Abstract
Free full text

Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling
Summary
Missense mutations in the p53 tumor suppressor inactivate its anti-proliferative properties but can also promote metastasis through a gain-of-function activity. We show that sustained expression of mutant p53 is required to maintain the pro-metastatic phenotype of a murine model of pancreatic cancer, a highly metastatic disease that frequently displays p53 mutations. Transcriptional profiling and functional screening identified the platelet-derived growth factor receptor b (PDGFRb) as both necessary and sufficient to mediate these effects. Mutant p53 induced PDGFRb through a cell-autonomous mechanism involving inhibition of a p73/NF-Y complex that represses PDGFRb expression in p53-deficient, non-invasive cells. Blocking PDGFRb signaling by RNA interference or small molecule inhibitors prevented pancreatic cancer cell invasion in vitro and metastasis formation in vivo. Finally, high PDGFRb expression correlates with poor disease-free survival in pancreatic, colon, and ovarian cancer patients, implicating PDGFRb as a prognostic marker and possible target for attenuating metastasis in p53 mutant tumors.
Introduction
Mutations in the p53 tumor suppressor gene represent the most common genetic lesions in cancer (Freed-Pastor and Prives, 2012). Functional studies indicate that wild-type p53 possesses a series of anti-proliferative activities that limit the proliferation and survival of pre-malignant cells. p53 exerts these activities, at least in part, through its ability to bind DNA in a sequence-specific manner to regulate gene expression, and the vast majority of mutations that occur in human tumors disable this property of p53 and, consequently, its anti-proliferative effects.
p53 mutations typically occur within the DNA-binding region and involve either DNA contact residues or residues important for conformational structure, both resulting in loss of DNA binding (Joerger and Fersht, 2007). Because p53 functions as a tetrameric transcription factor, mono-allelic p53 mutations can exert dominant-negative effects on a coexpressed wild-type p53 protein. p53 activates E3 ubiquitin ligases that feed back to trigger p53 destruction and its rapid turn over; however, p53 missense mutants defective in regulating gene expression lead to the stable accumulation of the variant proteins (Oren et al., 2010). Interestingly, genetically engineered mice harboring common p53 point mutations develop more aggressive and metastatic tumors compared to those arising in their p53 heterozygous or null counterparts (Lang et al., 2004; Olive et al., 2004, Hanel et al., 2013), suggesting that the mutant forms of p53 exert gain-of-function activities independent of their effects on wild-type p53. Accordingly, human tumors with mutant p53 are associated with poor patient prognosis (Soussi and Beroud, 2001) and drug resistance (Masciarelli et al., 2013).
Recently, targeting mutant p53 function has been proposed as an anti-metastatic measure. As p53 mutant proteins have to date proved undruggable (Levine and Oren, 2009; Lehmann and Pietenpol, 2012), efforts have focused on identifying the underlying mechanisms that mediate its effects. Such efforts have identified proteins involved in integrin recycling (Muller et al., 2009), the mevalonate pathway (Freed-Pastor et al., 2012) or miRNA biogenesis (Su et al., 2010) as potential mediators of mutant p53 action in invasion and metastasis. So far, most studies have been performed in breast cancer and the proposed mechanisms do not necessarily validate across cancer types. These observations underscore the importance of the cellular context in assessing mutant p53 action, and highlight the potential complexity of the effector network.
Pancreatic ductal adenocarcinoma (PDAC) is one cancer type in which mutant p53 impacts disease progression. PDAC arises from indolent pancreatic intraepithelial neoplasias (PanINs) that frequently go undetected and persist for many years. However, the conversion of PanINs to highly aggressive, frankly invasive and metastatic PDACs, in which p53 is mutated in 75% of cases, carries a dire prognosis due to late stage detection, the presence of metastases, and ineffective treatment options (Li et al., 2004). Even those patients with a surgically approachable pancreatic lesion develop recurrent and metastatic disease after local tumor resection (Hidalgo, 2010). Consistent with a role for mutant p53 in this process, mice harboring pancreatic cancers driven by oncogenic Kras and a mutant p53 allele show more metastases compared to identical mice harboring a p53 null allele (Morton et al., 2010). However, it is not known whether mutant p53 is needed to sustain the metastatic phenotype and how it is regulated. Such information would produce insights into p53 action and validate mutant p53 as a therapeutic target.
In this study, we combined several orthogonal approaches and models to systematically explore the molecular basis whereby mutant p53 promotes invasion and metastasis in PDAC and the clinical implications of its effects. These studies identified the platelet-derived growth factor receptor beta (PDGFRb) as necessary and sufficient to mediate the effects of mutant p53 on invasion and metastasis in both a murine model and human PDAC cells. Further, we identified elevated PDGFRb expression as an indicator of poor metastasis-free survival in human PDAC patients. Taken together, our data identify a key mediator of mutant p53 activity and suggest that PDGFRb inhibitors may act as anti-metastatic agents in some patients with tumors expressing mutant p53.
Results
Sustained Expression of Mutant p53 is Required for the Invasive Phenotype of Pancreatic Cancer Cells
Genetically engineered mouse models of pancreatic cancer harboring a latent oncogenic Kras allele (lox-stop-lox KrasG12D), a latent mutant p53R172H, and a tissue specific Cre recombinase (Pdx1-Cre), also known as KPC mice, develop highly metastatic pancreatic cancer that faithfully mimics the human disease (Hingorani et al., 2005). To understand the impact of p53 mutations on cell invasion and metastasis in this well-defined genetic system, we employed a murine KPC pancreatic cancer cell line that lost the remaining p53 wild type allele during disease progression (Morton et al., 2010). The behavior of KPC cell lines stably expressing shRNAs targeting (mutant) p53, or control shRNAs targeting Renilla (KPC+sh.Ctrl), were compared to one another and to a p53-null KPflC cell line (from a Pdx-Cre, LSL-KrasG12D/+, LSL-p53loxP/+) expressing the control shRNA (KPflC+sh.Ctrl).
We confirmed that KPC+sh.Ctrl cells expressing mutant p53 efficiently migrated in scratch-wound assays. This motility depended on mutant p53, as mutant p53 knockdown in KPC+sh.p53 cells reduced motility similarly to p53-/-KPflC+sh.Ctrl cells (Figure 1A). Next, we examined the invasive capacity of KPC cells into collagen gels in an inverted invasion assay. The presence of mutant p53 in KPC+sh.Ctrl cells enhanced invasiveness, which was significantly abrogated upon p53 knockdown (Figure 1B). The ability of mutant p53 to drive cell invasion was also evident following enforced expression of p53R175H and p53R273H, two mutants frequently found in human PDAC, in KPflC cells. This result indicates that the differences in invasiveness of KPC and KPflC cells depends on mutant p53 and were not acquired during generation and selection of cell populations expressing shRNAs or through RNAi off-target effects.
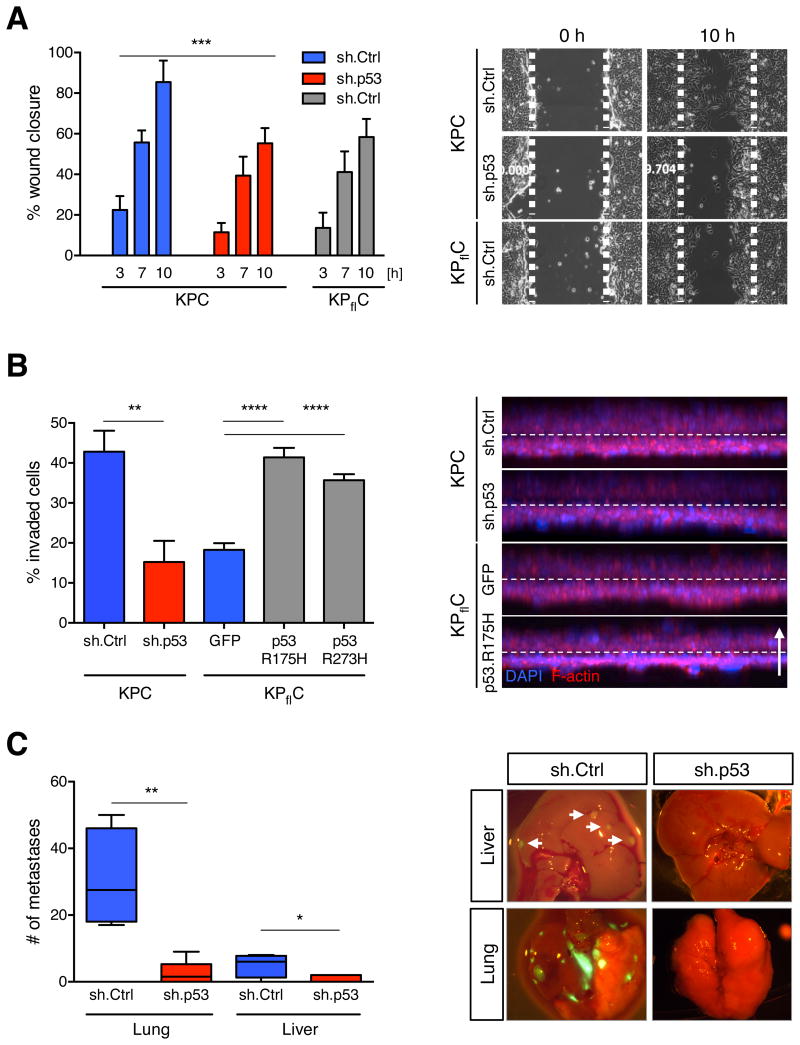
(A) Quantifications of wound distances in scratch-wound assays from 0, 3, 7, and 10 h after wounding of KPC cells stably expressing a nontargeting control shRNA (sh.Ctrl) or a shRNA targeting mutant p53 (sh.p53) or KPflC cells stably expressing a sh.Ctrl (left panel). Data presented as mean ±SD. ***p < 0.001. Representative phase contrast images from live cell recordings of each condition are shown at 0 and 10 h (right panel). (B) KPC+sh.p53 and +sh.Ctrl as well as KPflC cells expressing the GFP control and mutant p53 (175H and 273H) vector were allowed to invade into Collagen for 72 h before quantification as described in the Experimental Procedures (left panel). The average of invaded cells from 9 replicates ±SD is shown. A representative result of three repeated experiments is shown. **p < 0.01, ****p < 0.0001. Representative 3D reconstructions of each condition are shown (right panel). Cells were stained for F-actin (red) and DAPI (blue); dashed line indicates the approximate position of the Transwell membrane; the arrow indicates the direction of movement. (C) KPC+sh.p53 or +sh.Ctrl were orthotopically injected into the pancreata of athymic mice. When symptomatic, mice were euthanized and metastatic spread in lung and liver was quantified by counting GFP-positive macroscopic nodules (left panel). Data presented as mean ±SD. *p < 0.05, **p < 0.01. Panels show representative merged brightfield/GFP images from lung and liver (right panel).
To test whether mutant p53 expression was required to sustain the metastatic potential of KPC cells, we orthotopically injected KPC+sh.Ctrl and KPC+sh.p53 cells into the pancreata of athymic mice and scored the number of metastases formed in both lung and liver, the most common sites of pancreatic cancer spread in patients. Although the primary tumor burden was independent of p53 status, tumors originating from KPC+sh.Ctrl cells expressing mutant p53 were significantly more metastatic than those in which mutant p53 was silenced, and metastases in the lung and liver were detected to a greater extent in mice that had been injected with cells expressing mutant p53 (Figure 1C). Together, these results demonstrate that mutant p53 can contribute to PDAC invasion and metastasis and that inhibiting its activity can have an anti-metastatic effect.
Transcriptional Profiling and Functional Screening Identifies PDGFRb as a Downstream Mediator of Mutant p53 in Murine Pancreatic Cancer
To gain insight into how mutant p53 mediates the invasive phenotype of PDAC, we performed genome-wide transcriptome profiling of KPC cells by RNA sequencing (RNAseq). Four days following knockdown of mutant p53 in three independent clonal KPC populations, we observed a complex pattern of gene expression changes compared to three independent KPC+sh.Ctrl cell lines. We identified 441 genes either significantly up- or down regulated upon shRNA-mediated depletion of endogenous mutant p53 (Figures 2A and S1A). Ingenuity pathway analysis revealed that ~20% of the affected genes fall into the functional class of “cellular movement”, supporting our experimental observations that mutant p53 can govern the invasive phenotype of pancreatic cancer cells (Figures 2B and S1C).
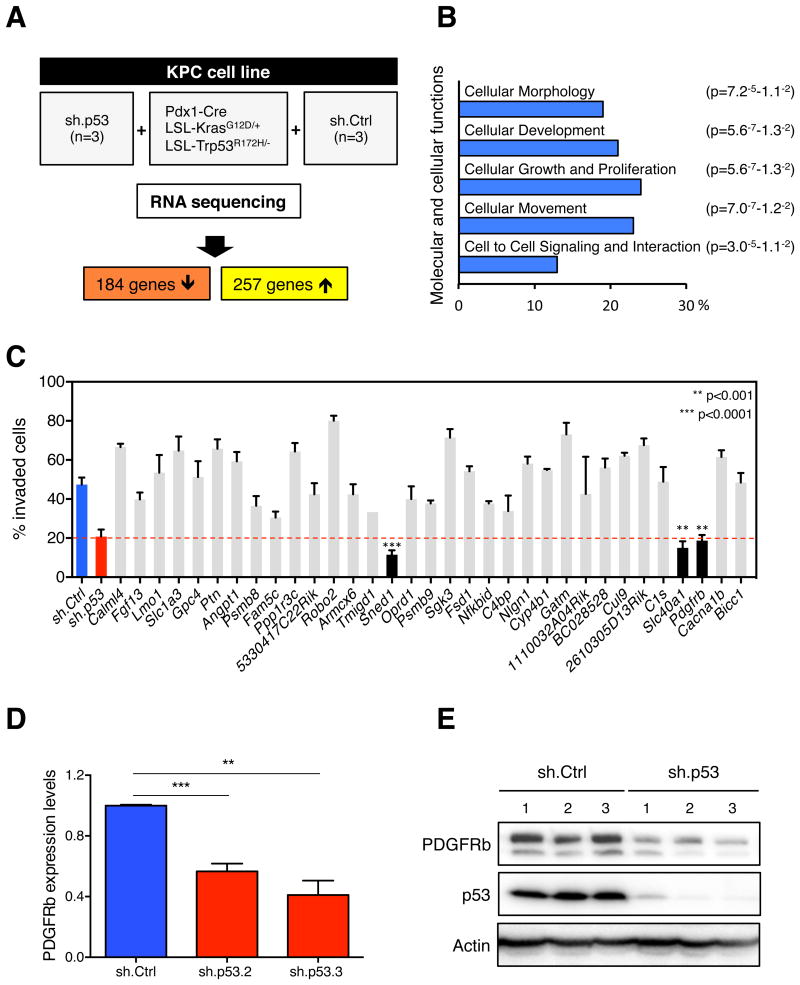
(A) Schematic workflow of RNA sequencing. (B) Ingenuity pathway analysis (Ingenuity Systems, www.ingenuity.com). Bars (p < 0.05) represent molecular and cellular functions that are significantly changed following mutant p53 depletion. (C) One-by-one invasion assay screen. Quantification of invaded KPC cells infected with individual shRNA-pools (~3.6 shRNAs/gene) targeting the top 40 upregulated genes identified by RNAseq. Data presented as mean ±SD. (D) qRT-PCR for PDGFRb in KPC+sh.p53 (2 or 3) or +sh.Ctrl cells. Data presented as mean normalized PDGFRb expression ±SD of triplicate samples. A representative result of three repeated experiments is shown. (E) Western blotting analysis of PDGFRb, p53, and Actin in sh.p53- or sh.Ctrl-expressing KPC cells. The two bands of PDGFRb represent differentially glycosylated forms of the protein. See also Figure S1 and Table S1.
To facilitate the identification of mediators of mutant p53 activity, we focused on genes whose expression was positively regulated by mutant p53, as such molecules might both mediate effects of mutant p53 and be targets for pharmacological inhibition. Therefore, we generated pools of 3-6 shRNAs targeting individual upregulated genes, and screened them one-by-one to identify those that phenocopied the decreased invasion seen upon downregulation of mutant p53 (Table S1). We identified three genes whose knockdown abrogated invasion driven by mutant p53 (Figure 2C). SLC40A1 is a cell membrane protein that has been shown to mediate cellular iron efflux (Montalbetti et al., 2013); SNED1 is a stromal marker that induces cisplatin-resistance in head and neck squamous carcinoma (Longati et al., 2013); and PDGFRb is a receptor tyrosine kinase that mediates PDGF-regulated proliferation, survival and chemotaxis (Dai, 2010).
Oncogenic properties of mutated or amplified PDGFRa have been extensively studied in several tumor types, whereas PDGFRb has been exclusively linked to tumor angiogenesis via paracrine effects (Pietras et al., 2003; Cao et al., 2004). Based on our screening results, we hypothesized that PDGFRb may also have a cell-autonomous impact on cell invasion in pancreatic cancer. First, we verified by RT-qPCR and western blotting that PDGFRb mRNA and protein were reduced upon knockdown of mutant p53 (Figures 2D and 2E). Expression of mutant p53 correlated with high PDGFRb expression levels and also with the expression of key downstream mediators of the PDGFRb signaling cascade (Figure S1B).
We next examined the effects of depleting each PDGFR isoform on the invasive potential of KPC cells in vitro (Figure 3A). Although knockdown of PDGFRa had no effect, depletion of PDGFRb decreased the ability of KPC cells to invade (Figure 3B). Conversely, overexpression of PDGFRb in p53-/- KPflC enhanced cell migration and invasion, similarly to cells over-expressing mutant forms of p53 (Figure S2A). Reduced levels of PDGFRb in KPC cells neither altered the rate of cell proliferation (Figure S2B) nor led to a competitive proliferative disadvantage over KPC cells expressing high PDGFRb levels (Figure S2C). When GFP-positive KPC+sh.PDGFRb cells were mixed with dsRED-positive KPC+sh.Ctrl cells and injected subcutaneously into athymic mice, the GFP:dsRED ratio of pre-injected cells was maintained in the established tumors, indicating that increased PDGFRb levels did not confer a selective advantage to tumor cell proliferation at the site of injection (Figure S2D). Thus, cell-autonomous activity of PDGFRb is not required for the proliferation and tumorigenic potential of p53 mutant murine cancer cells but specifically impacts their invasive potential.
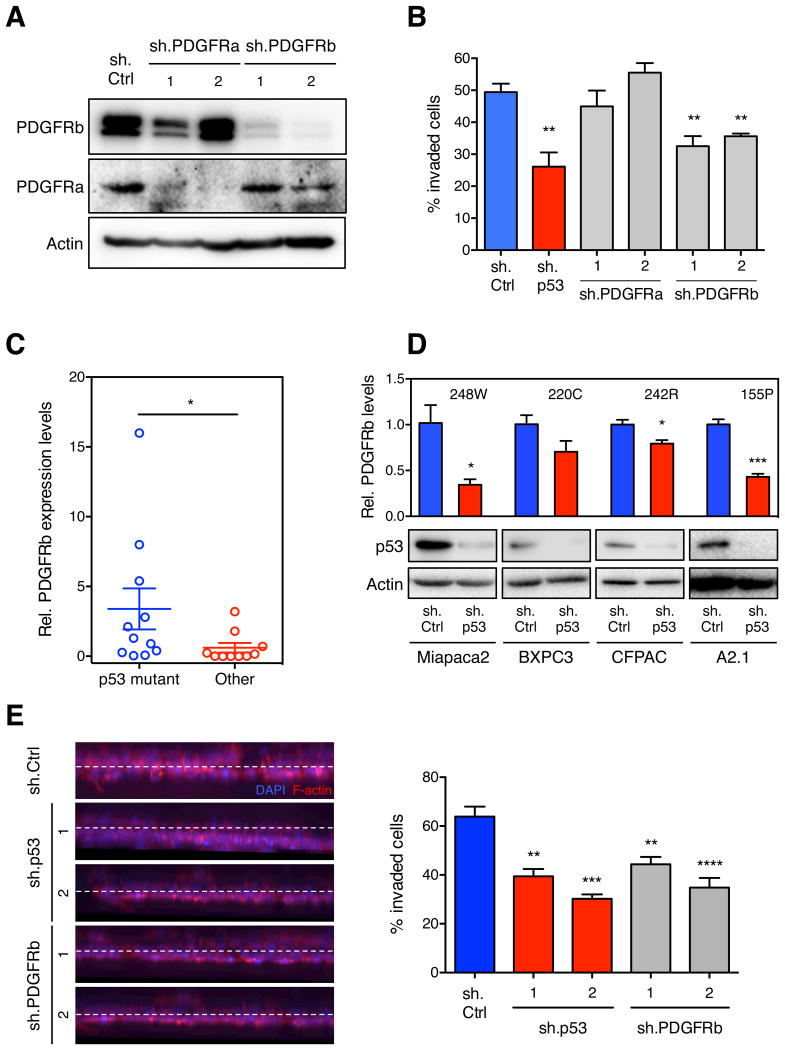
(A) PDGFRa, PDGFRb and Actin levels of KPC cells infected with shRNAs targeting PDGFRa, PDGFRb or a nontargeting control (Ctrl) as determined by western blotting. (B) Quantification of the invasion into collagen of cell lines from (A), compared to KPC+sh.p53. Data presented as mean ±SD. **p < 0.005. (C) qRT-PCR for PDGFRb in 21 human pancreatic cancer cell lines of different p53 status. Data presented as mean normalized PDGFRb expression ±SD. (D) qRT-PCR for PDGFRb in the human pancreatic cancer cell lines Miapaca2, BXPC3, CFPAC, and A2.1 expressing sh.p53 or sh.Ctrl. Data presented as mean normalized PDGFRb expression ±SD. *p < 0.05, ***p < 0.001. p53 mutation of each cell line as indicated. p53 and actin levels were determined by western blotting (lower panel). (E) Quantification of invasion of human A2.1 cells infected with sh.PDGFRb, sh.p53 or sh.Ctrl (right panel). Data presented as mean ±SD. **p < 0.005, ***p < 0.001, ****p < 0.0001. Representative 3D reconstructions of invaded cells are shown (Left panel). See also Figure S2.
PDGFRb Mediates Mutant p53 Action in Human Cancer Cells
To determine if the mutant p53-PDGFRb signaling axis acts in human cancer cells, we analyzed PDGFRb expression levels in a panel of human pancreatic cancer cell lines. As in our model, PDGFRb mRNA levels were significantly higher in cells expressing mutant p53 compared to those in cell lines that maintained or lost the wild type p53 allele (Figure 3C). Furthermore, knockdown of mutant p53 in Miapaca2, BXPC3, CFPAC and A2.1 cell lines (carrying the 248W, 220C, 242R, and 155P alleles, respectively) decreased PDGFRb mRNA levels to varying degrees (Figure 3D). Knockdown of mutant p53 also decreased PDGFRb expression in several human colon (SW620, p53273H/P309S), lung (H1975, p53273H), and breast (MDA-MB-231, p53280K) cancer cell lines (Figure S2E). Thus, the ability of mutant p53 to induce PDGFRb levels is not strictly confined to a particular p53 allele or tumor type.
We further analyzed the functional connection between mutant p53 and PDGFRb in promoting the invasiveness of human PDAC lines. Consistent with our studies in mouse PDAC lines, knockdown of either mutant p53 or PDGFRb reduced invasiveness of the A2.1 pancreatic cancer cell line (Figure 3E). Conversely, overexpression of PDGFRb in the human p53-/- ASPC pancreatic cancer cell line enhanced invasion compared to cells infected with a GFP control vector (Figure S2F). Collectively, these results confirm that upregulation of the PDGFRb receptor is important for the action of mutant p53 in PDAC and possibly other tumor types.
Mutant p53 disrupts the p73/NF-Y Complex to Mediate PDGFRb Expression and Tumor Cell Invasion
Several pro-oncogenic properties of mutant p53 depend on its ability to physically interact with and inhibit the p53 family members, p63 and p73 (Li and Prives, 2007). Since a previous report indicated that p73 can repress the transcription of PDGFRB (Hackzell et al., 2002), and because our KPC cells expressed p73 but not p63 as determined by RNA-seq (data not shown), we aimed to understand whether the physical interaction of mutant p53 with p73 might impair the ability of p73 to negatively regulate the expression of PDGFRb.
First, we verified the interaction between p73 and mutant p53 proteins by reciprocal co-immunoprecipitation (Figure 4A) and, consistent with previous reports, p73 binding to a “conformation” p53 mutant (R175H) appeared stronger than to a “DNA-binding” p53 mutant (R273H) (Gaiddon et al., 2001; Muller et al., 2009). Next, using a luciferase reporter driven from the PDGFRB promoter, we confirmed that overexpression of p73 in KPflC cells decreased transcriptional activity of PDGFRB and, conversely, that knockdown of endogenous p73 increased luciferase expression (Figures 4C and S3A). We also observed a similar increase in luciferase signal upon overexpression of two distinct forms of mutant p53 in KPflC cells (Figure 4C) as well as a significant decrease upon depletion of mutant p53 or overexpression of p73 in KPC cells (Figure S3C). Importantly, depletion of endogenous p73 in KPC cells expressing mutant p53 did not enhance the transcription of PDGFRB (Figure S3C), indicating that the repressing activity of p73 is regulated by its interaction with mutant p53. Hence, mutant p53 cancels the ability of p73 to repress PDGFRb transcription, leading to an increase in its expression.
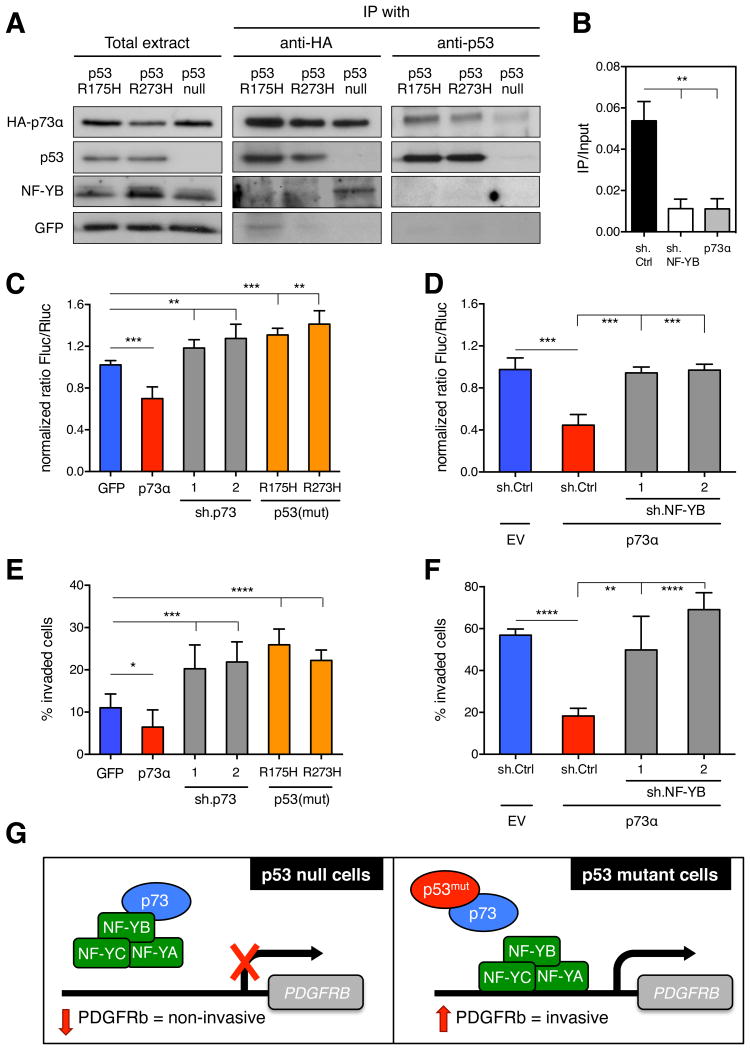
(A) KPflC cells stably expressing a GFP-, p53R175H-, or p53R273H vector were transfected with HA.TAp73α. Either p53 or HA were immunoprecipitated and the expression of HAp73α, p53, or NF-YB was determined in both the input (10% of lysates) and immunoprecipitation. (B) Chromatin immunoprecipitation (ChIP) using NF-YB antibodies in KPflC cells stably expressing sh.Ctrl, sh.NF-YB or HAp73α. Values are means ±SD. **p < 0.01. (C) KPflC cells stably expressing a GFP-, HAp73α-, p53R175H-, or p53R273H vector or sh.p73 (1 or 2) were co-transfected with the PDGFRB-promoter-luciferase construct and renilla-luciferase vector. Firefly-luciferase activity of GFP-vector cells was set to 1. Values are relative Firefly-luciferase (Fluc) units normalized by renilla expression (Rluc) ±SD of quadruplicate samples. **p < 0.01, ***p < 0.001. A representative result of three repeated experiments is shown. (D) KPflC+GFP cells as well as KPflC+HAp73α superinfected with sh.Ctrl, or sh.NF-YB (1 or 2) were co-transfected with the PDGFRB-promoter-luciferase construct and renilla-luciferase vector. Luciferase activity was measured as described above. ***p < 0.001. (E) Quantification of invasion of the same cells as in (C). Data presented as mean ±SD. *p < 0.05, ***p < 0.001, ****p < 0.0001. (F) Quantification of invasion of same cells as in (D). Data presented as mean ±SD. **p < 0.01, ****p < 0.0001. (G) Scheme summarizing the mechanism of action of mutant p53 in promoting invasiveness. See also Figure S3.
To better understand how p73 represses PDGFRB transcription, we performed chromatin immunoprecipitation (ChIP) analysis in KPflC cells but failed to detect direct binding of p73 to the PDGFRB promoter (data not shown), a result consistent with previous reports (Matys et al., 2006). Nevertheless, promoter analysis of PDGFRB (Transfec®, Biobase) identified a conserved CCAAT binding motif for NF-Y, a well-characterized heterotrimeric transcriptional activator (NF-YA, NF-YB, and NF-YC) of PDGFRB, where the NF-YB subunit interacts with p73 but is devoid of transcriptional activity (Ballagi et al., 1995; Ishisaki et al., 1997; Serra et al., 1998). We verified NF-Y binding to the PDGFRB promoter by use of ChIP analysis (Figure 4B). Remarkably, this binding was prevented by p73 overexpression, suggesting that the p73/NF-Y interaction hampers its ability to bind and activate the PDGFRB promoter (Figure 4B). Indeed, when we immunoprecipitated p73 in KPflC cells, we detected direct binding of NF-YB to p73 (Figure 4A). This interaction was abrogated upon expression of mutant p53, indicating that it disrupts or interferes with the formation of the inhibitory p73/NF-Y complex (Figure 4A).
Next, we tested whether the repressive action of p73 on PDGFRB transcription was mediated by NF-Y and modulated by mutant p53, and the implications of this regulatory circuit for invasion. Interestingly, the ability of p73 overexpression to inhibit the PDGFRB-luc reporter was abolished by depletion of NF-YB (Figures 4D and S3B), and NF-YB knockdown suppressed the ability of mutant p53 to enhance PDGFRb expression in KPflC cells (Figure S3D). As expected, p73 overexpression reduced the invasive potential of KPflC cells, whereas knockdown of p73 or overexpression of mutant p53 significantly increased invasiveness (Figure 4E). Conversely, as occurred in mutant p53 cells following p73 overexpression, depletion of p53 also reduced the invasive behavior in KPC cells (Figure S3E). Finally, NF-YB knockdown restored the invasive potential of KPflC cells that overexpressed p73 (Figure 4F) and suppressed the ability of mutant p53 to enhance cell invasion (Figure S3F). Together these results support a model in which mutant p53 promotes invasion in pancreatic cancer cells, in part, via an indirect mechanism that depends on its ability to enhance PDGFRb expression through the disruption of the inhibitory p73/NF-Y complex (Figure 4G).
Modulation of PDGFRb Expression Levels Mediates the Phenotypic Effects of Mutant p53 Depletion in vivo
Following the observation that p53 mutants induce the expression of PDGFRb to promote cell invasion in PDAC cultures, we investigated whether PDGFRb levels regulate metastatic behavior of PDAC cells in mice. To this end, we performed a lung colonization assay by injecting KPC+sh.p53, KPC+sh.PDGFRb, KPC+sh.PDGFRa or KPC+sh.Ctrl-cells intravenously via the tail vein into athymic mice and scored the number of colonies formed in the lungs. We found that, whereas KPC+sh.Ctrl and KPC+sh.PDGFRa cells expressing mutant p53 formed tumor nodules in the lungs at high frequency, PDGFRb depletion significantly reduced the number of lung colonies, phenocopying the anti-metastatic effect observed upon knocking down mutant p53 (Figures 5A and S4A). However, depletion of PDGFRb did not affect the size of the metastatic foci, suggesting that PDGFRb does not alter their capacity to grow and proliferate in a new environment (Figure S4B). In whole lung sections, GFP-positive signals coincided with metastatic nodules, indicating that metastases formed from cells expressing shRNAs, and likely not from the proliferation of tumor cells that lost shRNA expression (Figure S4C).
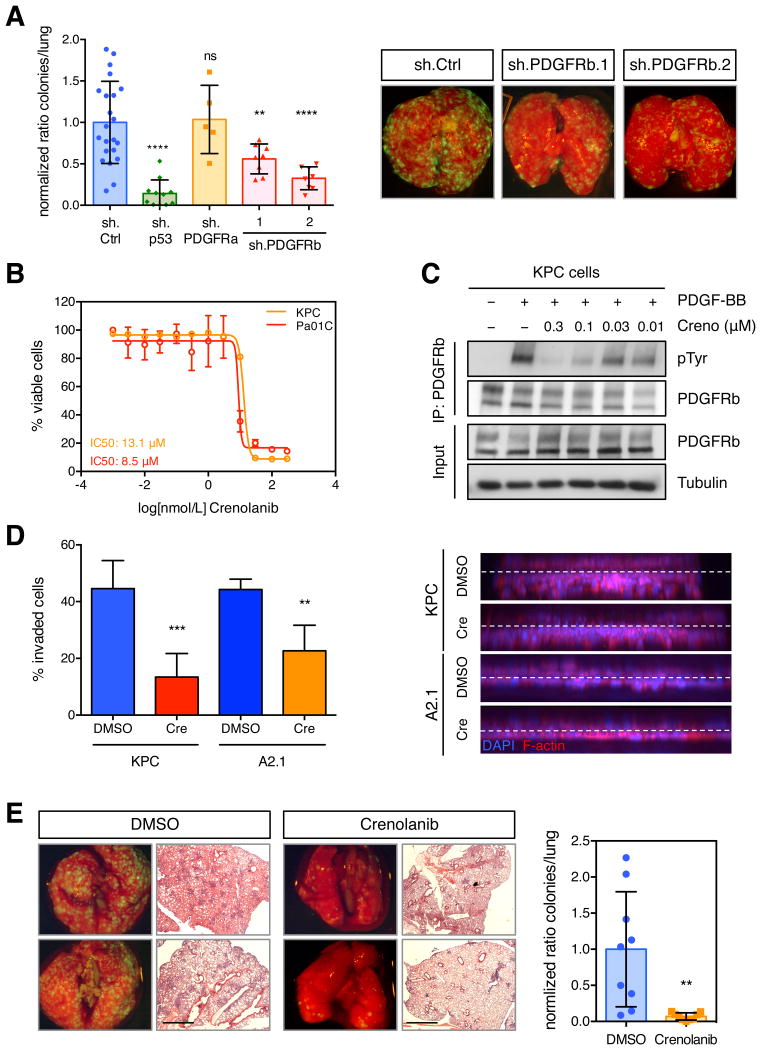
(A) Lung colonization assays after tail vein injection of KPC cells +sh.PDGFRa, +sh.PDGFRb (1 or 2), +sh.p53, or +sh.Ctrl. Total number of lung metastatic nodules in individual mice (n>6) was counted on serial histological sections (left panel). Data presented as mean ±SD. **p < 0.01, ****p < 0.0001. Representative merged brightfield/GFP images of whole lung from indicated mice (right panel). (B) MTS assay (E490) of murine KPC and human A2.1 cells treated with crenolanib with various doses for 72 h. Normalized values presented as mean ±SD form quadruple replicates. (C) Immunoprecipitation of PDGFRb from KPC cells stimulated with 50 ng/ml PDGF-BB, after crenolanib or DMSO treatment for 4 h. Protein levels of PDGFRb, phospho-Tyrosine and Tubulin were determined by western blotting. (D) Quantification of invasion of murine KPC and human A2.1 treated with either DMSO or crenolanib at 300 nM (left panel). Data presented as mean ±SD. **p < 0.01, ***p < 0.001. Representative 3D reconstructions of invaded cells are shown (right panel). (E) Lung colonization assays after tail vein injection of crenolanib (300 nM)- or DMSO-treated KPC cells. Representative merged brightfield/GFP imaged of whole lung as well as H&E stains of pulmonary lobes are shown (left panel). Quantification of total number of lung metastatic nodules in individual mice (n > 6) (right panel). Data presented as mean ±SD. **p < 0.01. Scale bars represent 1000 μm. See also Figure S4.
We next sought to examine whether pharmacologic inhibition of the PDGFRb pathway recapitulates the effects of PDGFRb or mutant p53 depletion (Figure 5A and S4A). We used the compound crenolanib, a small molecule inhibitor of type III tyrosine kinases, potent against PDGFRa, PDGFRb, and FLT3 but not other known receptor tyrosine kinases (VEGFR, FGFR), serine/threonine (RAF) or tyrosine kinases (ABL1) (Lewis et al., 2009). We assessed the potency and efficacy of crenolanib on inhibiting the viability of murine KPC and human A2.1 pancreatic cancer cells and found that the dose-response patterns were comparable between the two cell lines, with IC50 values of 13.1 μM and 8.5 μM, respectively (Figure 5B). Strong inhibition of PDGFRb activity, as measured by phospho-PDGFRb, was achieved in both cell lines at 0.3 μM, a dose at which no toxicity was observed (Figures 5C and S4D). Time course experiments revealed that strong target inhibition was achieved within 10 min of drug treatment (Figure S4E). Accordingly, crenolanib treatment of KPC and A2.1 cells substantially reduced invasion relative to that seen with cells treated with DMSO (Figure 5D).
To test whether crenolanib can suppress metastasis, KPC cells were pretreated with the drug overnight and injected intravenously into recipient mice that were subsequently assessed for colony formation in the lung. Although drug treatment had no effect on the viability of the injected cell population, mice injected with drug-treated KPC cells showed significantly fewer lung nodules compared to controls pre-treated with DMSO (Figures 5E and S4F). Conversely, the same concentration of crenolanib did not reduce the metastatic potential of KPflC cells in a lung colonization assay, suggesting that PDGFRb acts autonomously in KPC cells to potentiate cell invasion and metastasis (Figure S4G). Further supporting this notion, conditioned media from KPC cells and most human pancreatic cell lines tested triggered PDGFRb phosphorylation in serum-starved 3T3 cells, indicating pancreatic cancer cells can provide a source of PDGF ligand that could trigger autocrine activation of PDGFRb (Figure S4H and data not shown). Therefore, abrogation of this signaling by RNAi or small molecule inhibitors leads to a significant reduction of invasion and metastasis driven by mutant p53 in vitro and in vivo.
Imatinib Inhibits the Development of Metastases in a PDAC Mouse Model by targeting PDGFRb
The results described above imply that pharmacologic inhibition of PDGFRb could have anti-metastatic effects. We therefore sought to determine whether PDGFRb inhibition prevents metastasis in KPC mice that develop metastatic disease with a variable latency of 3 to 10 months in 50 - 80% of animals (Hingorani et al., 2005). For long-term treatment of KPC mice, we decided to use FDA-approved imatinib, a potent inhibitor of PDGFRb, c-KIT and BCR-ABL activity. Notably, the c-KIT and BCR-ABL kinases have not been linked to PDAC development (Jones et al., 2008).
Inhibition of PDGFRb by imatinib in KPC cells strongly reduced PDGFRb tyrosine phosphorylation at 3 uM (Figure 6A), a dose of the drug that is significantly lower than that required to inhibit cell proliferation (IC50 of 29.7 μM) (Figure S5A). Nonetheless, imatinib treatment significantly reduced the invasive potential of KPC cells in vitro (Figure S5B). More importantly, pre-treatment of KPC cells with imatinib decreased their potential to colonize the lungs of recipient athymic mice to a similar extent as that seen upon crenolanib treatment (Figure 6B).
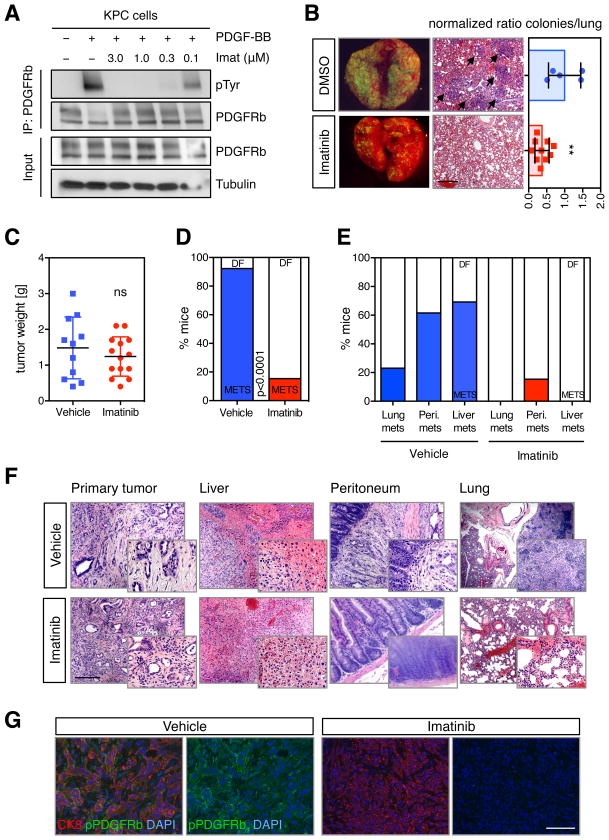
(A) Immunoprecipitation of PDGFRb from KPC cells stimulated with 50 ng/ml PDGF-BB, after imatinib or DMSO treatment for 4 h. Protein levels of PDGFRb, phospho-Tyrosine and Tubulin were determined by western blotting. (B) Lung colonization assays after tail vein injection of imatinib (3 μM)- or DMSO-treated KPC cells. Representative merged brightfield/GFP imaged of whole lung as well as H&E stains of pulmonary lobes are shown. Arrows indicate metastases (left panel). Quantification of total number of lung metastatic nodules in individual mice (n > 5) (right panel). Data presented as mean ±SD. **p < 0.01. Scale bars represent 100 μm. (C) Weight of pancreatic tumors of KPC mice treated with vehicle or imatinib at time of death. (D) Quantification of the number of mice with metastatic disease at the time of death. Values are percentages of the total number of mice in each cohort. Colored columns represent mice with metastases (METS) and white columns represent disease-free (DF) animals. (E) Quantification of the number of mice with lung, peritoneal (Peri.) or liver metastatic disease at the time of death. Values are percentages of the total number of mice in each cohort. (F) Representative H&E stains of harvested organs (primary tumor, lung, liver, peritoneal tissue) from vehicle and imatinib-treated animals. Scale bars represent 100 μm or 50 μm (insets). (G) Representative immunofluorescence images of pancreatic tumors of vehicle- or imatinib-treated KPC mice. DAPI, blue; CK8, red; and pPDGFRb, green. Scale bars represent 100 μm. See also Figure S5.
We next treated KPC mice with imatinib to assess its effects on metastasis. To this end, mice were treated with a dose of 50 mg/kg imatinib by oral gavage twice daily, a regimen previously shown to produce therapeutic concentrations of imatinib in mice (Wolff et al., 2003). Treatment was initiated in mice of 8 weeks of age, a time at which KPC mice have developed preneoplastic lesions (Hingorani et al., 2005), and mice were monitored until they became symptomatic. Imatinib had no impact on tumor volume in the pancreas or overall survival, suggesting that the high disease burden in the pancreas was the primary cause of death (Figures 6C and S5C).
However, Imatinib induced a striking reduction in the occurrence of metastasis. The incidence of metastasis was 92% in vehicle-treated animals compared to 15% in mice treated with imatinib, as assessed by macroscopic examination and confirmed by histopathological analyses (χ2 test, p<0.0001) (Figures 6D and S5D). The anti-metastatic effect was observed across several organs such as liver, peritoneum and lung (Figures 6E and 6F). As expected, imatinib was able to effectively inhibit PDGFRb activity in primary tumors based on reduced levels of phospho-PDGFRb in the tumor cells (Figures 6G and S5E). Together, these data suggest that by inhibiting the kinase activity of PDGFRb, imatinib significantly diminishes the metastatic potential of pancreatic cancer cells.
PDGFRb Expression Correlates with Disease Free-Survival in Human Pancreatic, Colorectal, and Ovarian Cancer Patients
To investigate the clinical significance of PDGFRb expression, we examined whether upregulation of this gene is correlated with prognosis or with the clinicopathological characteristics of PDACs in patients. To avoid confounding signals from the tumor stroma, PDGFRb mRNA levels were assessed in tumor samples with high purity score. Strikingly, we observed that pancreatic cancer patients with tumors expressing high levels of PDGFRb showed a poor disease-free survival and, hence, shorter time to relapse including metastases in distant organs (p=0.019) (Figure 7A). Additionally, PDGFRb expression levels were significantly elevated in late-stage PDAC as compared to the earlier stages (Figure 7B). Patients with high PDGFRb levels also displayed an increase in tumor cells invading the vascular space, another clinicopathological characteristic of tumor dissemination (Figure 7C).
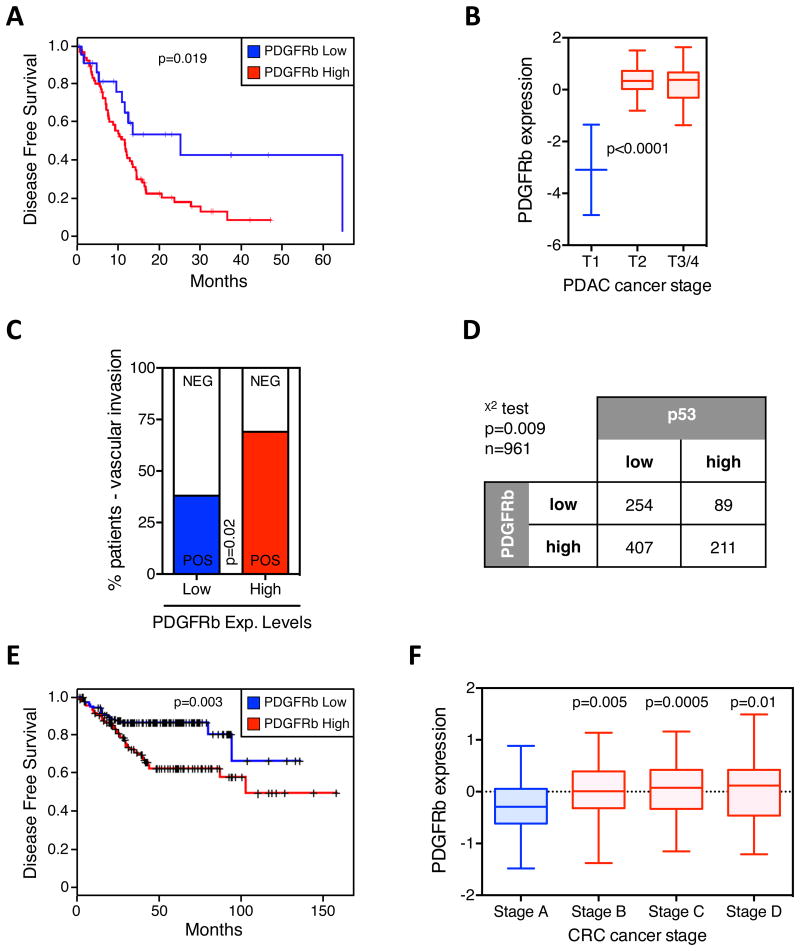
(A) Kaplan-Meier survival curves of 103 pancreatic cancer patients (clinical variable = DFS) as a function of PDGFRb-high versus PDGFRb-low expressing tumors. (B) Box plot of PDGFRb expression versus tumor grade of pancreatic tumors. (C) Clinicopathologic analysis of vascular space invasion of pancreatic cancer patients stratified by the expression levels of PDGFRb in the primary tumor. (D) Stratification of human PDAC samples (n = 961) based on high and low pPDGFRb and p53 expression levels. Chi-Square test was performed (p = 0.009). p53 and pPDGFRb levels were assessed by IHC and scored using a relative scale from 0 to 3. (E) Kaplan-Meier survival curves of colorectal cancer patients (clinical variable = DFS) as a function of PDGFRb-high versus PDGFRb-low expressing tumors. (F) Box plot of PDGFRb expression versus tumor grade of colon tumors. See also Figure S6.
Next, we tested whether PDGFRb levels correlate with the status of p53 by analyzing a panel of PDAC tissue microarrays (TMAs). Of importance, we observed significantly higher levels of activated PDGFRb in those tumors that showed an accumulation of p53 (p=0.009), which generally represents tumors with p53 mutation (Cooks et al., 2013) (Figures 7D and S6A). These data confirmed results obtained with mice and underscore a role of mutant p53 in regulating the PDGFRb signaling in human pancreatic cancer.
Other tumor types for which p53 mutations are predictive of metastatic disease are colorectal and ovarian cancer (Russo et al., 2005; Levesque et al., 1995). Thus, we analyzed the clinical significance of PDGFRb in these cancer types and found that levels of PDGFRb significantly stratified colorectal and ovarian tumor patients into two distinct cohorts. Patients with tumors expressing low PDGFRb levels exhibit a lower probability to form metastases compared to patients with PDGFRb high-expressing tumors (p=0.003 and p<0.0001) (Figures 7E and S6C). As in PDAC, a significant increase in PDGFRb expression was observed in higher stage colorectal cancers (Figure 7F). In addition to PDGFRb, our mutant p53 gene signature significantly scored as a prognostic marker in colorectal and ovarian tumor patients (top 40 genes upregulated in KPC cells; Figure 2C and Table S1). When we analyzed the three genes that scored in our invasion assay screen, we found that the PDGFRB gene was the strongest predictor for the probability to develop metastasis in colorectal and ovarian cancer patients (Figures S6B and S6C). In summary, consistent with our functional studies, elevated PDGFRb expression correlated significantly with the status of p53, higher tumor stage, and a poorer disease-free survival rate in pancreatic, colorectal and ovarian cancer patients.
Discussion
Mutations that occur in the p53 tumor suppressor inactivate wild-type p53 functions but can also produce “gain-of-function” oncogenic properties that can contribute to cell proliferation, survival and metastasis. Here, we explored the phenotypic effects of mutant p53 in pancreatic cancer and showed that the sustained expression of the mutant p53 allele is necessary to maintain the invasive phenotype of PDAC cells by increasing the expression of PDGFRb. These results have several ramifications for our understanding of mutant p53 action as well as the behavior and potential treatment of pancreatic cancer.
Signaling through PDGFRb contributes to multiple tumor-associated processes including cell invasion and metastasis. Given the generally restricted expression of PDGFRb to mesenchymal cell types, most of its oncogenic properties are thought to reflect paracrine effects of tumor cell-secreted PDGF. Indeed, previous work on the role of PDGFRb in carcinoma progression and metastasis suggest that it mainly elicits responses in the tumor stroma by promoting tumor angiogenesis (Pietras et al., 2003; Cao et al., 2004). In contrast, our study provides new evidence for tumor cell-specific expression of PDGFRb in promoting metastasis. We show that genetic or pharmacological inhibition of PDGFRb in the pancreatic cancer cells themselves dramatically reduces their invasive and metastatic potential, and that treatment of mice harboring genetic and histologically relevant tumors prevents metastatic spread in vivo. Together, our data indicate that pancreatic tumor cells expressing mutant p53 not only synthesize PDGF but also up-regulate PDGFRb, leading to a tumor autocrine, cell-autonomous effect.
Though increases in PDGFRb expression were necessary and sufficient to mediate mutant p53 effects in our model, we identified at least two additional genes (SNED1 and SLC40A1) that also contribute to the invasive phenotype through as yet unknown mechanisms. Studies in other systems, primarily breast cancer, have suggested that CXCR4, cyclin-G2, and the mevalonate pathway are important mediators of the pro-metastatic activities of mutant p53 (Mehta et al., 2007; Adorno et al., 2009; Freed-Pastor et al., 2012). In addition, mutant p53 has also been reported to drive invasion by regulating several miRNAs such as miR155 and miR130b (Neilsen et al., 2013; Dong et al., 2013). In agreement, we found that miR155 is positively regulated by mutant p53 in KPC cells and can promote metastasis in our model (data not shown); however, in contrast to a previous report (Su et al., 2010), no effects on DICER expression were observed, arguing that in pancreatic cancer the mutant p53-associated changes in microRNA expression and metastasis are DICER independent. Additionally, miR34a expression levels were not dependent on p53 status in our system (data not shown), even though this miRNA acts as negative regulator of PDGFRb in lung cancer (Garofalo at al., 2013). Regardless, as for wild type p53, mutant p53 exerts effects through the regulation of multiple genes rather than by modulating a single signaling pathway.
Most of our understanding of how mutant p53 mediates its oncogenic activity has been derived from exploring the consequences of the physical interaction between the mutant protein and the p53 family members, p63 and p73. Whereas the mutant p53-p63 interaction modulates the expression of p63 target genes to enhance invasion and metastasis (Adorno et al., 2009; Muller et al., 2009), how p53-p73 interactions produce similar outcomes is poorly understood. Here, we show that mutant p53 enhances pancreatic cancer cell metastasis by modulating p73 and its interaction with the transcriptional activator NF-Y. This model is consistent with previous studies showing that: (1) loss of p73 in a p53-null background might be functionally equivalent to the expression of mutant p53 (Lang et al., 2004); (2) aberrant transcriptional regulation by mutant p53 is mediated through the transcriptional activator NF-Y (Di Agostino et al., 2006); and (3) mutant p53 promotes recycling of receptor tyrosine kinases to initiate invasion (Muller et al., 2009). Whether structurally distinct p53 mutants enhance metastasis to the same extent and through the same mechanism remains unclear, and certainly most truncating mutants arising from the nonsense mutations occurring in a fraction of pancreas cancers are predicted to behave as if p53 null. Still, in our study both conformational (e.g. R175H) and structural mutants (e.g. R273H) were capable of inducing PDGFRB through a similar mechanism.
Questions remain as to how the mutant p53/p73/NF-Y regulatory axis acts mechanistically. For instance, it remains unclear whether p73 acts to suppress the transactivation capacity of NF-Y or whether it sequesters the activator and prevents its binding to the PDGFRB promoter. Even though our results indicate that p73 overexpression hampers the ability of NF-Y to bind to the PDGFRB promoter, further studies will be required to distinguish between mechanisms. In addition, we noted slightly stronger induction of PDGFRB transcription as well as higher levels of invasion upon overexpression of mutant p53 compared to depletion of p73, indicating that mutant p53 may exert additional regulatory effects on PDGFRb expression. Although studies suggest that mutant p53 can directly bind to NF-Y to regulate its transcriptional activity (Di Agostino et al., 2006), we failed to observe any physical interaction between mutant p53 and NF-Y in our cells. The discrepancy could reflect different extraction conditions or biological settings, since the mutant p53/NF-Y interaction has been shown to occur upon DNA damage (Liu et al., 2011).
Mutations in KRAS, p53, CDKN2A, BRCA2 and SMAD4 define the genetic landscape of PDAC; however, it remains unclear how each mutation contributes to the malignant evolution of this aggressive disease. We present evidence for a crucial role of mutant p53 in metastasis formation, which supports the attractive concept of targeting its gain-of-function activities to limit cancer cell dissemination and metastasis. However, mutant p53 is neither a targetable cell surface protein nor a druggable enzyme (Levine and Oren, 2009), and novel therapeutic modalities such as RNAi or restoring wild type p53 conformations have yet to show efficacy in clinical studies (Lehmann and Pietenpol, 2012). Hence, targeting downstream pathways or genes that mediate the activity of mutant p53, such as PDGFRb, pose an alternative treatment strategy.
Owing to the early metastatic spread of pancreatic cancer, widespread use of PDGFRb inhibitors might require advances in early detection or combination with other therapies. Nonetheless, our study suggests that PDGFRb inhibition might prove immediately useful in pancreatic patients harboring p53 missense mutations either before (neoadjuvant) or after surgical resection (adjuvant) of localized disease (10-15% of PDAC cases), in patients with locally advanced inoperable non-metastatic disease (~30% of PDAC cases), or as a preventative approach in patients with familial predisposition to cancer development. Moreover, PDGFRb inhibition as a therapeutic approach could be extended to other metastatic cancer types, e.g. colorectal, where the disease is often diagnosed before tumor cell dissemination.
High levels of PDGFRb expression have recently been associated with tumor recurrence in primary colorectal cancer, another gastrointestinal tumor in which p53 is frequently mutated (Stellar et al., 2013). Accordingly, we noted correlations between elevated PDGFRb levels, more advanced tumor stage, and poorer disease-free survival in pancreatic as well as colorectal and ovarian cancer patients. These results indicate that PDGFRb levels might be used as a prognostic biomarker for cancer progression, and eventually used in conjunction with p53 to identify patient cohorts most likely to respond to therapies targeting this axis. Although further studies will be required to explore this notion, pharmacological inhibition of PDGFRb with the tyrosine kinase inhibitor imatinib, in combination with standard chemotherapy, has shown promise in treating metastatic colorectal cancer patients (Hoehler et al., 2013).
In summary, we describe a gain-of-function activity of mutant p53 that promotes invasion and metastasis through increasing PDGFRB transcription and reverting the repressive function of the p73/NF-Y complex. While other activities of mutant p53 on cell behavior and survival exist (Freed-Pastor and Prives, 2012), our study provides a detailed molecular understanding of at least one aspect of the invasive behavior of cells expressing mutant p53, and offers a potential new target for therapy that might interfere with this activity.
Experimental Procedures
Wound healing and Invasion Assays
Wound healing and three-dimensional invasion assays were conducted as previously described (Goulimari et al., 2005; Kitzing et al., 2007). In brief, invasion assays were carried out in 24-well transwell inserts, lined with collagen type 1, and cells were seeded on the inverted inserts.
Immunostaining and Microscopy
Invasion assay inserts were fixed using 4% formaldehyde before confocal microscopy (Perkin Elmer Spinning Disk) was conducted. Images were analyzed using Imaris software.
RT–qPCR
Real-time PCR was carried out in triplicate using SYBR Green PCR Master Mix on the ViiA™ 7 Real-Time PCR System.
RNA sequencing and Data Analysis
Directional (stranded) libraries for Paired End sequencing of KPC cells were conducted on the Illumina platform. Differential expression analysis for sequence count data (FPKM values) was conducted using DESeq.
PDGFRb Luciferase Reporter Assay
Cells were transiently transfected with expression plasmid, reporter plasmid, and renilla-luciferase vector. After 36 h, firefly luciferase and renilla activities were measured on a Varioskan Flash Multimode Reader.
Chromatin Immunoprecipitation
As previously described in Beckerman et al. (2009). In brief, Protein A/G Sepharose beads conjugated to anti-NF-YB antibody were used to immunoprecipitate NF-YB from whole cell lysates. Quantitative ChIP was carried out on an ABI StepOne Plus using SYBR green dye.
Mouse Studies
All animal experiments were performed in accordance with a protocol approved by the Memorial Sloan-Kettering Institutional Animal Care and Use Committee.
Human Data Sets
Gene expression data and survival analyses of ovarian, colorectal, and pancreatic cancer patients with annotated clinical outcomes were downloaded from the Gene Expression Omnibus database (GSE50827, GSE9899, GSE17537, and GSE28735). For survival analyses, gene expression data was clustered into groups using kmeans and Kaplan-Meier analyses was performed. Significance for these plots was determined using the logrank test.
Supplementary Material
01
figure s1
figure s2
figure s3
figure s4
figure s5
figure s6
Acknowledgments
We thank K. Funa for sharing plasmids; A. Lujambio, M. Taylor, J. Ahn, and other members of the Lowe laboratory for critical discussions and/or technical help; APGI for providing clinical data; J. P. Morton for providing cell lines. C. J. Sherr and L. Dow for advice on experimental design and for editing the manuscript. S.W. is the recipient of the Annette Kade Fellowship from the Watson School of Biological Sciences. E.M. is supported by The Jane Coffin Childs Memorial Fund for Medical Research. S.W.L. is the Geoffrey Beene Chair of Cancer Biology and a Howard Hughes Medical Institute investigator. This work was supported by CA 013106 from the National Cancer Institute.
Footnotes
Accession Numbers: The Sequence Read Archive (SRA) accession number of the RNA sequencing data reported in this paper is SRP033333.
Supplemental Information: Supplemental Information includes six figures, one table, and extended experimental procedures.
Author Contributions: S.W. and E.M. designed and performed the majority of the experiments and contributed equally to this work. M.S., J.P.M., D.F.T., and T.K. contributed intellectually to the design of the project and helped performing experiments (treatment of KPC mice, IHC/IF, TMAs and invasion assay, respectively.). E.W. and C.A.D. conducted and analyzed RNA sequencing. S.M., N.T.P., and C.P. conducted and analyzed ChIP experiments. E.K.M., J.W., S.M.G., and A.V.B. collected, analyzed, and interpreted human data. C.P. and D.A. provided TMAs and their p53 status. S.W.L., S.W., and E.M. wrote the manuscript with the assistance from all authors. S.W.L. conceived and supervised the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, et al. A Mutantp53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. [Abstract] [Google Scholar]
- Ballagi AE, Ishizaki A, Nehlin JO, Funa K. Isolation and characterization of the mouse PDGF beta-receptor promoter. Biochem Biophys Res Commun. 1995;210:165–173. [Abstract] [Google Scholar]
- Beckerman R, Donner AJ, Mattia M, Peart MJ, Manley JL, Espinosa JM, Prives C. A role for Chk1 in blocking transcriptional elongation of p21 RNA during the S-phase checkpoint. Genes Dev. 2009;23:1364–1377. [Europe PMC free article] [Abstract] [Google Scholar]
- Cao R, Björndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. [Abstract] [Google Scholar]
- Dai Y. Platelet-derived growth factor receptor tyrosine kinase inhibitors: a review of the recent patent literature. Expert Opin Ther Pat. 2010;20:885–897. [Abstract] [Google Scholar]
- Di Agostino S, Strano S, Emiliozzi V, Zerbini V, Mottolese M, Sacchi A, Blandino G, Piaggio G. Gain of function of mutant p53: the mutant p53/NF-Y protein complex reveals an aberrant transcriptional mechanism of cell cycle regulation. Cancer Cell. 2006;10:191–202. [Abstract] [Google Scholar]
- Dong P, Karaayvaz M, Jia N, Kaneuchi M, Hamada J, Watari H, Sudo S, Ju J, Sakuragi N. Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene. 2013;32:3286–3295. [Europe PMC free article] [Abstract] [Google Scholar]
- Freed-Pastor WA, Mizuno H, Zhao X, Langerød A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li W, Polotskaia A, et al. Mutant p53 Disrupts Mammary Tissue Architecture via the Mevalonate Pathway. Cell. 2012;148:244–258. [Europe PMC free article] [Abstract] [Google Scholar]
- Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;12:1268–1286. [Europe PMC free article] [Abstract] [Google Scholar]
- Gaiddon C, Lokshin M, Ahn J, Zhang T, Prives C. A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol Cell Biol. 2001;21:1874–1887. [Europe PMC free article] [Abstract] [Google Scholar]
- Garofalo M, Jeon YJ, Nuovo GJ, Middleton J, Secchiero P, Joshi P, Alder H, Nazaryan N, Di Leva G, Romano G, et al. MiR-34a/c-Dependent PDGFR-α/β Downregulation Inhibits Tumorigenesis and Enhances TRAIL-Induced Apoptosis in Lung Cancer. PLoS One. 2013;8:e67581. [Europe PMC free article] [Abstract] [Google Scholar]
- Goulimari P, Kitzing T, Knieling H, Brandt DT, Offermanns S, Grosse R. Galpha12/13 is essential for directed cell migration and localized Rho-Dia1 function. J Biol Chem. 2005;280:42242–42251. [Abstract] [Google Scholar]
- Hackzell A, Uramoto H, Izumi H, Kohno K, Funa K. p73 independent of c-Myc represses transcription of platelet-derived growth factor beta-receptor through interaction with NF-Y. J Biol Chem. 2002;277:39769–39776. [Abstract] [Google Scholar]
- Hanel W, Marchenko N, Xu S, Yu SX, Weng W, Moll U. Two hot spot mutant p53 mouse models display differential gain of function in tumorigenesis. Cell Death Differ. 2013;20:898–909. [Europe PMC free article] [Abstract] [Google Scholar]
- Hingorani SR, Petricoin E, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. [Abstract] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. [Abstract] [Google Scholar]
- Hoehler T, von Wichert G, Schimanski C, Kanzler S, Moehler MH, Hinke A, Seufferlein T, Siebler J, Hochhaus A, Arnold D, et al. Phase I/II trial of capecitabine and oxaliplatin in combination with bevacizumab and imatinib in patients with metastatic colorectal cancer: AIO KRK 0205. Br J Cancer. 2013;17:1408–1413. [Europe PMC free article] [Abstract] [Google Scholar]
- Ishisaki A, Muramaya T, Ballagi AE, Funa K. Nuclear factor Y controls the basal transcription activity of the mouse platelet-derived-growth-factor beta-receptor gene. Eur J Biochem. 1997;246:142–146. [Abstract] [Google Scholar]
- Joerger AC, Fersht AR. Structural biology of the tumor suppressor p53 and cancer-associated mutants. Adv Cancer Res. 2007;97:1–23. [Abstract] [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. [Europe PMC free article] [Abstract] [Google Scholar]
- Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. [Abstract] [Google Scholar]
- Lehmann BD, Pietenpol JA. Targeting Mutant p53 in Human Tumors. J Clin Oncol. 2012;30:3648–3650. [Abstract] [Google Scholar]
- Levesque MA, Katsaros D, Yu H, Zola P, Sismondi P, Giardina G, Diamandis EP. Mutant p53 protein overexpression is associated with poor outcome in patients with well or moderately differentiated ovarian carcinoma. Cancer. 1995;15:1327–38. [Abstract] [Google Scholar]
- Levin AR, Oren M. The first 30 years of p53: growing ever more complex. Nature Reviews Cancer. 2009;9:749–758. [Europe PMC free article] [Abstract] [Google Scholar]
- Lewis NL, Lewis LD, Eder JP, Reddy NJ, Guo F, Pierce KJ, Olszanski AJ, Cohen RB. Phase I study of the safety, tolerability, and pharmacokinetics of oral CP-868,596, a highly specific platelet-derived growth factor receptor tyrosine kinase inhibitor in patients with advanced cancers. J Clin Oncol. 2009;27:5262–5269. [Europe PMC free article] [Abstract] [Google Scholar]
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic Cancer. Lancet. 2004;363:1049–1057. [Abstract] [Google Scholar]
- Li Y, Prives C. Are interactions with p63 and p73 involved in mutant p53 gain of oncogenic function? Oncogene. 2007;26:2220–2225. [Abstract] [Google Scholar]
- Liu K, Ling S, Lin WC. TopBP1 mediates mutant p53 gain of function through NF-Y and p63/p73. Mol Cell Biol. 2011;31:4464–4481. [Europe PMC free article] [Abstract] [Google Scholar]
- Longati P, Jia X, Eimer J, Wagman A, Witt MR, Rehnmark S, Verbeke C, Toftgård R, Löhr M, Heuchel RL. 3D pancreatic carcinoma spheroids induce a matrix-rich, chemoresistant phenotype offering a better model for drug testing. BMC Cancer. 2013;13:95. [Europe PMC free article] [Abstract] [Google Scholar]
- Masciarelli S, Fontemaggi G, Di Agostino S, Donzelli S, Carcarino E, Strano S, Blandino G. Gain-of-function mutant p53 downregulates miR-223 contributing to chemoresistance of cultured tumor cells. Oncogene. 2013 advance online publication. [Abstract] [Google Scholar]
- Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, Barre-Dirrie A, Reuter I, Chekmenev D, Krull M, Hornischer K, et al. TRANSFAC® and its module TRANSCompel®: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 2006;34:D108–110. [Europe PMC free article] [Abstract] [Google Scholar]
- Mehta SA, Christopherson KW, Bhat-Nakshatri P, Goulet RJ, Jr, Broxmeyer HE, Kopelovich L, Nakshatri H. Negative regulation of chemokine receptor CXCR4 by tumor suppressor p53 in breast cancer cells: implications of p53 mutation or isoform expression on breast cancer cell invasion. Oncogene. 2007;17:3329–3337. [Abstract] [Google Scholar]
- Montalbetti N, Simonin A, Kovacs G, Hediger MA. Mammalian iron transporters: families SLC11 and SLC40. Mol Aspects. 2013;34:270–287. [Abstract] [Google Scholar]
- Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, Jamieson NB, Oien KA, Lowy AM, Brunton VG, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. PNAS. 2010;107:246–251. [Europe PMC free article] [Abstract] [Google Scholar]
- Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, et al. Mutant p53 Drives Invasion by Promoting Integrin Recycling. Cell. 2009;139:1327–1341. [Abstract] [Google Scholar]
- Neilsen PM, Noll JE, Mattiske S, Bracken CP, Gregory PA, Schulz RB, Lim SP, Kumar R, Suetani RJ, Goodall GJ, et al. Mutant p53 drives invasion in breast tumors through up-regulation of miR-155. Oncogene. 2013;13:2992–3000. [Abstract] [Google Scholar]
- Olive KP, Tuveson D, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. [Abstract] [Google Scholar]
- Pietras K, Sjoblom T, Rubin K, Heldin CH, Ostman A. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3:439–443. [Abstract] [Google Scholar]
- Serra E, Zemzoumi K, di Silvio A, Mantovani R, Lardans V, Dissous C. Conservation and divergence of NF-Y transcriptional activation function. Nucleic Acids Res. 1998;26:3800–3805. [Europe PMC free article] [Abstract] [Google Scholar]
- Soussi T, Béroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1:233–240. [Abstract] [Google Scholar]
- Su X, Chakravarti D, Cho MS, Liu L, Gi YJ, Lin YL, Leung ML, El-Naggar A, Creighton CJ, Suraokar MB, et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature. 2010;467:986–990. [Europe PMC free article] [Abstract] [Google Scholar]
- Wolff NC, Richardson JA, Egorin M, Ilaria RL., Jr The CNS is a sanctuary for leukemic cells in mice receiving imatinib mesylate for Bcr/Abl-induced leukemia. Blood. 2003;101:5010–5013. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.cell.2014.01.066
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S0092867414002141/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.cell.2014.01.066
Article citations
From regulation to deregulation of p53 in hematologic malignancies: implications for diagnosis, prognosis and therapy.
Biomark Res, 12(1):137, 14 Nov 2024
Cited by: 0 articles | PMID: 39538363
Review
A recent update on the connection between dietary phytochemicals and skin cancer: emerging understanding of the molecular mechanism.
Ann Med Surg (Lond), 86(10):5877-5913, 07 Aug 2024
Cited by: 0 articles | PMID: 39359831 | PMCID: PMC11444613
Review Free full text in Europe PMC
p53R172H and p53R245W Hotspot Mutations Drive Distinct Transcriptomes in Mouse Mammary Tumors Through a Convergent Transcriptional Mediator.
Cancer Res Commun, 4(8):1991-2007, 01 Aug 2024
Cited by: 0 articles | PMID: 38994678 | PMCID: PMC11310746
The anti-tumor effect of trifluridine via induction of aberrant mitosis is unaffected by mutations modulating p53 activity.
Cell Death Discov, 10(1):307, 02 Jul 2024
Cited by: 0 articles | PMID: 38956056 | PMCID: PMC11219725
Understanding the complexity of p53 in a new era of tumor suppression.
Cancer Cell, 42(6):946-967, 09 May 2024
Cited by: 16 articles | PMID: 38729160 | PMCID: PMC11190820
Review Free full text in Europe PMC
Go to all (309) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
GEO - Gene Expression Omnibus (4)
- (1 citation) GEO - GSE50827
- (1 citation) GEO - GSE17537
- (1 citation) GEO - GSE9899
- (1 citation) GEO - GSE28735
Nucleotide Sequences
- (1 citation) ENA - SRP033333
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Pancreatic Cancer Progression Relies upon Mutant p53-Induced Oncogenic Signaling Mediated by NOP14.
Cancer Res, 77(10):2661-2673, 09 Mar 2017
Cited by: 23 articles | PMID: 28280038
Impact of p53 and PDGFR-β Expression on Metastasis and Prognosis of Patients with Pancreatic Cancer.
World J Surg, 40(8):1977-1984, 01 Aug 2016
Cited by: 19 articles | PMID: 26940582
Loss of HIF1A From Pancreatic Cancer Cells Increases Expression of PPP1R1B and Degradation of p53 to Promote Invasion and Metastasis.
Gastroenterology, 159(5):1882-1897.e5, 05 Aug 2020
Cited by: 61 articles | PMID: 32768595 | PMCID: PMC7680408
Metastasis: understanding the prowess of mutant p53.
Nat Rev Cancer, 14(6):385, 28 Apr 2014
Cited by: 4 articles | PMID: 24769756
Funding
Funders who supported this work.
Cancer Research UK (1)
Advancing Genotype Guided Stratified Therapy for Pancreatic Cancer
Professor Andrew Biankin, University of Glasgow
Grant ID: 17263
NCI NIH HHS (5)
Grant ID: P01 CA087497
Grant ID: P30 CA008748
Grant ID: P01 CA013106
Grant ID: CA 013106
Grant ID: P01 CA129243





