Abstract
Free full text

Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films
Abstract
Since the discovery of graphene, the quest for two-dimensional (2D) materials has intensified greatly. Recently, a new family of 2D transition metal carbides and carbonitrides (MXenes) was discovered that is both conducting and hydrophilic, an uncommon combination. To date MXenes have been produced as powders, flakes, and colloidal solutions. Herein, we report on the fabrication of ~1 × 1 cm2 Ti3C2 films by selective etching of Al, from sputter-deposited epitaxial Ti3AlC2 films, in aqueous HF or NH4HF2. Films that were about 19 nm thick, etched with NH4HF2, transmit ~90% of the light in the visible-to-infrared range and exhibit metallic conductivity down to ~100 K. Below 100 K, the films’ resistivity increases with decreasing temperature and they exhibit negative magnetoresistance—both observations consistent with a weak localization phenomenon characteristic of many 2D defective solids. This advance opens the door for the use of MXenes in electronic, photonic, and sensing applications.
Introduction
Since the discovery of graphene,1−3 two-dimensional (2D) solids have attracted considerable attention due to the unique properties bestowed upon them by their reduced dimensionality. These are currently being considered for a multitude of applications, including electronic, photonic, and energy storage devices.4−6 For instance, graphene has an electron mobility of 2 × 105 cm2 V–1 s–1 at room temperature, which shows a weak dependence on temperature.3,7 Furthermore, a single layer of graphene transmits 97.7% of light in the near-infrared to ultraviolet range.8 This combination of unique electronic and optical properties has positioned graphene as a promising material for transparent conductive electrodes.
The immense interest generated by graphene has renewed efforts to identify and characterize other 2D solids such as BN,9 MoS210,11 that may possess equally attractive properties. Recently, we discovered a new family of 2D materials that is both metallically conducting and hydrophilic, an uncommon combination indeed. This new family of materials was labeled MXenes6,12 to emphasize that they are produced by selective etching of the A layers from the MAX phases and their similarity to graphene.13 The latter are a large family of more than 60 phases, with the general formula of Mn+1AXn, where n = 1, 2, 3, where M is an early transition metals, A is an A-group (12–16) element, and X is carbon and/or nitrogen.14 The MAX phases are nanolaminated, wherein every n-layers of M atoms are interleaved with layers of pure A; the X atoms occupy the octahedral sites between the M atoms. To date the following MXenes have been synthesized: Ti3C2,13 Ti2C, Ta4C3, TiNbC, (V0.5,Cr0.5)3C2, Ti3CN,15 and most recently Nb2C and V2C.16
By varying the M and X elements, as well as the surface chemistries and/or the number of layers, n, in Mn+1Xn, it is possible to tune the MXene properties. This wealth of new 2D materials has launched experimental and theoretical activities worldwide17,18 (see ref (12) for a recent review). MXenes show promise as anodes for lithium ion batteries; a result supported by ab initio calculations.19−21 More recently, Lukatskaya et al.,22 have shown that a host of cations (Na+, Mg2+, Al3+, NH4+, etc.) can be readily intercalated, from aqueous solutions, between the Ti3C2 layers. Volumetric capacitances exceeding 300 F/cm3 were reported. These values are much higher than those of porous carbon currently used in electrochemical capacitors.
However, thin films are needed to explore electronic or photonic applications. Herein, we report on the synthesis of ~1 × 1 cm2 epitaxial Ti3C2 thin films. The materials described here represent a substantial advance in several ways: (1) they are produced as continuous epitaxial thin films; (2) in all previous studies, the etchant was hydrofluoric acid (HF). Here, it is shown that ammonium bifluoride, NH4HF2, can be used instead; (3) the one-step synthesis of a MXene, intercalated with ammonia, is demonstrated; (4) the availability of epitaxial films on transparent and insulating sapphire substrates enabled the measurement of some of the fundamental physical properties, such as optical absorption, in a broad wavelength range, and the temperature dependence of conductivity and magnetoresistance down to 2 K. These films show high transparency for wavelengths in the visible to infrared range.
Methods
Deposition of Ti3AlC2
The Ti3AlC2 thin films were deposited from three elemental targets (Ti, Al, and C with diameters of 75, 50, and 75 mm, respectively) using DC magnetron sputtering in an ultrahigh vacuum system described elsewhere.38,39 The sputtering process gas was Ar (99.9999% purity) at a constant pressure of 4.8 mbar. The substrates were c-axis-oriented sapphire, Al2O3 (0001), with surface areas of 10 × 10 mm2 and thicknesses of 0.5 cm (MTI Corp. CA). Prior to deposition, the substrates were cleaned using acetone, rinsed with isopropanol, dried by nitrogen gas, and finally preheated inside the deposition chamber at 780 °C for 60 min. Deposition was performed at 780 °C. Titanium and carbon targets were ignited for 5 s followed by the ignition of the aluminum target. This procedure resulted in the formation of a TiC (111) incubation layer 5–10 nm thick followed by the growth of Ti3AlC2. Previous work have shown that a TiC incubation layer facilitates the growth of epitaxial Ti3AlC2.29,38,40
Synthesis of Ti3C2
Two chemicals were used to etch, at room temperature, the Ti3AlC2 films. The first was 50% concentrated HF (Sigma Aldrich, Stockholm, Sweden). Samples of nominal thickness of 15, 28, 43, and 60 nm were etched for 10, 15, 60, and 160 min, respectively. The second was 1 M NH4HF2 (Sigma Aldrich, Stockholm, Sweden). Samples of the same thickness as those mentioned above were etched for 150, 160, 420, and 660 min, respectively. After etching, the samples were rinsed in deionized water, then in ethanol.
Chemical and Morphological Characterization
X-ray diffraction (XRD) of the films was performed using an X’Pert Powder diffractometer (PANalytical, Almelo, The Netherlands), with a θ–2θ continuous scan of a step size with 0.017° and 40 s dwell time. XRD of the Ti3C2Tx-IC powders and deintercalated Ti3C2Tx thin films were carried out using a diffractometer (SmartLab, Rigaku, Tokyo, Japan) with a θ-2θ continuous scan of a step size of 0.02° and 1 s dwell time.
X-ray reflectometry (XRR) continuous scans were performed using an X’Pert Powder diffractometer (PANalytical, Almelo, The Netherlands), with a step size of 0.01° and 1.76 s dwell time. Simulation for the XRR results was carried out using the X’Pert Reflectivity software produced by PANalytical B.V.
To characterize the chemical states of elements in the thin films before and after etching, X-ray photoelectron spectroscopy (XPS) was performed using a surface analysis system (Kratos AXIS Ultra DLD, Manchester, U.K.). Monochromatic Al Kα X-rays irradiated the samples at an angle of 45°, with respect to the surface; X-ray spot size was 300 × 800 μm. The electron energy analyzer accepted the photoelectrons perpendicular to the sample surface with an acceptance angle of ±15°. The high-resolution spectra were recorded using a pass energy of 20 eV and a step size of 0.1 eV. To avoid broadening of the XPS spectra caused by sample charging, an electron flood gun was used while recording the data. The binding energy scale of all XPS spectra was therefore referenced to the Fermi level which was set to a binding energy of 0 eV. Peak assignments of the spectra were supported through an analysis of a TiC bulk sample of known stoichiometry (1:1) and a TiO2 thin film of known stoichiometry (1:2). The quantification and peak fitting were carried out using CasaXPS Version 2.3.16 RP 1.6.
Transmission electron microscopy (TEM) imaging, film thickness measurement, and selected area electron diffraction (SAED) acquisition was carried out using a TEM (FEI Tecnai G2 TF20 UT) operated at 200 kV with a point resolution of 0.19 nm. High-resolution scanning TEM imaging (HR STEM), and energy-dispersive X-ray spectroscopy (EDX) were performed using a HRTEM instrument (FEI image/probe double Cs corrected Titan3 G2 60–300, Eindhoven, The Netherlands) operated at 300 kV with an ultrathin window silicon drift detection X-ray energy-dispersive spectrometer and a monochromator.
Cross-sectional TEM samples were prepared by sandwiching two cross-sectioned samples in a Ti grid that was in turn mechanically polished down to 70 μm, followed by ion milling to electron transparency. Scanning electron microscopy (SEM) (Zeiss Supra 50VP, Germany) was used to investigate the morphology of the Ti3AlC2 and Ti3C2Tx films.
Optical and Electrical Characterization
Transmittance values of the films were obtained using a spectrophotometer (Perkin-Elmer Lambda 950 UV–vis) with a 2-nm slit width and resolution. Spectra were corrected with both 100% and 0% transmittance background spectra. A bare sapphire substrate was used as a reference. The number of MXene layers obtained for Figure Figure3b3b were calculated by dividing the total film thicknesses by c/2, where c is the lattice parameters obtained from XRD.
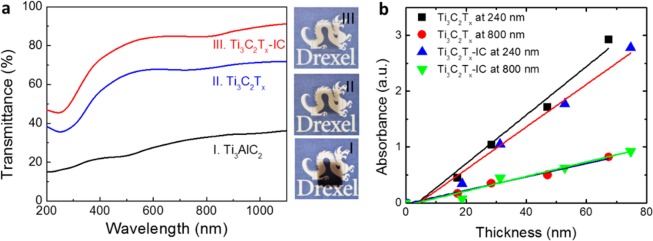
(a) Transmittance spectra and visual images (on right) for (I) Ti3AlC2, (II) Ti3C2Tx, and (III) Ti3C2Tx-IC films of 15 nm nominal thickness. The films are ≈1 × 1 cm2 in area; (b) light absorbance at wavelengths of 240 and 800 nm vs thickness of Ti3C2Tx and Ti3C2Tx-IC films.
Room-temperature resistivities were measured using a four-point probe method. Three sheet-resistance measurements were taken for each sample. The errors reported in Table 1 and Supporting Information Table S4 were calculated from these three measurements. The resistivity was obtained by multiplying the sheet resistance with the corresponding average film thickness.
The temperature-dependent in-plane resistivity measurements were performed in a Physical Property Measurement System (Quantum Design, San Diego) using an external current source (Keithley 6220, Ohio) and a nanovoltmeter (Keithley 2182A). A linear four-point probe geometry was used. Gold wires were attached to the films using silver paint. Positive and negative currents were applied at each temperature to eliminate any thermal offsets. The magnetoresistance, MR, measurements were performed with the magnetic field—up to 10 T—applied out of the plane of the film.
Results and Discussion
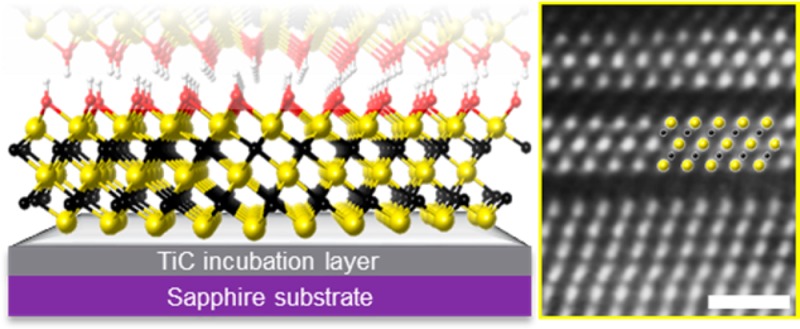
The films used were 15 to 60 nm thick Ti3AlC2 films deposited onto sapphire (000 ) substrates
by magnetron sputtering. More
details can be found in Supporting Information
Section I. Scheme 1a shows the process
starting from the sputter-deposition of Ti3AlC2 (with initial formation of a TiC incubation layer). This is followed
by etching of the Al layers resulting in 2D Ti3C2Tx layers (Scheme. 1b), where Tx stands for the surface −O,
−OH, or −F terminations resulting from the aqueous HF
etchant. In Scheme 1b, the Ti3C2 surfaces are presumed to be OH-terminated. STEM image of
the interface between the TiC incubation layer and Ti3C2Tx is shown in Scheme 1c. The fact that the very first MXene layer has
an ordered structure bodes well for the production of single layer
MXene films.
) substrates
by magnetron sputtering. More
details can be found in Supporting Information
Section I. Scheme 1a shows the process
starting from the sputter-deposition of Ti3AlC2 (with initial formation of a TiC incubation layer). This is followed
by etching of the Al layers resulting in 2D Ti3C2Tx layers (Scheme. 1b), where Tx stands for the surface −O,
−OH, or −F terminations resulting from the aqueous HF
etchant. In Scheme 1b, the Ti3C2 surfaces are presumed to be OH-terminated. STEM image of
the interface between the TiC incubation layer and Ti3C2Tx is shown in Scheme 1c. The fact that the very first MXene layer has
an ordered structure bodes well for the production of single layer
MXene films.
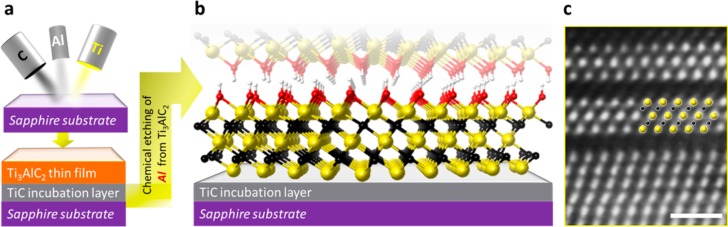
(a) Magnetron sputtering of Ti, Al and C forming a few-nanometer TiC incubation layer on a (0001) sapphire substrate, followed by the deposition of Ti3AlC2; (b) schematic diagram of OH-terminated Ti3C2 after selective etching of Al from Ti3AlC2 (Ti atoms are yellow, C atoms are black, O atoms are red, and H atoms are white); (c) STEM image of the first two Ti3C2Tx layers after applying Wiener filter; scale bar is equal to 1 nm. Inset shows Ti atoms in yellow and C atoms in black.
To date, the only etchant reported for producing MXenes has been HF.13,15,16 Herein, we show that, NH4HF2 can be used for the same purpose. The main advantage of the latter is that it is less hazardous than HF23 and is a milder etchant. Its use leads to the concomitant intercalation of cations during the etching process. For the sake of brevity, these films will be referred to as Ti3C2Tx-IC, where the IC represents the intercalated species, viz. NH3 and NH4+ (see below).
A typical XRD pattern of an
as-deposited Ti3AlC2 film (Figure (Figure1a,1a, I) shows the (000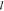 ) peaks from
Ti3AlC2, a TiC incubation layer) and the sapphire
substrate.23 The presence of only peaks
corresponding to
basal-plane oriented Ti3AlC2 indicates epitaxial
growth, a fact also confirmed by TEM and SAED (Figure (Figure2a).2a). The Ti3C2Tx XRD pattern (Figure (Figure1a,1a, II) on the
other hand, shows a shift to a lower angles of the 000
) peaks from
Ti3AlC2, a TiC incubation layer) and the sapphire
substrate.23 The presence of only peaks
corresponding to
basal-plane oriented Ti3AlC2 indicates epitaxial
growth, a fact also confirmed by TEM and SAED (Figure (Figure2a).2a). The Ti3C2Tx XRD pattern (Figure (Figure1a,1a, II) on the
other hand, shows a shift to a lower angles of the 000 peaks corresponding
to an increase in the c lattice parameter from 18.6
Å for Ti3AlC2 to 19.8 Å for Ti3C2Tx. The latter value
agrees with previous work on Ti3C2Tx synthesized from
Ti3AlC2 powders.13 The XRD pattern of Ti3C2Tx-IC (Figure (Figure1a,1a, III), is similar to
the other two, except that now c is further increased
to 24.7 Å.
peaks corresponding
to an increase in the c lattice parameter from 18.6
Å for Ti3AlC2 to 19.8 Å for Ti3C2Tx. The latter value
agrees with previous work on Ti3C2Tx synthesized from
Ti3AlC2 powders.13 The XRD pattern of Ti3C2Tx-IC (Figure (Figure1a,1a, III), is similar to
the other two, except that now c is further increased
to 24.7 Å.
(a) XRD patterns of as-deposited −60 nm nominal thickness - Ti3AlC2 thin films (I), Ti3C2Tx after etching in 50% HF for 2 h 40 min (II), and Ti3C2Tx-IC after etching in 1 M NH4HF2 for 11 h (III). XPS spectra of, (b) Ti 2p, (c) C 1s, and (d) Al 2p for Ti3AlC2, Ti3C2Tx, and Ti3C2Tx-IC thin films, respectively. The vertical lines in panels b and c indicate the positions of Ti (3/2p and 1/2p) and C (1s) binding energies in TiC, respectively. (e) High resolution XPS spectra for N 1s region for Ti3C2Tx-IC, best fitted by symmetric Gaussian–Lorentzian curves resting on a Shirley background. The two components correspond to (NH4+1)24 and (NH3).25
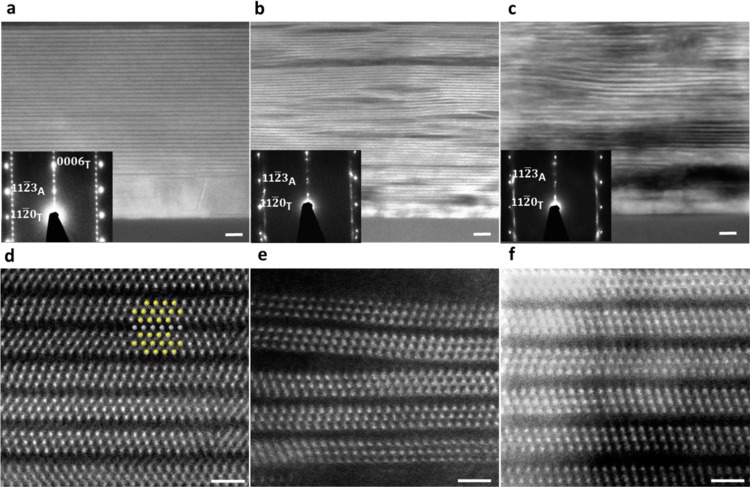
Cross-sectional STEM images of (a) Ti3AlC2,
(b) Ti3C2Tx,
(c) and Ti3C2Tx-IC
films (60 nm nominal thickness) grown on a sapphire substrate with
a TiC incubation layer. Insets show SAED of the film and the substrate.
The subscripts A and T correspond to Al2O3 and
Ti3AlC2, respectively. High-resolution STEM
images of (d) Ti3AlC2, (e) Ti3C2Tx, and (f) Ti3C2Tx-IC films along the [11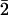 0]
zone axis. The inset in panel d shows Ti, Al, and C atoms in yellow,
gray, and black, respectively. Scale bars for low resolution (a, b,
and c) and high-resolution (d, e, and f) images correspond to 5 and
1 nm, respectively.
0]
zone axis. The inset in panel d shows Ti, Al, and C atoms in yellow,
gray, and black, respectively. Scale bars for low resolution (a, b,
and c) and high-resolution (d, e, and f) images correspond to 5 and
1 nm, respectively.
Similar behavior was observed when Ti3AlC2 powders were intercalated with NH4OH or NH4F after HF etching. In both cases, the c lattice expansion was of the order of 25% (see Supporting Information Figure S5a). The independence of the increase in the c lattice parameter on the nature of the anion of the etching solution strongly suggests that the cations (NH4+) and/or (NH3), and not the anions, are the intercalated species. We note in passing that the present work is in contradistinction to the recent work by Lukatskaya et al. who intercalated NH4OH into Ti3C2Tx,22 in a two-step process. Herein, the etching and intercalation occur in a single step. This is an important result because it considerably simplifies the intercalation process.
The XPS results, shown in Figure Figure1b–d for1b–d for films, with a nominal thickness of 60 nm, demonstrate a shift in the Ti 2p and C 1s (Figure (Figure1b1b and c) toward higher binding energies for the titanium carbide species in Ti3AlC2, Ti3C2Tx, and Ti3C2Tx-IC, compared to those of binary TiC (shown in Figure Figure1b1b and c as thin vertical lines), indicating the change in the nature of bonding between the Ti and C atoms in Ti3AlC2 and the corresponding MXenes. The latter most likely occurs because valence electrons are withdrawn from the Ti atoms, and subsequently from the C atoms, in the MXene layers by the surface functional groups, as well as from the interaction of the surface with the intercalated compounds. The removal of Al is verified by the high-resolution spectra in the Al 2p region for Ti3C2Tx and Ti3C2Tx-IC (Figure (Figure1d),1d), in which a very weak Al signal—most probably originating from aluminum fluoride (see Supporting Information section I)—is recorded. The Ti3AlC2, Al 2p signal corresponds to Al bonded to Ti, as well as, to surface aluminum oxide.
The reactions of HF with Ti3AlC2 have been postulated by Naguib et al.15 to be
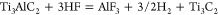
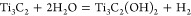
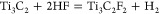
Reaction 1 is followed by reactions 2 and 3, which result in OH and F terminated Ti3C2 surfaces or Ti3C2Tx. The elemental ratio obtained from the analysis of high-resolution (XPS) spectra is Ti3C2.2O2F0.6 (see Supporting Information section II). As indicated by XPS, terminal hydroxyl and fluoride groups exist on the surface of the material, thereby indirectly confirming the aforementioned reactions. EDX mapping in the TEM (Supporting Information Figure S7) also confirms the presence of F and O atoms between the Ti3C2 layers.
As discussed above for the NH4HF2 etched Ti3AlC2, the etching of the Al and the intercalation of ammonium species occur concomitantly. It is thus reasonable to conclude that in this case the following reactions are operative:
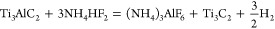
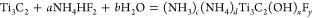
Unlike HF etching, NH4HF2 etching results in formation of (NH4)3AlF6 according to reaction 4 (see Supporting Information section III). Reaction 5 depicts the intercalation of NH3 and NH4+1 between the Ti3C2Tx layers.26 In order to confirm the nature of the intercalating species in Ti3C2Tx-IC, a high-resolution XPS spectrum of the N 1s region was recorded (Figure (Figure1e).1e). The latter was best fitted by two components: one for NH4+1 (55.8% of N 1s; peak position, 402 eV; fwhm, 1.8 eV);24 the other for NH3 (44.2% of N 1s; peak position, 400.1 eV; fwhm, 1.8 eV).25 It is thus reasonable to conclude that both species intercalate this MXene. We note in passing that both NH3 and NH4+ intercalate between the 2D layers of transition metal dichalcogenides, such as TiSe2 and TiS2.27,28
The elemental ratio obtained from the analysis of high-resolution XPS spectra (see Supporting Information section II) of Ti3C2 produced by NH4HF2 etching is Ti3C2.3O1.2F0.7N0.2. Here again, the XPS analysis indicates the presence of terminal hydroxyl and fluoride groups.
Cross-sectional scanning TEM micrographs of as-deposited Ti3AlC2 films, before (Figure (Figure2a2a and d) and after etching with HF (Figure (Figure2b2b and e) or NH4HF2 (Figure (Figure3c3c and f) clearly show the presence of the TiC incubation layers and the effects of etching on the microstructures of the films. The SAED patterns confirm the out-of-plane epitaxial relationship Ti3AlC2(0001)//TiC(111)//Al2O3(0001).29 At 18.6 Å, the c lattice parameter for Ti3AlC2, obtained from the SAED pattern and TEM micrographs (see Supporting Information section VIII), is in excellent agreement with that calculated from XRD (18.6 Å). At 19.5–20 Å, the c lattice parameters of Ti3C2Tx obtained from the SAED patterns match the ones obtained from XRD (19.8 Å). However, at 21 ± 0.5 Å, the average c for Ti3C2Tx-IC measured from the SAED pattern is considerably lower than that obtained from XRD (25 Å). The most probable reason for this state of affairs is the deintercalation of the ammonium species during TEM sample preparation and/or observation (see Methods and Supporting Information section IV).
The light elements of the surface termination groups (O, H, and F) cannot be seen between the layers, but the larger and nonuniform spacing seen in Figure Figure2b,2b, c, e, and f indirectly confirm the weak interactions between the MXene layers after etching and the formation of a 2D structure. The nonuniform interlayer spacing observed in the STEM images of the HF-etched sample (Figure (Figure2b)2b) could also account for the peak broadening observed in XRD (Figure (Figure22a).
Prior to etching, the initial thicknesses of the films examined in TEM were 60 nm (Figure (Figure2a).2a). However, as a result of the increase in c and the separation between the MXene layers, due to exfoliation, the etched films were thicker than the initial films (Table 1). Comparing the atomic layers in Ti3C2Tx-IC (Figure (Figure2c2c and f) to those of the Ti3C2Tx layers (Figures (Figures3b,3b, and e), it is obvious that the former are more uniformly spaced. This result most probably reflects the milder nature of NH4HF2 as compared to HF. For the latter, the reaction is faster (Table 1) and more vigorous than the former. Another possible explanation is that the intercalation of ammonia species leads to stronger interactions between MXene layers, essentially “gluing” them together as observed for other MXene intercalation compounds.22,30
In terms of light transmittance, both Ti3C2Tx and Ti3C2Tx-IC films are significantly more transparent than Ti3AlC2 of the same initial thickness, 15 nm (Figure (Figure3a,3a, and Table 1). The increased transparency of Ti3C2Tx and Ti3C2Tx-IC, compared to that of Ti3AlC2 is also evident visually (Figure (Figure3,3, middle insets).
With 90% transmittance, the Ti3C2Tx-IC films were the most transparent, followed by the Ti3C2Tx films at 70%. With a transmittance of 30%, the Ti3AlC2 films were the least transparent. It is worth noting here that the transmittance of all films would have been higher had the TiC incubation layer been absent.
A linear dependence of the absorbance—that is independent of the wavelength of the light—on the thickness of the Ti3C2Tx and Ti3C2Tx-IC films was observed (Figure (Figure3b).3b). Given the similarities in the transmittance curves and the linear dependencies of absorbance values for both samples, it is reasonable to conclude that Ti3C2Tx and Ti3C2Tx-IC are quite similar in structure. A crude estimation of the transmittance of a single MXene layer, d, (since each length c is comprised of two MXene layers, d is approximately equal to the film thickness divided by 2c) could be obtained from the linear fits of absorbance vs d. The transmittances, calculated at a wavelength of 240 nm, for single layers of Ti3C2Tx and Ti3C2Tx-IC are about 90.5% and 91.5%, respectively; the corresponding transmittances, at a wavelength of 800 nm, are 97.3% and 97.1% respectively. The latter values are quite close to those reported for graphene single layers.8 Note that to obtain these values, both the thickness and absorbance of the TiC incubation layer were neglected.
Table 1
| deposition time [min] | thickness [nm] | etching duration [minutes] | resistivity [μΩm] | transmittance [%] | ||
|---|---|---|---|---|---|---|
| set 1 | Ti3AlC2 | 15.2 ± 0.5a | 0.45 ± 0.01 | 31 | ||
| Ti3C2Tx | 5 | 17.2 ± 0.8a | 9.5 | 39.23 ± 1.21 | 68 | |
| Ti3C2Tx-IC | 18.7 ± 0.6a | 150 | 4472 ± 323 | 85 | ||
| set 2 | Ti3AlC2 | 27.7 ± 0.8a | 0.34 ± 0.01 | 14 | ||
| Ti3C2Tx | 10 | 28.4 ± 1.8a | 15 | 2.28 ± 0.04 | 49 | |
| Ti3C2Tx-IC | 31.3 ± 1.2a | 160 | 5.01 ± 0.03 | 37 | ||
| set 3 | Ti3AlC2 | 43.4 ± 3.6b | 0.31 ± 0.01 | 5.2 | ||
| Ti3C2Tx | 20 | 47.1 ± 3.5b | 60 | 22.27 ± 0.43 | 30 | |
| Ti3C2Tx-IC | 52.8 ± 2.5b | 420 | 31 ± 2.8 | 28 | ||
| set 4 | Ti3AlC2 | 60.0 ± 5.4c | 0.35 ± 0.01 | 3.4 | ||
| Ti3C2Tx | 30 | 67.4 ± 5.3c | 160 | 1.76 ± 0.02 | 15 | |
| Ti3C2Tx-IC | 74.7 ± 3.7d | 660 | 54 ± 4.51 | 14 |
We next turn to the electrical properties, which confirm the metallic-like nature of the conductivities of all etched films despite their optical transparency. As expected, and consistent with previous work,31 the Ti3AlC2 films are metallic with resistivity, ρ, values in the range from 0.37 to 0.45 μΩ m. The latter increase linearly with increasing temperature (Figure (Figure3a).3a). Furthermore, ρ increases with decreasing film thickness (Table 1 and Supporting Information Figure S10). The resistivity values of the Ti3C2Tx-IC films are systematically higher than those produced by HF etching. For instance, 28 nm nominally thick Ti3C2Tx and Ti3C2Tx-IC films have ρ values of 2.3 and 5.0 μΩ m, respectively. This result is also consistent with previous work that has shown that intercalation of MXenes with organic compounds increases their resistivity.30 The resistivities of the etched films also depend significantly on etching time; longer etching times lead to higher ρ values presumably due to the formation of defects (see Supporting Information Table S2), in agreement with previous work.32 The results listed in Table 1 are those obtained upon the full MAX to MXene conversion. The latter was determined by intermittently etching each film, followed by XRD. When the Ti3AlC2 peaks disappeared, the etching process was halted (Supporting Information Figure S6). We note in passing that there were no changes in the c lattice parameter with etching time. Furthermore, the fact that the conductivities are affected by the intercalants, suggests that MXenes can potentially be used as sensors.
At 1.8 μΩ m, a 60 nm nominally thick Ti3C2Tx sample is the most conductive of the HF etched films Ti3C2Tx films (Table 1). However, at 700 nm wavelength, its transmittance is only 15%. The 15 nm nominally thick Ti3C2Tx sample exhibited the highest transmittance (68% at 700 nm wavelength) with a ρ of 39.2 μΩ m. For the Ti3C2Tx-IC films, the lowest resistivity was 5.0 μΩ m, with a transmittance of about 37%; the most transparent (>85% at 700 nm wavelength) had a resistivity of ≈4.5 mΩ m.
At this juncture, it is worth comparing our results with other conductive electrodes at a wavelength of 550 nm. Referring to Supporting Information Figure S10, it is obvious that while the transmittance of our thinnest films is higher than that of indium tin oxide, ITO, their sheet resistance values are orders of magnitude higher. When compared to graphene transparent conductive electrodes, again MXene transmittance values are slightly higher, but the resistivity is about 2 orders of magnitude higher.
Why the resistivities measured herein are as high as they are, is unclear at this time. One possibility is the film morphology. As noted above, the Ti3AlC2 films are predominantly c-axis oriented (Figure (Figure2a).2a). However, a secondary grain population, whose basal planes are not parallel to the substrate, also exists (see Supporting Information Figure S9). If the reasonable assumption is made that after etching the conductivity along [0001] is significantly lower than along [100], this secondary grain population, will act as insulating islands. Reducing the fraction of such grains should result in films that are more conductive when etched. Deintercalation of films by heat treatment can alone increase the conductivity by an order of magnitude or more.30
Theoretical calculations predict that it is possible to alter the electronic properties of MXenes by altering their surface terminations.21,33 For example, pure Ti3C2 is predicted to exhibit a metallic behavior, whereas Ti3C2F2 and Ti3C2(OH)2 are predicted to have band gaps of 0.05 and 0.1 eV, respectively.4,13 Thus, another potential avenue for enhancing the films’ conductivities is to eliminate the surface groups. We note in passing that several applications, such as touch screen, electromagnetic shielding for cathode ray tubes and electrostatic dissipation, require sheet resistance values which are comparable to what we report for MXene thin films, viz. 1000 to 1 MΩ/sq.34
To elucidate the conduction mechanisms of the MXene layers, their resistivities, and magnetoresistances (MRs) from room temperature down to about 2.5 K were measured. Figure Figure4a4a shows the temperature dependent resistivity for Ti3AlC2, Ti3C2Tx, and Ti3C2Tx-IC films of 28 nm nominal thickness. The Ti3AlC2 films exhibit metallic behavior from 300 K down to about 10 K. For the Ti3C2Tx and Ti3C2Tx-IC films, on the other hand, metallic behavior is observed from 300 to about 100 K; below 100 K the resistivity increases with decreasing temperature (Figure (Figure4b).4b). Similar low-temperature behavior was observed in other Ti3C2Tx and Ti3C2Tx-IC films (see Supporting Information Figure S11). The low temperature transport data can best be fit assuming ρ ~ ln T (inset in Figure Figure4b). As4b). As shown in Supporting Information Figure S7, other mechanisms associated with insulating behavior, such as thermally activated processes, 3D variable range hopping and others, do not accurately reflect the ρ (T) data (see Supporting Information Figure S12). The logarithmic dependence on temperature is consistent with weak localization, a phenomenon caused by electron backscattering and often observed in 2D metals.35 To provide further insight into the transport properties, MR measurements were performed in the low temperature (dρ/dT < 0) and high temperature (dρ/dT > 0) regimes. The appearance of negative MR in the low temperature regime (Figure (Figure4c)4c) is again consistent with weak localization, verifying that these materials are indeed 2D.35−37
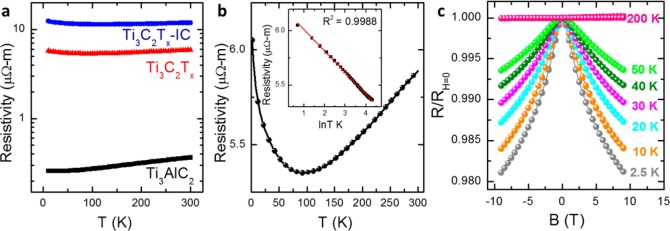
Dependence of the electrical behavior of Ti3AlC2, Ti3C2Tx, and Ti3C2Tx-IC films on temperature and magnetic field. (a) Resistivity vs temperature for Ti3AlC2, Ti3C2Tx, and Ti3C2Tx–IC films of 20 nm nominal thickness. (b) Resistivity vs temperature for Ti3C2Tx of 28 nm nominal thickness. Inset shows fitting of resistivity, over the temperature range of 2 to 74 K, to the weak localization model (ρ ~ ln T). (c) Comparison of normalized magnetoresistance curves for Ti3C2Tx of 28 nm nominal thickness at various temperatures ranging from 2.5 to 200 K. RH=0 refers to the film resistance in the absence of applied magnetic field.
Conclusions
In conclusion, epitaxial Ti3C2Tx films can be readily produced by the room temperature etching of epitaxial Ti3AlC2 thin films in HF or NH4HF2 solutions. The latter etchant yields films intercalated with NH3 and NH4+ species, that have c lattice parameters (~ 25 Å) that are 25% larger than films etched with HF. The Ti3C2Tx-IC films have higher transparencies and resistivities than their Ti3C2Tx counterparts. Ti3C2Tx and Ti3C2Tx-IC films of ~15-nm nominal thickness were found to be 68 and 85% transparent, respectively. Both films also exhibited metallic conductivity down to 100 K; below 100 K, the resistivities increase with decreasing temperatures and exhibit negative MRs at the lowest temperatures, both attributes consistent with, and evidence for, their 2D metallic nature.
The MXene films produced herein are promising materials for transparent conductive electrodes, sensors and other applications. By better control of the deposition process, such that nonbasal growth is eliminated or minimized, the potential exists for enhancing their conductivities. A parallel approach is to modify, or eliminate, the surface terminations such as F, O, or OH.
Synthesis of single-layer Ti3C2 films is the next frontier. Other MXenes (Ti-based and others containing other transition metals such as Nb, V, Ta, etc. or nitrogen in addition to carbon) may also show attractive optical and electrical properties and should be produced and studied in their thin-film state. It is vital to note here that the production of epitaxial uniform multilayer MXene films is a necessary and crucial first step to applying this novel and unique family of materials in the field of electronics, optoelectronics and photonics. Given the vast richness of MXene chemistries, together with the multiple different intercalants (from cations to polymers to organic molecules), it is obvious that we are standing at the edge of a truly vast terra incognita.
Acknowledgments
Hossein Fashandi is acknowledged for his help and useful discussions about growth of Ti3AlC2 thin films. The authors acknowledge funding from the Swedish Research Council (VR) Grant Nos. 621-2012-4430 and 621-2011-4420, the VR Linnaeus Strong Research Environment LiLi-NFM. M.W.B., J.H., and P.E. also acknowledge the Swedish Foundation for Strategic Research (SSF) through the Synergy Grant FUNCASE (M.W.B., J.H., and P.E.) and the Ingvar Carlsson Award 3 (P.E.). The Knut and Alice Wallenberg Foundation supported the Ultra-Electron Microscopy Laboratory at Linköping University operated by the Thin Film Physics Division. S.J.M. and C.R.S. were supported by the U.S. Office of Naval Research (ONR N00014-11-1-0109); the acquisition of the Physical Properties Measurement System was supported by the U.S. Army Research Office under grant number W911NF-11-1-0283. M.L. and Y.G. were supported by the US Department of Energy, Energy Storage Systems Research Program through Sandia National Laboratory.
Supporting Information Available
XRR, thickness determination, XPS analysis of Ti3AlC2, Ti3C2Tx and Ti3C2Tx-IC thin films, XRD analysis of byproducts from etching Ti3AlC2 with NH4HF2, intercalation and deintercalation of Ti3C2Tx thin films, optimization of the etching process, EDX mapping of Ti3C2Tx, SEM and TEM for Ti3AlC2 and Ti3C2Tx thin films, and electrical transport measurements and analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
J.H. planned and performed the thin film depositions; performed and developed the etching process; performed XRD of the films before and after etching; and measured the room temperature resistivities. M.R.L. performed the intercalation and deintercalation experiments; carried out the XRD scans and analysis of the intercalated, and deintercalated samples; performed the SEM; and made the figures for the manuscript. K.M.C. measured and analyzed the optical transmittance properties. C.R.S. and S.J.M. performed and analyzed the temperature dependent resistivity and MR measurements. J.L. performed and analyzed the TEM micrographs with contributions from J.H., L.H., and P.E. XPS experiments were performed by L.-Å.N. Additionally, J. H. analyzed the results with contributions from K.M.C. and L.-Å.N. The manuscript draft was written by J.H. and P.E. All authors were involved in the discussions and commented on and revised successive drafts of the manuscript. M.W.B., L.H., and Y.G. conceived and initiated the research. M.W.B, L.H., Y.G., and P.E. supervised the work.
References
- Novoselov K. S.; Geim A. K.; Morozov S. V.; Jiang D.; Zhang Y.; Dubonos S. V.; Grigorieva I. V.; Firsov A. A. Science 2004, 306, 666–669. [Abstract] [Google Scholar]
- Novoselov K.; Jiang D.; Schedin F.; Booth T.; Khotkevich V.; Morozov S.; Geim A. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 10451–10453. [Europe PMC free article] [Abstract] [Google Scholar]
- Novoselov K. S.; Fal’ko V. I.; Colombo L.; Gellert P. R.; Schwab M. G.; Kim K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Abstract] [Google Scholar]
- Tang Q.; Zhou Z.Progress in Materials Science 2013, 58. [Google Scholar]
- Nicolosi V.; Chhowalla M.; Kanatzidis M. G.; Strano M. S.; Coleman J. N. Science 2013, 340, 1420–1438. [Google Scholar]
- Zhang X.; Xie Y. Chem. Soc. Rev. 2013, 42, 8187–8199. [Abstract] [Google Scholar]
- Bolotin K. I.; Sikes K. J.; Jiang Z.; Klima M.; Fudenberg G.; Hone J.; Kim P.; Stormer H. L. Solid State Commun. 2008, 146, 351–355. [Google Scholar]
- Nair R. R.; Blake P.; Grigorenko A. N.; Novoselov K. S.; Booth T. J.; Stauber T.; Peres N. M.; Geim A. K. Science 2008, 320, 1308. [Abstract] [Google Scholar]
- Ci L.; Song L.; Jin C.; Jariwala D.; Wu D.; Li Y.; Srivastava A.; Wang Z. F.; Storr K.; Balicas L.; Liu F.; Ajayan P. M. Nat. Mater. 2010, 9, 430–435. [Abstract] [Google Scholar]
- van der Zande A. M.; Huang P. Y.; Chenet D. A.; Berkelbach T. C.; You Y.; Lee G. H.; Heinz T. F.; Reichman D. R.; Muller D. A.; Hone J. C. Nat. Mater. 2013, 12, 554–561. [Abstract] [Google Scholar]
- Najmaei S.; Liu Z.; Zhou W.; Zou X.; Shi G.; Lei S.; Yakobson B. I.; Idrobo J. C.; Ajayan P. M.; Lou J. Nat. Mater. 2013, 12, 754–759. [Abstract] [Google Scholar]
- Naguib M.; Mochalin V. N.; Barsoum M. W.; Gogotsi Y. Adv. Mater. 2014, 26, 992–1005. [Abstract] [Google Scholar]
- Naguib M.; Kurtoglu M.; Presser V.; Lu J.; Niu J.; Heon M.; Hultman L.; Gogotsi Y.; Barsoum M. W. Adv. Mater. 2011, 23, 4248–4253. [Abstract] [Google Scholar]
- Barsoum M. W.. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides; John Wiley & Sons: New York, 2013. [Google Scholar]
- Naguib M.; Mashtalir O.; Carle J.; Presser V.; Lu J.; Hultman L.; Gogotsi Y.; Barsoum M. W. ACS Nano 2012, 6, 1322–1331. [Abstract] [Google Scholar]
- Naguib M.; Halim J.; Lu J.; Cook K. M.; Hultman L.; Gogotsi Y.; Barsoum M. W. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Abstract] [Google Scholar]
- Enyashin A.; Ivanovskii A. J. Solid State Chem. 2013, 207, 42–48. [Google Scholar]
- Enyashin A. N.; Ivanovskii A. L. J. Phys. Chem. C 2013, 117, 13637–13643. [Google Scholar]
- Naguib M.; Come J.; Dyatkin B.; Presser V.; Taberna P. L.; Simon P.; Barsoum M. W.; Gogotsi Y. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar]
- Tang Q.; Zhou Z.; Shen P. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Abstract] [Google Scholar]
- Shein I. R.; Ivanovskii A. L. Micro Nano Lett. 2013, 8, 59–62. [Google Scholar]
- Lukatskaya M. R.; Mashtalir O.; Ren C. E.; Dall’Agnese Y.; Rozier P.; Taberna P. L.; Naguib M.; Simon P.; Barsoum M. W.; Gogotsi Y. Science 2013, 341, 1502–1505. [Abstract] [Google Scholar]
- Misra A.; Prasad J.; Sees J. A.; Hall L. H.Benign Method for Etching Silicon Dioxide. U.S. Patent No. 6048406, 2000.
- Bourbigot S.; Le Bras M.; Gengembre L.; Delobel R. Appl. Surf. Sci. 1994, 81, 299–307. [Google Scholar]
- Egawa C.; Naito S.; Tamaru K. Surf. Sci. 1983, 131, 49–60. [Google Scholar]
- Rakov E. G. e.; Mel’nichenko E. Russ. Chem. Rev. 1984, 53, 851–869. [Google Scholar]
- Friend R. H.; Yoffe A. D. Adv. Phys. 1987, 36, 1–94. [Google Scholar]
- Bernard L.; Mckelvy M.; Glaunsinger W.; Colombet P. Solid State Ionics 1985, 15, 301–310. [Google Scholar]
- Wilhelmsson O.; Palmquist J.-P.; Lewin E.; Emmerlich J.; Eklund P.; Persson P.; Högberg H.; Li S.; Ahuja R.; Eriksson O. J. Cryst. Growth 2006, 291, 290–300. [Google Scholar]
- Mashtalir O.; Naguib M.; Mochalin V. N.; Dall’Agnese Y.; Heon M.; Barsoum M. W.; Gogotsi Y. Nat. Commun. 2013, 4, 1716. [Abstract] [Google Scholar]
- Wilhelmsson O.; Palmquist J.-P.; Nyberg T.; Jansson U. Appl. Phys. Lett. 2004, 85, 1066–1068. [Google Scholar]
- Mashtalir O.; Naguib M.; Dyatkin B.; Gogotsi Y.; Barsoum M. W. Mater. Chem. Phys. 2013, 139, 147–152. [Google Scholar]
- Khazaei M.; Arai M.; Sasaki T.; Chung C. Y.; Venkataramanan N. S.; Estili M.; Sakka Y.; Kawazoe Y. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar]
- Geng H.-Z.; Kim K. K.; So K. P.; Lee Y. S.; Chang Y.; Lee Y. H. J. Am. Chem. Soc. 2007, 129, 7758–7759. [Abstract] [Google Scholar]
- Bergmann G. Phys Rev B 1982, 25, 2937–2939. [Google Scholar]
- Bergmann G. Phys. Rep. 1984, 107, 1–58. [Google Scholar]
- Henzler M.; Lüer T.; Heitmann J. Phys Rev B 1999, 59, 2383–2387. [Google Scholar]
- Emmerlich J.; Högberg H.; Sasvári S.; Persson P. O. Å.; Hultman L.; Palmquist J.-P.; Jansson U.; Molina-Aldareguia J. M.; Czigány Z. J. Appl. Phys. 2004, 96, 4817–4826. [Google Scholar]
- Frodelius J.; Eklund P.; Beckers M.; Persson P.; Högberg H.; Hultman L. Thin Solid Films 2010, 518, 1621–1626. [Google Scholar]
- Eklund P.; Beckers M.; Frodelius J.; Högberg H.; Hultman L. J. Vac. Sci. Technol., A 2007, 25, 1381–1388. [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1021/cm500641a
Read article for free, from open access legal sources, via Unpaywall:
https://pubs.acs.org/doi/pdf/10.1021/cm500641a
Linköping University Digital Archive
https://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-106852
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1021/cm500641a
Article citations
A comprehensive overview of recent progress in MXene-based polymer composites: Their fabrication processes, advanced applications, and prospects.
Heliyon, 10(17):e37030, 30 Aug 2024
Cited by: 0 articles | PMID: 39319124 | PMCID: PMC11419932
Review Free full text in Europe PMC
Advances on MXene-Based Memristors for Neuromorphic Computing: A Review on Synthesis, Mechanisms, and Future Directions.
ACS Nano, 18(33):21685-21713, 07 Aug 2024
Cited by: 0 articles | PMID: 39110686 | PMCID: PMC11342387
Review Free full text in Europe PMC
Research Progress on Ammonia Sensors Based on Ti3C2Tx MXene at Room Temperature: A Review.
Sensors (Basel), 24(14):4465, 10 Jul 2024
Cited by: 0 articles | PMID: 39065863 | PMCID: PMC11280721
Review Free full text in Europe PMC
Hydrophilicity and surface charge modulation of Ti3C2T x MXene based membranes for water desalination.
RSC Adv, 14(30):21635-21643, 08 Jul 2024
Cited by: 0 articles | PMID: 38979456 | PMCID: PMC11229083
M<sub>4</sub>X<sub>3</sub> MXenes: Application in Energy Storage Devices.
Nanomicro Lett, 16(1):215, 14 Jun 2024
Cited by: 0 articles | PMID: 38874816 | PMCID: PMC11178707
Review Free full text in Europe PMC
Go to all (137) article citations
Other citations
Wikipedia
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Electronic and optical characterization of 2D Ti2C and Nb2C (MXene) thin films.
J Phys Condens Matter, 31(16):165301, 22 Jan 2019
Cited by: 15 articles | PMID: 30669136
25th anniversary article: MXenes: a new family of two-dimensional materials.
Adv Mater, 26(7):992-1005, 19 Dec 2013
Cited by: 631 articles | PMID: 24357390
Transparent, Flexible, and Conductive 2D Titanium Carbide (MXene) Films with High Volumetric Capacitance.
Adv Mater, 29(36), 25 Jul 2017
Cited by: 112 articles | PMID: 28741695
Two-dimensional Ti3C2 MXene-based nanostructures for emerging optoelectronic applications.
Mater Horiz, 8(11):2929-2963, 01 Nov 2021
Cited by: 1 article | PMID: 34558566
Review
Funding
Funders who supported this work.
Army Research Office (1)
Grant ID: W911NF-11-0283
Grant ID: ONR N 00014-11-1-0109
Swedish Foundation for Strategic Research (2)
Grant ID: Synergy Grant FUNCASE
Grant ID: Ingvar Carlsson Award 3
Swedish Research Council Formas (2)
Grant ID: 621-2011-4420
Grant ID: 621-201204430





