needs to be done to identify novel mechanisms such as the role of microbial pathogenicity factors, alterations in host commensal microbiota, and post-infectious plasticity in the neuromuscular apparatus of the gut. From the stand-point of the host, alterations in gut physiology using deep phenotyping measures in studies of GI transit, sensation and permeability and central nervous system changes using functional brain imaging will be necessary to find subsets of PI-IBS patients with unique physiological alterations. Overall, this subset of IBS provides an opportunity to prospectively recruit patients and study mechanism for PI-IBS and IBS in general. Additionally, studies involving isolated, identifiable pathogens will likely provide more relevant mechanistic information.
References
Articles from The Indian Journal of Medical Research are provided here courtesy of Scientific Scholar
Citations & impact
Impact metrics
Citations of article over time
Article citations
The efficacy and neural mechanism of acupuncture therapy in the treatment of visceral hypersensitivity in irritable bowel syndrome.
Front Neurosci, 17:1251470, 04 Sep 2023
Cited by: 3 articles | PMID: 37732301 | PMCID: PMC10507180
Review Free full text in Europe PMC
Post-Infectious Irritable Bowel Syndrome after an Epidemic of Gastroenteritis in South of Iran.
Middle East J Dig Dis, 14(3):304-309, 30 Jul 2022
Cited by: 1 article | PMID: 36619262 | PMCID: PMC9489434
The characteristics of intestinal flora of IBS-D with different syndromes.
Immun Inflamm Dis, 8(4):615-628, 17 Sep 2020
Cited by: 6 articles | PMID: 32940426 | PMCID: PMC7654421
Genome-Wide Identification of Host-Segregating Single-Nucleotide Polymorphisms for Source Attribution of Clinical Campylobacter coli Isolates.
Appl Environ Microbiol, 86(24):e01787-20, 24 Nov 2020
Cited by: 13 articles | PMID: 33036986 | PMCID: PMC7688228
An Investigation of the Effect of Catecholamines and Glucocorticoids on the Growth and Pathogenicity of Campylobacter jejuni.
Pathogens, 9(7):E555, 10 Jul 2020
Cited by: 7 articles | PMID: 32664224 | PMCID: PMC7400237
Go to all (14) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
On the fiftieth anniversary. Postinfectious irritable bowel syndrome: mechanisms related to pathogens.
Neurogastroenterol Motil, 26(2):156-167, 01 Feb 2014
Cited by: 28 articles | PMID: 24438587
Review
Post-infectious irritable bowel syndrome following water contamination.
Kidney Int Suppl, (112):S42-3, 01 Feb 2009
Cited by: 8 articles | PMID: 19180133
Review
Intestinal Barrier in Post-Campylobacter jejuni Irritable Bowel Syndrome.
Biomolecules, 13(3):449, 28 Feb 2023
Cited by: 6 articles | PMID: 36979384 | PMCID: PMC10046606
Role of infection in irritable bowel syndrome.
J Gastroenterol, 42 Suppl 17:41-47, 01 Jan 2007
Cited by: 103 articles | PMID: 17238025
Review



