Abstract
Free full text

The metabolite alpha-ketoglutarate extends lifespan by inhibiting the ATP synthase and TOR
Abstract
Metabolism and ageing are intimately linked. Compared to ad libitum feeding, dietary restriction (DR) or calorie restriction (CR) consistently extends lifespan and delays age-related diseases in evolutionarily diverse organisms1,2. Similar conditions of nutrient limitation and genetic or pharmacological perturbations of nutrient or energy metabolism also have longevity benefits3,4. Recently, several metabolites have been identified that modulate ageing5,6 with largely undefined molecular mechanisms. Here we show that the tricarboxylic acid (TCA) cycle intermediate α-ketoglutarate (α-KG) extends the lifespan of adult C. elegans. ATP synthase subunit beta is identified as a novel binding protein of α-KG using a small-molecule target identification strategy called DARTS (drug affinity responsive target stability)7. The ATP synthase, also known as Complex V of the mitochondrial electron transport chain (ETC), is the main cellular energy-generating machinery and is highly conserved throughout evolution8,9. Although complete loss of mitochondrial function is detrimental, partial suppression of the ETC has been shown to extend C. elegans lifespan10–13. We show that α-KG inhibits ATP synthase and, similar to ATP synthase knockdown, inhibition by α-KG leads to reduced ATP content, decreased oxygen consumption, and increased autophagy in both C. elegans and mammalian cells. We provide evidence that the lifespan increase by α-KG requires ATP synthase subunit beta and is dependent on the target of rapamycin (TOR) downstream. Endogenous α-KG levels are increased upon starvation and α-KG does not extend the lifespan of DR animals, indicating that α-KG is a key metabolite that mediates longevity by DR. Our analyses uncover new molecular links between a common metabolite, a universal cellular energy generator, and DR in the regulation of organismal lifespan, thus suggesting new strategies for the prevention and treatment of ageing and age-related diseases.
To gain insight into the regulation of ageing by endogenous small molecules, we screened normal metabolites and aberrant disease-associated metabolites for their effects on the adult lifespan using the C. elegans model. We discovered that the TCA cycle intermediate α-KG (but not isocitrate or citrate) delays ageing and extends the lifespan of C. elegans by ~50% (Fig. 1a, Extended Data Fig. 1a). In the cell, α-KG (or 2-oxoglutarate, Fig. 1b) is produced from isocitrate by oxidative decarboxylation catalyzed by isocitrate dehydrogenase (IDH). α-KG can also be produced anaplerotically from glutamate by oxidative deamination using glutamate dehydrogenase, and as a product of pyridoxal phosphate-dependent transamination reactions where glutamate is a common amino donor. α-KG extended wildtype N2 lifespan in a concentration-dependent manner, with 8 mM α-KG producing the maximal lifespan extension (Fig. 1c); 8 mM was the concentration used in all subsequent C. elegans experiments. There is a ~50% increase in α-KG concentration in worms on 8 mM α-KG plates compared to those on vehicle plates (Extended Data Fig. 1b), or ~160 µM vs. ~110 µM assuming homogenous distribution (Methods). α-KG not only extends lifespan, but also delays age-related phenotypes, such as the decline in rapid, coordinated body movement (Supplementary Videos 1–2). α-KG supplementation in the adult stage is sufficient for longevity (Extended Data Fig. 1c).
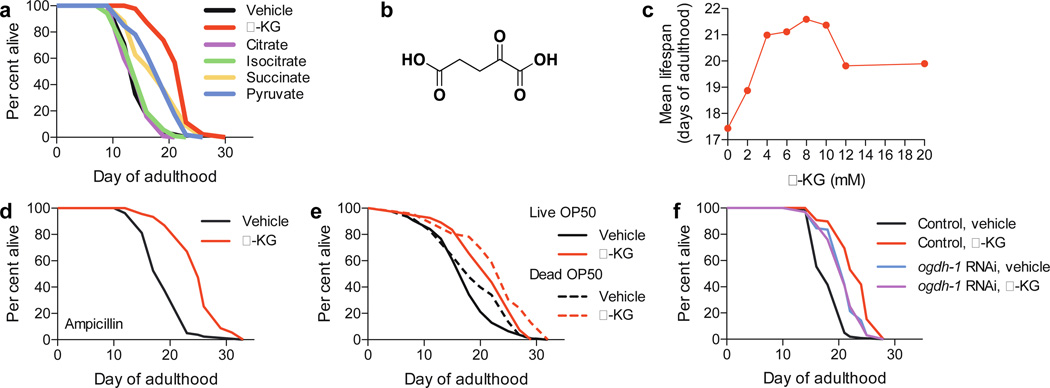
a, α-KG extends the lifespan of adult worms in the metabolite longevity screen. 8 mM was used for all metabolites. b, Structure of α-KG. c, Dose response of α-KG in longevity. d–e, α-KG extends the lifespan of worms fed bacteria that have been d, ampicillin-arrested, mveh = 19.4 (n = 80), mα-KG = 25.1 (n = 91), P < 0.0001 (log-rank test); or e, γ-irradiation-killed, mveh = 19.0 (n = 88), mα-KG = 23.0 (n = 46), P < 0.0001 (log-rank test). f, α-KG does not further extend the lifespan of ogdh-1(RNAi) worms, mveh = 21.2 (n = 98), mα-KG = 21.1 (n = 100), P = 0.65 (log-rank test). m, mean lifespan (days of adulthood); n, number of animals tested.
The dilution or killing of the bacterial food has been shown to extend worm lifespan14, but the lifespan increase by α-KG is not due to altered bacterial proliferation or metabolism (Fig. 1d–e, Extended Data Fig. 1d). Animals also did not view α-KGtreated food as less favorable (Extended Data Fig. 1e–f), and there was no significant change in food intake, pharyngeal pumping, foraging behavior, body size, or brood size in the presence of α-KG (Extended Data Fig. 1e–h, data not shown).
In the cell, α-KG is decarboxylated to succinyl-CoA and CO2 by α-KG dehydrogenase (encoded by ogdh-1), a key control point in the TCA cycle. Increasing α-KG levels by ogdh-1 RNAi (Extended Data Fig. 1b) also extends worm lifespan (Fig. 1f; Supplementary Notes), consistent with a direct effect of α-KG on longevity independent of the bacterial food.
To investigate the molecular mechanism(s) of longevity by α-KG, we took advantage of an unbiased biochemical approach, DARTS7. Since we hypothesized that key target(s) of α-KG are likely to be conserved and ubiquitously expressed, we used a human cell line (Jurkat) that is easy to culture as the protein source for DARTS (Fig. 2a). Mass spectrometry identified ATP5B, the beta subunit of the catalytic core of the ATP synthase, among the most abundant and enriched proteins present in the α-KG treated sample (Extended Data Table 1); the homologous alpha subunit ATP5A was also enriched albeit to a lesser extent. The interaction between α-KG and ATP5B was verified using additional cell lines (Fig. 2b, data not shown), and corroborated for the C. elegans ortholog ATP-2 (Extended Data Fig. 2a).
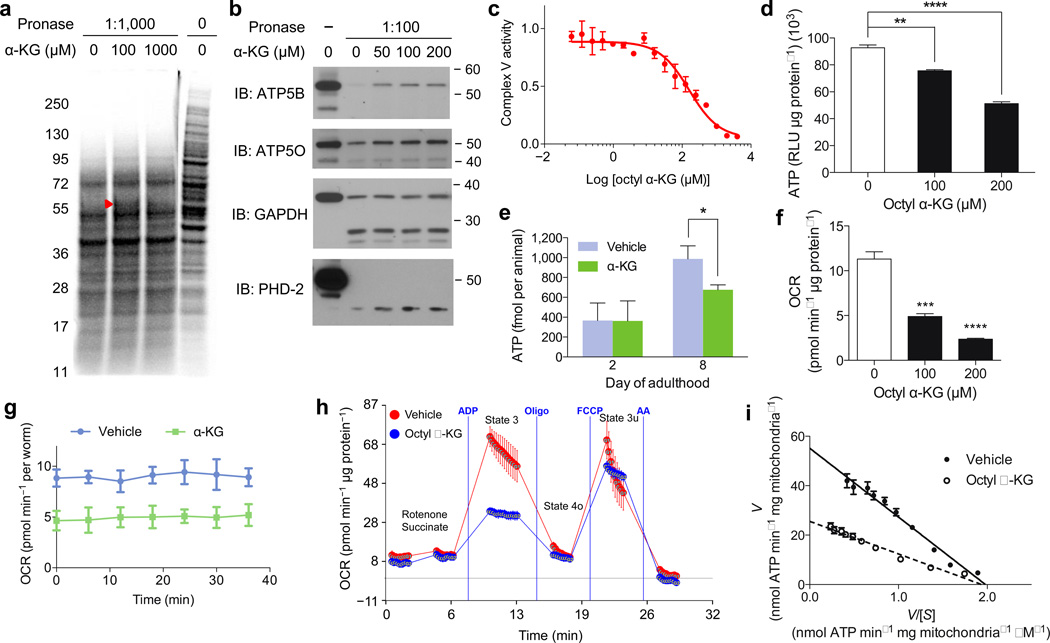
a, DARTS identifies ATP5B as an α-KG-binding protein. Red arrowhead, protected band. b, DARTS confirms α-KG binding specifically to ATP5B. c, Inhibition of ATP synthase by α-KG (released from octyl α-KG; Supplementary Notes). This inhibition was reversible (not shown). d–g, Reduced ATP levels in (d) octyl α-KG treated normal human fibroblasts (**P = 0.0016, ****P < 0.0001; by t-test, two-tailed, two-sample unequal variance) and (e) α-KG treated worms (day 2, P = 0.969; day 8, *P = 0.012; by t-test, two-tailed, two-sample unequal variance). Decreased oxygen consumption rates in (f) octyl α-KG treated cells (***P = 0.0004, ****P < 0.0001; by t-test, two-tailed, two-sample unequal variance) and (g) α-KG treated worms (P < 0.0001; by t-test, two-tailed, two-sample unequal variance). RLU, relative luminescence unit. h, α-KG, released from octyl α-KG (800 µM), decreases state 3, but not state 4o or 3u (P = 0.997), respiration in mitochondria isolated from mouse liver. The respiratory control ratio is decreased in the octyl α-KG (3.1 ± 0.6) vs. vehicle (5.2 ± 1.0) (*P = 0.015; by t-test, two-tailed, two-sample unequal variance). i, Eadie-Hofstee plot of steady-state inhibition kinetics of ATP synthase by α-KG (produced by in situ hydrolysis of octyl α-KG). [S] is the substrate (ADP) concentration, and V is the initial velocity of ATP synthesis in the presence of 200 µM octanol (vehicle control) or octyl α-KG. α-KG (produced from octyl α-KG) decreases the apparent Vmax (53.9 to 26.7) and Km (25.9 to 15.4), by nonlinear regression least squares fit. Number of independent experiments, c–i: 2. Mean ± s.d. is plotted in all cases.
α-KG inhibits the activity of Complex V, but not Complex IV, from bovine heart mitochondria (Fig. 2c, Extended Data Fig. 2b, data not shown). This inhibition is also readily detected in live mammalian cells (Fig. 2d, data not shown) and in live nematodes (Fig. 2e), as evidenced by the reduced ATP levels. Concomitantly, oxygen consumption rates are lowered (Fig. 2f–g), similar to the scenario with atp-2 knockdown (Extended Data Fig. 2c). Specific inhibition of Complex V, but not the other ETC complexes, by α-KG is further confirmed by respiratory control analysis15 (Fig. 2h, Extended Data Fig. 2d–h). To understand the mechanism of inhibition by α-KG, we studied the enzyme inhibition kinetics of ATP synthase. α-KG (released from octyl α-KG) decreases both the effective Vmax and Km of ATP synthase, indicative of uncompetitive inhibition (Fig. 2i, Supplementary Notes).
To determine the significance of ATP-2 to the longevity by α-KG, we measured the lifespan of atp-2(RNAi) adults given α-KG. As reported13, atp-2(RNAi) animals live longer (Fig. 3a). However, their lifespan is not further extended by α-KG (Fig. 3a), indicating that ATP-2 is required for the longevity benefit of α-KG. This requirement is specific because, in contrast, the lifespan of the even longer-lived insulin/IGF-1 receptor daf-2(e1370) mutant worms3 is further increased by α-KG (Fig. 3b). Remarkably, oligomycin, an inhibitor of ATP synthase, also extends the lifespan of adult worms (Extended Data Fig. 3a). Together, the direct binding of ATP-2 by α-KG, the related enzymatic inhibition, reduction in ATP levels and oxygen consumption, lifespan analysis, and other similarities (see also Supplementary Notes, Extended Data Fig. 4) to atp-2 knockdown or oligomycin treatment demonstrate that α-KG likely extends lifespan primarily by targeting ATP-2.
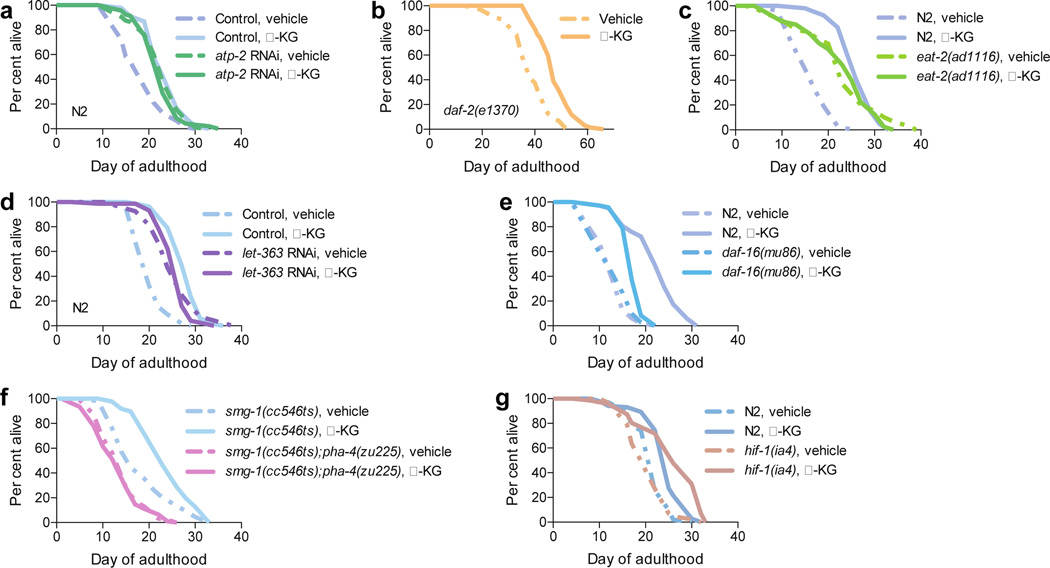
Effect of α-KG on the lifespan of mutant or RNAi worms: a, atp-2(RNAi), mveh = 22.8 (n = 97), mα-KG = 22.5 (n = 94), P = 0.35; or RNAi control, mveh = 18.6 (n = 94), mα-KG = 23.4 (n = 91), P < 0.0001. b, daf-2(e1370), mveh = 38.0 (n = 72), mα-KG = 47.6 (n = 69), P < 0.0001. c, eat-2(ad1116), mveh = 22.8 (n = 59), mα-KG = 22.9 (n = 40), P = 0.79. d, CeTOR(RNAi), mveh = 25.1 (n = 96), mα-KG = 25.7 (n = 74), P = 0.95; or gfp RNAi control, mveh = 20.2 (n = 99), mα-KG = 27.7 (n = 81), P < 0.0001. e, daf-16(mu86), mveh = 13.4 (n = 71), mα-KG = 17.4 (n = 72), P < 0.0001; or N2, mveh = 13.2 (n = 100), mα-KG = 22.3 (n = 104), P < 0.0001. f, pha-4(zu225), mveh = 14.2 (n = 94), mα-KG = 13.5 (n = 109), P = 0.55. g, hif-1(ia4), mveh = 20.5 (n = 85), mα-KG = 26.0 (n = 71), P < 0.0001; or N2, mveh = 21.5 (n = 101), mα-KG = 24.6 (n = 102), P < 0.0001. m, mean lifespan (days of adulthood); n, number of animals tested. P-values were determined by the log-rank test. Number of independent experiments: RNAi control (6), atp-2 (2), CeTOR (3), N2 (5), daf-2 (2), eat-2 (2), pha-4 (2), daf-16 (2), hif-1 (5).
The lower ATP content in α-KG treated animals suggests that longevity by α-KG may involve a DR-like state. Consistent with this idea, we found that α-KG does not extend the lifespan of eat-2(ad1116) animals (Fig. 3c), which is a model of DR with impaired pharyngeal pumping and therefore reduced food intake16. The longevity of eat-2 mutants requires TOR/let-36317, an important mediator of the effects of DR on longevity18. Likewise, α-KG fails to increase the lifespan of CeTOR(RNAi) animals (Fig. 3d). The AMP-activated protein kinase (AMPK) is another conserved major sensor of cellular energy status19. Both AMPK/aak-2 and the FoxO transcription factor DAF-16 mediate DR-induced longevity in C. elegans fed diluted bacteria20, but neither is required for lifespan extension in the eat-2 model16,20. We found that in aak-2 (Extended Data Fig. 5a) and daf-16 (Fig. 3e) mutants the longevity effect of α-KG is smaller than in N2 (P < 0.0001), suggesting that α-KG longevity partially depends on AMPK and FoxO; nonetheless, lifespan is significantly increased by α-KG in aak-2 (24.3%, P < 0.0001) and daf-16 (29.5%, P < 0.0001) mutant or RNAi animals (Fig. 3e, Extended Data Fig. 5a–b, data not shown), indicating an AMPK-FoxO independent effect by α-KG in longevity.
The inability of α-KG to further extend the lifespan of CeTOR(RNAi) animals suggests that α-KG treatment and TOR inactivation extend lifespan either through the same pathway (with α-KG acting on or upstream of TOR), or through independent mechanisms or parallel pathways that converge on a downstream effector. The first model predicts that the TOR pathway will be less active upon α-KG treatment, whereas if the latter model were true then TOR would be unaffected by α-KG treatment. In support of the first model, we found that TOR pathway activity is decreased in human cells treated with octyl α-KG (Fig. 4a, Extended Data Fig. 6a–b). However, α-KG does not interact with TOR directly (Extended Data Fig. 6d–e). Consistent with the involvement of TOR in α-KG longevity, the FoxA transcription factor PHA-4, which is required to extend adult lifespan in response to reduced CeTOR signaling21 and for DR-induced longevity in C. elegans22, is likewise required for α-KG-induced longevity (Fig. 3f). Moreover, autophagy, which is activated both by TOR inhibition18,23 and by DR24, is markedly increased in worms treated with α-KG (or ogdh-1 RNAi) and in atp-2(RNAi) animals (Fig. 4b–c, Extended Data Fig. 6c, Extended Data Fig. 7, Supplementary Notes), as indicated by the prevalence of GFP::LGG-1 puncta (Methods). Autophagy was also induced in mammalian cells treated with octyl α-KG (Extended Data Fig. 6f). Furthermore, α-KG does not result in significantly more autophagy in either atp-2(RNAi) or CeTOR(RNAi) worms (Fig. 4b–c). The data provide further evidence that α-KG decreases TOR pathway activity through the inhibition of ATP synthase. Similarly, autophagy is induced by oligomycin, and oligomycin does not augment autophagy in CeTOR(RNAi) worms (Extended Data Fig. 3b–c).
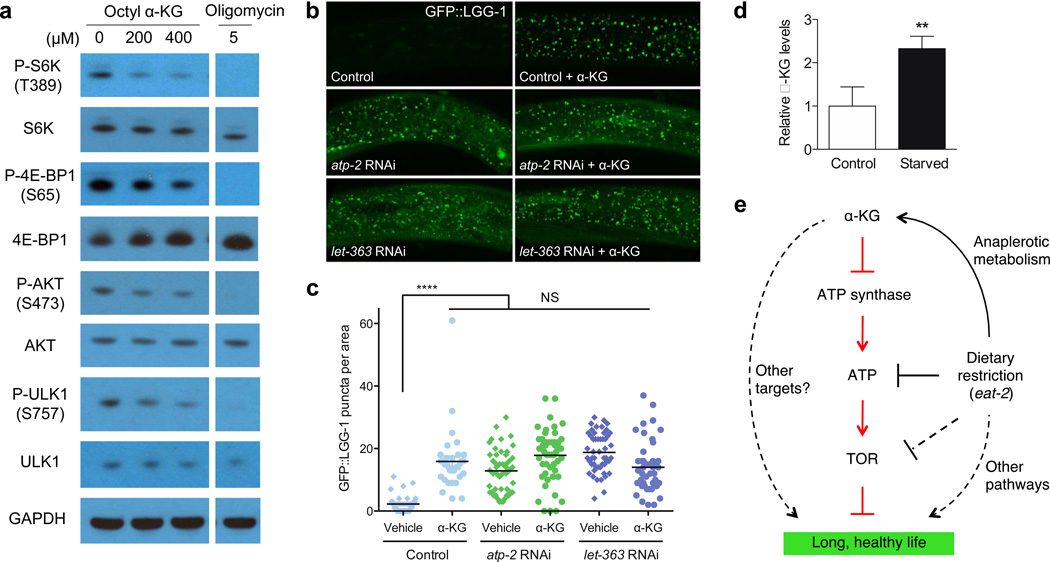
a, Decreased phosphorylation of mTOR substrates in U87 cells treated with octyl α-KG or oligomycin. Similar results were obtained in HEK-293, normal human fibroblasts, and MEFs (not shown). b, Increased autophagy in animals treated with α-KG or RNAi for atp-2 or CeTOR. c, GFP::LGG-1 puncta quantitated using ImageJ (Methods). 2–3 independent experiments. Bars indicate the mean. ****P < 0.0001; n.s., not significant (t-test, two-tailed, two-sample unequal variance). d, α-KG levels are increased in starved worms. **P < 0.01 (t-test, two-tailed, two-sample unequal variance). Mean ± s.d. is plotted. e, Model of α-KG-mediated longevity.
α-KG is not only a metabolite, but also a co-substrate for a large family of dioxygenases25. The hypoxia inducible factor (HIF-1) is modified by one of these enzymes, the prolyl 4-hydroxylase (PHD) EGL-9, and thereafter degraded by the von Hippel-Lindau (VHL) protein26,27. α-KG extends the lifespan of animals with loss-offunction mutations in hif-1, egl-9, and vhl-1 (Fig. 3g, Extended Data Fig. 5c), suggesting that this pathway does not play a major role in lifespan extension by α-KG. However, it is prudent to acknowledge that the formal possibility of other α-KG binding targets playing an additional role in the extension of lifespan by α-KG cannot be eliminated at this time.
In summary, we show that ageing in C. elegans is delayed by α-KG supplementation to adult animals. This longevity effect is likely mediated by the ATP synthase, which we identified as a direct target of α-KG, and TOR, a major effector of DR. Identification of new protein targets of α-KG illustrates that regulatory networks acted upon by the metabolites are likely more complex than currently appreciated, and that DARTS is a useful method for discovering new protein targets and regulatory functions of metabolites. Our findings demonstrate a novel mechanism for extending lifespan that is mediated by the regulation of cellular energy metabolism by a key metabolite. Such moderation of ATP synthesis by metabolite(s) has likely evolved to ensure energy efficiency by the organism in response to nutrient availability. We suggest that this system may be exploited to confer a DR-like state that favors maintenance over growth, and thereby delay ageing and prevent age-related diseases. In fact, the TOR pathway is often hyperactivated in human cancer; inhibition of TOR function by α-KG in normal human cells suggests an exciting role for α-KG as an endogenous tumor suppressor metabolite. Interestingly, physiological increases in α-KG levels have been reported in starved yeast and bacteria28, in the liver of starved pigeons29, and in humans after physical exercise30. The biochemical basis for this increase of α-KG is explained by starvation-based anaplerotic gluconeogenesis, which activates glutamate-linked transaminases in the liver to provide carbon derived from amino acid catabolism. Consistent with this idea, α-KG levels are elevated in starved C. elegans (Fig. 4d). These findings suggest a model in which α-KG is a key metabolite mediating lifespan extension by starvation/DR (Fig. 4e).
Longevity molecules that delay ageing and extend lifespan have profound implications and have long been a dream of humanity. Endogenous metabolites such as α-KG that can alter C. elegans lifespan suggest that an internal mechanism may exist that is accessible to a myriad of regulations and interventions.
METHODS
Nematode strains and maintenance
Caenorhabditis elegans strains were maintained using standard methods31. The following strains were used.
| Strain | Genotype | Source |
|---|---|---|
| Bristol N2 | wildtype | Caenorhabditis Genetics Center (CGC), University of Minnesota |
| DA1116 | eat-2(ad1116)II | CGC |
| CB1370 | daf-2(e1370)III | CGC |
| CF1038 | daf-16(mu86)I | CGC |
| PD8120 | smg-1(cc546ts)I | CGC |
| SM190 | smg-1(cc546ts)I;pha-4(zu225)V | CGC |
| RB754 | aak-2(ok524)X | CGC |
| ZG31 | hif-1(ia4)V | CGC |
| ZG596 | hif-1(ia7)V | CGC |
| JT307 | egl-9(sa307)V | CGC |
| CB5602 | vhl-1(ok161)X | CGC |
| DA2123 | adIs2122[lgg-1 GFP + rol-6(su1006)] GFP + rol-6(su1006)] | CGC |
RNAi in C. elegans
RNAi in C. elegans was accomplished by feeding worms HT115(DE3) bacteria expressing target-gene double-stranded RNA (dsRNA) from the pL4440 vector32. dsRNA production was induced overnight on plates containing 1 mM IPTG. All RNAi feeding clones were obtained from the C. elegans ORF-RNAi Library (Thermo Scientific/Open Biosystems) unless otherwise stated. The C. elegans TOR (CeTOR) RNAi clone33 was obtained from Joseph Avruch (MGH/Harvard). Efficient knockdown was confirmed by Western blotting of the corresponding protein or by qRT-PCR of the mRNA. The primer sequences used for qRT-PCR are as follows.
atp-2 forward: TGACAACATTTTCCGTTTCACC
atp-2 reverse: AAATAGCCTGGACGGATGTGAT
let-363/CeTOR forward: GATCCGAGACAAGATGAACGTG
let-363/CeTOR reverse: ACAATTTGGAACCCAACCAATC
ogdh-1 forward: TGATTTGGACCGAGAATTCCTT
ogdh-1 reverse: GGATCAGACGTTTGAACAGCAC
We validated the RNAi knockdown of both ogdh-1 and atp-2 by quantitative RT-PCR and also of atp-2 by Western blotting. Transcripts of ogdh-1 were reduced by 85%, and transcripts and protein levels of atp-2 were reduced by 52% and 83%, respectively, in larvae that were cultivated on bacteria that expressed the corresponding dsRNAs. In addition, RNAi of atp-2 in our hands was associated with delayed post-embryonic development and larval arrest, consistent with the phenotypes of atp-2(ua2) animals. Analysis by qRT-PCR indicated a modest but significant decrease by 26% in transcripts of CeTOR in larvae undergoing RNAi; moreover, molecular markers for autophagy were induced in these animals, and the lifespan of adults was extended, consistent with partial inactivation of the kinase.
In lifespan experiments, we used RNAi to inactivate atp-2, ogdh-1, and CeTOR in mature animals in the presence or absence of exogenous α-KG. The concentration of α-KG used in these experiments (8 mM) was empirically determined to be most beneficial for wild-type animals (Fig. 1c). This approach enabled us to evaluate the contribution of essential proteins and pathways to the longevity conferred by supplemental α-KG. Specifically, we were able to substantially but not fully inactivate atp-2 in adult animals that had completed embryonic and larval development. As described in our manuscript, supplementation with 8 mM α-KG did not further extend (and in fact, in one occasion, even decreased) the lifespan of atp-2(RNAi) animals (Extended Data Table 2), indicating that atp-2 is required for α-KG to promote longevity. On the other hand, a complete inactivation of atp-2 would be lethal, and thereby mask the benefit of ATP synthase inhibition by α-KG.
Lifespan analysis
Lifespan assays were conducted at 20 °C on solid nematode growth media (NGM) using standard protocols and were replicated in at least two independent experiments. C. elegans were synchronized by performing either a timed egg lay34 or an egg preparation (lysing ~100 gravid worms in 70 µl M9 buffer31, 25 µl bleach (10% sodium hypochlorite solution), and 5 µl 10 N NaOH). Young adult animals were picked onto NGM assay plates containing 1.5% dimethyl sulfoxide (DMSO; Sigma, D8418), 49.5 µM 5-fluoro-2’-deoxyuridine34 (FUDR; Sigma, F0503), and α-KG (Sigma, K1128) or vehicle control (H2O). FUDR was included to prevent progeny production. Media containing α-KG were adjusted to pH 6.0 (i.e., the same pH as the control plates) by the addition of NaOH. All compounds were mixed into the NGM media after autoclaving and before solidification of the media. Assay plates were seeded with OP50 (or a designated RNAi feeding clone, see below). Worms were moved to new assay plates every 4 days (to ensure sufficient food was present at all times and to reduce the risk of mold contamination). To assess the survival of the worms, the animals were prodded with a platinum wire every 2–3 days, and those that failed to respond were scored as dead. For analysis concerning mutant strains, the corresponding parent strain was used as a control in the same experiment.
For lifespan experiments involving RNAi, the plates also contained 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG; Acros, CAS 367-93-1) and 50 µg/mL ampicillin (Fisher, BP1760-25). RNAi was accomplished by feeding N2 worms HT115(DE3) bacteria expressing target-gene dsRNA from pL444032; control RNAi was done in parallel for every experiment by feeding N2 worms HT115(DE3) bacteria expressing either GFP dsRNA or empty vector (which gave identical lifespan results).
Lifespan experiments with oligomycin (Cell signaling, 9996) were performed as described for α-KG (i.e., NGM plates with 1.5% DMSO and 49.5 µM FUDR; N2 worms; OP50 bacteria).
For lifespan experiments concerning smg-1(cc546ts);pha-4(zu225) and smg-1(cc546ts)22,35, from egg to L4 stage the strains were grown from egg to L4 stage at 24°C, which inactivates the smg-1 temperature-sensitive allele, preventing mRNA surveillance-mediated degradation of the pha-4(zu225) mRNA which contains a premature stop codon, and thus produces a truncated but fully functional PHA-4 transcription factor35). Then at the L4 stage the temperature was shifted to 20°C, which restores smg-1 function and thereby results in the degradation of pha-4(zu225) mRNA. Treatment with α-KG began at the L4 stage.
All lifespan data are available in Extended Data Table 2, including sample sizes. The sample size was chosen on the basis of standards done in the field in published manuscripts. No statistical method was used to predetermine the sample size. Animals were assigned randomly to the experimental groups. Worms that ruptured, bagged (i.e., exhibited internal progeny hatching), or crawled off the plates were censored. Lifespan data were analyzed using GraphPad Prism; P-values were calculated using the log-rank (Mantel-Cox) test.
Statistical analyses
All experiments have been repeated at least two times with identical or similar results. Data represent biological replicates. Appropriate statistical tests are used for every figure. Data meet the assumptions of the statistical tests described for each figure. Mean ± s.d. is plotted in all figures unless stated otherwise.
Food preference assay
Protocol adapted from Abada et al.36. A 10 cm NGM plate was seeded with two spots of OP50 as shown in extended data figure 1e. After letting the OP50 lawns to dry over 2 days at room temperature, vehicle (H2O) or α-KG (8 mM) was added to the top of the lawn and allowed to dry over 2 days at room temperature. ~50–100 synchronized adult day 1 worms were placed onto the center of the plate and their preference for either bacterial lawn was recorded after 3 h at room temperature.
Target identification using drug affinity responsive target stability (DARTS)
For unbiased target ID (Fig. 2a), human Jurkat cells were lysed using M-PER (Thermo Scientific, 78501) with the addition of protease inhibitors (Roche, 11836153001) and phosphatase inhibitors37. TNC buffer (50 mM Tris-HCl pH 8.0, 50 mM NaCl, 10 mM CaCl2) was added to the lysate and protein concentration was then determined using the BCA Protein Assay kit (Pierce, 23227). Cell lysates were incubated with either vehicle (H2O) or α-KG for 1 h on ice followed by an additional 20 min at room temperature. Digestion was performed using Pronase (Roche, 10165921001) at room temperature for 30 min and stopped using excess protease inhibitors with immediate transfer to ice. The resulting digests were separated by SDS-PAGE and visualized using SYPRO Ruby Protein Gel Stain (Invitrogen, S12000). The band with increased staining from the α-KG lane (corresponding to potential protein targets that are protected from proteolysis by the binding of α-KG) and the matching area of the control lane were excised, in-gel trypsin digested, and subjected to LC-MS/MS analysis as described7,38. Mass spectrometry results were searched against the human Swissprot database (release 57.15) using Mascot version 2.3.0, with all peptides meeting a significance threshold of 0.05.
For target verification by DARTS-Western blotting (Fig. 2b), HeLa cells were lysed in M-PER buffer (Thermo Scientific, 78501) with the addition of protease inhibitors (Roche, 11836153001) and phosphatase inhibitors (50 mM NaF, 10 mM β-glycerophosphate, 5 mM sodium pyrophosphate, 2 mM Na3VO4). Chilled TNC buffer (50 mM Tris-HCl pH 8.0, 50 mM NaCl, 10 mM CaCl2) was added to the protein lysate, and protein concentration of the lysate was measured by the BCA Protein Assay kit (Pierce, 23227). The protein lysate was then incubated with vehicle control (H2O) or varying concentrations of α-KG for 3 h at room temperature with shaking at 600 rpm in an Eppendorf Thermomixer. Pronase (Roche, 10165921001) digestions were performed for 20 min at room temperature, and stopped by adding SDS loading buffer and immediately heating at 70 °C for 10 min. Samples were subjected to SDS-PAGE on 4–12% Bis-Tris gradient gel (Invitrogen, NP0322BOX) and Western blotted for ATP synthase subunits ATP5B (Sigma, AV48185), ATP5O (Abcam, ab91400), and ATP5A (Abcam, ab110273). Binding between α-KG and PHD-2/Egln1 (Cell Signaling, 4835), for which α-KG is a co-substrate39, was confirmed by DARTS. GAPDH (Ambion, AM4300) was used as a negative control.
For DARTS using C. elegans (Extended Data Fig. 2a), wildtype animals of various ages were grown on NGM/OP50 plates, washed 4 times with M9 buffer, and immediately placed in the −80 °C freezer. Animals were lysed in HEPES buffer (40 mM HEPES pH 8.0, 120 mM NaCl, 10% glycerol, 0.5 % Triton X-100, 10 mM β-glycerophosphate, 50 mM NaF, 0.2 mM Na3VO4, protease inhibitors (Roche, 11836153001)) using Lysing Matrix C tubes (MP Biomedicals, 6912-100) and the FastPrep-24 (MP Biomedicals) high-speed benchtop homogenizer in the 4 °C room (disrupt worms for 20 seconds at 6.5 m/s, rest on ice for 1 min; repeat twice). Lysed animals were centrifuged at 14,000 rpm for 10 min at 4 °C to pellet worm debris, and supernatant was collected for DARTS. Protein concentration was determined by BCA Protein Assay kit (Pierce, 23223). A worm lysate concentration of 1.13 µg/µL was used for the DARTS experiment. All steps were performed on ice or at 4 °C to help prevent premature protein degradation. TNC buffer (50 mM Tris-HCl pH 8.0, 50 mM NaCl, 10 mM CaCl2) was added to the worm lysates. Worm lysates were incubated with vehicle control (H2O) or α-KG for 1 h on ice and then 50 min at room temperature. Pronase (Roche, 10165921001) digestions were performed for 30 min at room temperature and stopped by adding SDS loading buffer and heating at 70 °C for 10 min. Samples were then subjected to SDS-PAGE on NuPAGE Novex 4–12% Bis-Tris gradient gels (Invitrogen, NP0322BOX), and Western blotting was carried out with an antibody against ATP5B (Sigma, AV48185) that also recognizes ATP-2.
Complex V activity assay
Complex V activity was assayed using the MitoTox OXPHOS Complex V Activity Kit (Abcam, ab109907). Vehicle (H2O) or α-KG was mixed with the enzyme prior to the addition of phospholipids. In experiments using octyl α-KG, vehicle (1% DMSO) or octyl α-KG was added with the phospholipids. Relative complex V activity was compared to vehicle. Oligomycin (Sigma, O4876) was used as a positive control for the assay.
Isolation of mitochondria from mouse liver
Animal studies were performed under approved UCLA animal research protocols. Mitochondria from 3-month-old C57BL/6 mice were isolated as described40. Briefly, livers were extracted, minced at 4 °C in MSHE+BSA (70 mM sucrose, 210 mM mannitol, 5 mM HEPES, 1 mM EGTA, and 0.5% fatty acid free BSA, pH 7.2), and rinsed several times to remove blood. All subsequent steps were performed on ice or at 4 °C. The tissue was disrupted in 10 volumes of MSHE+BSA with a glass Dounce homogenizer (5–6 strokes) and the homogenate was centrifuged at 800 × g for 10 min to remove tissue debris and nuclei. The supernatant was decanted through a cell strainer and centrifuged at 8,000 × g for 10 min. The dark mitochondrial pellet was resuspended in MSHE+BSA and re-centrifuged at 8,000 × g for 10 min. The final mitochondrial pellets were used for various assays as described below.
Submitochondrial particle (SMP) ATPase assay
ATP hydrolysis by ATP synthase was measured using submitochondrial particles (see41 and refs therein). Mitochondria were isolated from mouse liver as described above. The final mitochondrial pellet was resuspended in buffer A (250 mM sucrose, 10 mM Tris-HCl, 1 mM ATP, 5 mM MgCl2, and 0.1 mM EGTA, pH 7.4) at 10 µg/µL, subjected to sonication on ice (Fisher Scientific Model 550 Sonic Dismembrator; medium power, alternating between 10 s intervals of sonication and resting on ice for a total of 60 s of sonication), and then centrifuged at 18,000 × g for 10 min at 4 °C. The supernatant was collected and centrifuged at 100,000 × g for 45 min at 4 °C. The final pellet (submitochondrial particles) was resuspended in buffer B (250 mM sucrose, 10 mM Tris-HCl, and 0.02 mM EGTA, pH 7.4).
The SMP ATPase activity was assayed using the Complex V Activity Buffer as above. The production of ADP is coupled to the oxidation of NADH to NAD+ through pyruvate kinase and lactate dehydrogenase. The addition of α-KG (up to 10 mM) did not affect the activity of pyruvate kinase or lactate dehydrogenase when external ADP was added. The absorbance decrease of NADH at 340 nm correlates to ATPase activity. SMPs (2.18 ng/uL) were incubated with vehicle or α-KG for 90 min at room temperature prior to the addition of activity buffer, and then the absorbance decrease of NADH at 340 nm was measured every 1 min for 1 h. Oligomycin (Cell signaling, 9996) was used as a positive control for the assay.
Assay for ATP levels
Normal human diploid fibroblast WI-38 (ATCC, CCL-75) cells were seeded in 96-well plates at 2 × 104 cells per well. Cells were treated with either DMSO (vehicle control) or octyl α-KG at varying concentrations for 2 h in triplicate. ATP levels were measured using the CellTiter-Glo luminescent ATP assay (Promega, G7572); luminescence was read using Analyst HT (Molecular Devices). In parallel, identically treated cells were lysed in M-PER (Thermo Scientific, 78501) to obtain protein concentration by BCA Protein Assay kit (Pierce, 23223). ATP levels were normalized to protein content. Statistical analysis was performed using GraphPad Prism (unpaired t-test).
Assay for ATP levels in C. elegans. Synchronized day 1 adult wildtype C. elegans were placed on NGM plates containing either vehicle or 8 mM α-KG. On day 2 and 8 of adulthood, 9 replicates and 4 replicates, respectively, of about 100 worms were collected from α-KG or vehicle control plates, washed 4 times in M9 buffer, and frozen in −80 °C. Animals were lysed using Lysing Matrix C tubes (MP Biomedicals, 6912-100) and the FastPrep-24 (MP Biomedicals) high-speed benchtop homogenizer (disrupt worms for 20 s at 6.5 m/s, rest on ice for 1 min; repeat twice). Lysed animals were centrifuged at 14,000 rpm for 10 min at 4 °C to pellet worm debris, and supernatant was saved for ATP quantitation using the Kinase-Glo Luminescent Kinase Assay Platform (Promega, V6713) according to manufacturer’s instructions. Assay was performed in white opaque 96 well tissue culture plates (Falcon, 353296), and luminescence was measured using Analyst HT (Molecular Devices). ATP levels were normalized to number of worms. Statistical analysis was performed using Microsoft Excel (t-test, two-tailed, two-sample unequal variance).
Measurement of oxygen consumption rates (OCR)
OCR measurements were made using a Seahorse XF-24 analyzer (Seahorse Bioscience)42. Cells were seeded in Seahorse XF-24 cell culture microplates at 50,000 cells/well in DMEM media supplemented with 10% FBS and 10 mM glucose, and incubated at 37 °C and 5% CO2 for overnight. Treatment with octyl α-KG or DMSO (vehicle control) was for 1 h. Cells were washed in unbuffered DMEM medium (pH 7.4, 10 mM glucose) just prior to measurement, and maintained in this buffer with indicated concentrations of octyl α-KG. Oxygen consumption rates were measured 3 times under basal conditions and normalized to protein concentration per well. Statistical analysis was performed using GraphPad Prism.
Measurement of oxygen consumption rates (OCR) in living C. elegans. Protocol adapted from43,44. Wildtype day 1 adult N2 worms were placed on NGM plates containing 8 mM α-KG or H2O (vehicle control) seeded with OP50 or HT115 E. coli. OCR was assessed on day 2 of adulthood. On day 2 of adulthood, worms were collected and washed 4 times with M9 to rid the samples of bacteria (we further verified that α-KG does not affect oxygen consumption of the bacteria – therefore, even if there were any leftover bacteria after the washes, the changes in OCR observed would still be worm-specific), and then the animals were seeded in quadruplicates in Seahorse XF-24 cell culture microplates (Seahorse Bioscience, V7-PS) in 200 µL M9 at ~200 worms per well. Oxygen consumption rates were measured 7 times under basal conditions and normalized to the number of worms counted per well. The experiment was repeated twice. Statistical analysis was performed using Microsoft Excel (t-test, two-tailed, two-sample unequal variance).
Measurement of mitochondrial respiratory control ratio (RCR)
Mitochondrial RCR was analyzed using isolated mouse liver mitochondria (see15 and refs therein). Mitochondria were isolated from mouse liver as described above. The final mitochondrial pellet was resuspended in 30 µL of MAS buffer (70 mM sucrose, 220 mM mannitol, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA, and 0.2% fatty acid free BSA, pH 7.2).
Isolated mitochondrial respiration was measured by running coupling and electron flow assays as described40. For the coupling assay, 20 µg of mitochondria in complete MAS buffer (MAS buffer supplemented with 10 mM succinate and 2 µM rotenone) were seeded into a XF24 Seahorse plate by centrifugation at 2,000 × g for 20 min at 4 °C. Just before the assay, the mitochondria were supplemented with complete MAS buffer for a total of 500 µL (with 1% DMSO or octyl α-KG), and warmed at 37 °C for 30 min before starting the oxygen consumption rate measurements. Mitochondrial respiration begins in a coupled State 2; State 3 is initiated by 2 mM ADP; State 4o (oligomycinin-sensitive, i.e., Complex V-independent) is induced by 2.5 µM oligomycin and State 3u (FCCP uncoupled maximal respiratory capacity) by 4 µM FCCP. Finally, 1.5 µg/mL antimycin A was injected at the end of the assay. The State 3/State 4o ratio gives the respiratory control ratio (RCR).
For the electron flow assay, the MAS buffer was supplemented with 10 mM sodium pyruvate (Complex I substrate), 2 mM malate (Complex II inhibitor), and 4 µM FCCP, and the mitochondria are seeded the same way as described for the coupling assay. After basal readings, the sequential injections were as follows: 2 µM rotenone (Complex I inhibitor), 10 mM succinate (Complex II substrate), 4 µM antimycin A (Complex III inhibitor), and 10 mM/100 µM ascorbate/tetramethylphenylenediamine (Complex IV substrate).
ATP synthase enzyme inhibition kinetics
ATP synthesis enzyme inhibition kinetic analysis was performed using isolated mitochondria. Mitochondria were isolated from mouse liver as described above. The final mitochondrial pellet was resuspended in MAS buffer supplemented with 5 mM sodium ascorbate (Sigma, A7631) and 5 mM TMPD (Sigma, T7394).
The reaction was carried out in MAS buffer containing 5 mM sodium ascorbate, 5 mM TMPD, luciferase reagent (Roche, 11699695001), octanol or octyl α-KG, variable amounts of ADP (Sigma, A2754), and 3.75 ng/µL mitochondria. ATP synthesis was monitored by the increase in luminescence over time by a luminometer (Analyst HT, Molecular Devices). ATP synthase-independent ATP formation, derived from the oligomycin-insensitive luminescence, was subtracted as background. The initial velocity of ATP synthesis was calculated from the slope of the first 3 min of the reaction, before the velocity begins to decrease. Enzyme inhibition kinetics was analyzed by nonlinear regression least squares fit using GraphPad Prism.
Assay for mammalian TOR (mTOR) pathway activity
mTOR pathway activity in cells treated with octyl α-KG or oligomycin was determined by the levels of phosphorylation of known mTOR substrates, including S6K (T389), 4E-BP1 (S65), AKT (S473), and ULK1 (S757)45–49. Specific antibodies used: P-S6K T389 (Cell Signaling, 9234), S6K (Cell Signaling, 9202S), P-4E-BP1 S65 (Cell Signaling, 9451S), 4E-BP1 (Cell Signaling, 9452S), P-AKT S473 (Cell Signaling, 4060S), AKT (Cell Signaling, 4691S), P-ULK1 S757 (Cell Signaling, 6888), ULK1 (Cell Signaling, 4773S), and GAPDH (Santa Cruz Biotechnology, 25778).
Assay for autophagy
DA2123 animals carrying an integrated GFP::LGG-1 translational fusion gene50–52, were used to quantify levels of autophagy. To obtain a synchronized population of DA2123, we performed an egg preparation of gravid adults (by lysing ~100 gravid worms in 70 µL M9 buffer, 25 µL bleach and 5 µL 10 N NaOH), and allowed the eggs to hatch overnight in M9 causing starvation induced L1 diapause. L1 larvae were deposited onto NGM treatment plates containing vehicle, 8 mM α-KG, or 40 µM oligomycin, and seeded with either E. coli OP50, HT115(DE3) with an empty vector, or HT115(DE3) expressing dsRNAs targeting atp-2, CeTOR/let-363, or ogdh-1 as indicated. When the majority of animals in a given sample first reached the mid L3 stage, individual L3 larvae were mounted onto microscope slides and anesthetized with 1.6 mM levamisole (Sigma, 31742). Nematodes were observed using an Axiovert 200M Zeiss confocal microscope with a LSM5 Pascal laser, and images were captured using the LSM Image Examiner (Zeiss). For each specimen, GFP::LGG-1 puncta (autophagosomes) in the epidermis, including the lateral seam cells and Hyp7, were counted in three separate regions of 140.97 µm2 using analyze particles in ImageJ53. Measurements were made blind to both the genotype and supplement. Statistical analysis was performed using Microsoft Excel (t-test, two-tailed, two-sample unequal variance).
Assay for autophagy in mammalian cells. HEK-293 cells were seeded in 6-well plates at 2.5 × 105 cells/well in DMEM media supplemented with 10% FBS and 10 mM glucose, and incubated overnight before treatment with either octanol (vehicle control) or octyl α-KG for 72 h. Cells were lysed in M-PER buffer with protease and phosphatase inhibitors. Lysates were subjected to SDS-PAGE on a 4–12% Bis-Tris gradient gel with MES running buffer and Western blotted for LC3 (Novus, NB100-2220). LC3 is the mammalian homolog of worm LGG-1, and conversion of the soluble LC3-I to the lipidated LC3-II is activated in autophagy, e.g., upon starvation54.
Pharyngeal pumping rates of C. elegans treated with 8 mM α-KG
The pharyngeal pumping rates of 20 wildtype N2 worms per condition were assessed. Pharyngeal contractions were recorded for 1 min using a Zeiss M2BioDiscovery microscope and an attached Sony NDR-XR500V video camera at 12-fold optical zoom. The resulting videos were played back at 0.3× speed using MPlayerX and pharyngeal pumps were counted. Statistical analysis was performed using Microsoft Excel (t-test, two-tailed, two-sample unequal variance).
Assay for α-KG levels in C. elegans
Synchronized adult worms were collected from plates with vehicle (H2O) or 8 mM α-KG, washed 3 times with M9 buffer, and flash frozen. Worms were lysed in M9 using Lysing Matrix C tubes (MP Biomedicals, 6912-100) and the FastPrep-24 (MP Biomedicals) high-speed benchtop homogenizer in the 4 °C room (disrupt worms for 20 seconds at 6.5 m/s, rest on ice for 1 min; repeat three times). Lysed animals were centrifuged at 14,000 rpm for 10 min at 4 °C to pellet worm debris, and the supernatant was saved. The protein concentration of the supernatant was determined by the BCA Protein Assay kit (Pierce, 23223); there was no difference in protein level per worm in α-KG treated and vehicle treated animals (data not shown). α-KG content was assessed as described previously55 with modifications. Worm lysates were incubated at 37 °C in 100 mM KH2PO4 (pH 7.2), 10 mM NH4Cl, 5 mM MgCl2, and 0.3 mM NADH for 10 minutes. Glutamate dehydrogenase (Sigma, G2501) was then added to reach a final concentration of 1.83 units/mL. Under these conditions glutamate dehydrogenase uses α-KG and NADH to make glutamate. The absorbance decrease was monitored at 340 nm. The intracellular level of α-KG was determined from the absorbance decrease in NADH. The approximate molarity of α-KG present inside the animals was estimated using average protein content (~245 ng/worm, from BCA assay) and volume (~3 nL for adult worms 1.1 mm in length and 60 µm in diameter (http://www.wormatlas.org/hermaphrodite/introduction/Introframeset.html)).
For quantitative analysis of α-KG in worms using UHPLC-ESI/MS/MS, synchronized day 1 adult worms were placed on vehicle plates with or without bacteria for 24 h, and then collected and lysed in the same manner as above. α-KG analysis by LC/MS/MS was carried out on an Agilent 1290 Infinity UHPLC system and 6460 Triple Quadrupole mass spectrometer (Agilent Technologies) using an electrospray ionization (ESI) source with Agilent Jet Stream technology. Data were acquired with Agilent MassHunter Data Acquisition software version B.06.00, and processed for precursor and product ions selection with MassHunter Qualitative Analysis software version B.06.00 and for calibration and quantification with MassHunter Quantitative Analysis for QQQ software version B.06.00.
For UHPLC, 3 µL calibration standards and samples were injected onto the UHPLC system including a G4220A binary pump with a built-in vacuum degasser and a thermostatted G4226A high performance autosampler. An ACQUITY UPLC BEH Amide analytical column (2.1 × 50 mm, 1.7 µm) and a VanGuard BEH Amide Pre-column (2.1 × 5 mm, 1.7 µm) from Waters Corporation were used at the flow rate of 0.6 mL/min using 50/50/0.04 acetonitrile/water/ammonium hydroxide with 10 mM ammonium acetate as mobile phase A and 95/5/0.04 acetonitrile/water/ammonium hydroxide with 10 mM ammonium acetate as mobile phase B. The column was maintained at room temperature. The following gradient was applied: 0–0.41 min: 100% B isocratic; 0.41–5.30 min: 100–30% B; 5.3–5.35 min: 30-0% B; 5.35–7.35 min: 0% B isocratic; 7.35–7.55 min: 0–100%B; 7.55–9.55 min: 100% B isocratic.
For the MS detection, the ESI mass spectra data were recorded on a negative ionization mode by MRM. MRM transitions of α-KG and its ISTD 13C4-α-KG (Cambridge Isotope Laboratories) were determined using a 1-min 37% B isocratic UHPLC method through the column at flow rate of 0.6 mL/min. The precursor ion of [M-H]− and the product ion of [M-CO2-H]− were observed to have the highest signal to noise ratios. The precursor and product ions are respectively 145.0 and 100.9 for AKG, and 149.0 and 104.9 for ISTD 13C4-α-KG. Nitrogen was used as the drying, sheath, and collision gas. All the source and analyzer parameters were optimized using Agilent MassHunter Source and iFunnel Optimizer and Optimizer software respectively. The source parameters are as follows: drying gas temperature 120 °C, drying gas flow 13 L/min, nebulizer pressure 55 psi, sheath gas temperature 400 °C, sheath gas flow 12 L/min, capillary voltage 2000 V, and nozzle voltage 0 V. The analyzer parameters are as follows: fragmentor voltage 55 V, collision energy 2 V, and cell accelerator voltage 1 V. The UHPLC eluants before 1 min and after 5.3 min were diverted to waste.
Membrane-permeable esters of α-KG
Octyl α-KG, a commonly used membrane-permeable ester of α-KG55–58, was used to deliver α-KG across lipid membranes in experiments using cells and mitochondria. Upon hydrolysis by cellular esterases, octyl α-KG yields α-KG and the byproduct octanol. We showed that, whereas octanol control has no effect (Extended Data Fig. 2e–f and Extended Data Fig. 6a), α-KG alone can bind and inhibit ATP synthase (Fig. 2a–b, Extended Data Fig. 2a–b, and data not shown), decrease ATP and OCR (Fig. 2e, and Fig. 2g), induce autophagy (Fig. 4b), and increase C. elegans lifespan (Fig. 1, Fig. 3, and Extended Data Figs. 1, ,5,5, and Table 2). The existence and activity of esterases in our mitochondrial and cell culture experiments have been confirmed using calcein AM (C1430, Molecular Probes), an esterase substrate that fluoresces upon hydrolysis, and also by mass spectrometry (data not shown). The hydrolysis by esterases explains why distinct esters of α-KG, such as 1-octyl α-KG, 5-octyl α-KG, and dimethyl α-KG, have similar effects to α-KG (Extended Data Fig. 2g–h and Extended Data Table 2).
Synthesis of octyl α-KG
Synthesis of 1-octyl α-KG has been published by GD and MEJ59. Briefly, 1-octanol (0.95 mL, 6.0 mmol), DMAP (37 mg, 0.3 mmol), and DCC (0.743 g, 3.6 mmol) were added to a solution of 1-cyclobutene-1-carboxylic acid (0.295 g, 3.0 mmol) in dry CH2Cl2 (6.0 mL) at 0 °C. After it had stirred for 1 h, the solution was allowed to warm to room temperature and stirred for another 8 h. The precipitate was filtered and washed with ethyl acetate (3 × 100 mL). The combined organic phases were washed with water and brine, and dried over anhydrous Na2SO4. Flash column chromatography on silica gel eluting with 80/1 hexane/ethyl acetate gave octyl cyclobut-1-enecarboxylate as a clear oil (0.604 g, 96%). To a −78 °C solution of this oil (0.211 g, 1.0 mmol) in CH2Cl2 (10 mL) was bubbled O3/O2 until the solution turned blue. The residual ozone was discharged by bubbling with O2 and the reaction was warmed to room temperature and stirred for another 1 h. Dimethyl sulfide (Me2S, 0.11 mL, 1.5 mmol) was added to the mixture and it was stirred for another 2 h. The CH2Cl2 was removed in vacuo and the crude product was dissolved in a solution of 2-methyl-2-butene (0.8 mL) in t-BuOH (3.0 mL). To this was added dropwise a solution containing sodium chlorite (0.147 g, 1.3 mmol) and sodium dihydrogen phosphate monohydrate (0.179 g, 1.3 mmol) in H2O (1.0 mL). The mixture was stirred at room temperature overnight, and then extracted with ethyl acetate (3 × 50 mL). The combined organic phases were washed with water and brine, and dried over anhydrous Na2SO4. Flash column chromatography on silica gel eluting with 5/1 hexane/ethyl acetate gave octyl α-KG which became a pale solid when stored in the refrigerator (0.216 g, 84%).
Synthesis of 5-octyl L-Glu ((S)-2-amino-5-(octyloxy)-5-oxopentanoic acid)
L-Glutamic acid (0.147 g, 1.0 mmol) and anhydrous sodium sulfate (0.1 g) was dissolved in octanol (2.0 mL), and then tetrafluoroboric acid-dimethyl ether complex (0.17 mL) was added. The suspended mixture was stirred at 21 °C overnight. Anhydrous THF (5 mL) was added to the mixture and it was filtered through a thick pad of activated charcoal. Anhydrous triethylamine (0.4 mL) was added to the clear filtrate to obtain a milky white slurry. Upon trituration with ethyl acetate (10 mL), the monoester monoacid precipitated. The precipitate was collected, washed with additional ethyl acetate (2 × 5 mL), and dried in vacuo to give the desired product 5-octyl L-Glu (0.249 g, 96%) as a white solid. 1H NMR (500 MHz, Acetic acid-d4): δ 4.12 (dd, J = 6.6, 6.6 Hz, 1H), 4.11 (t, J = 6.8 Hz, 2H), 2.64 (m, 2H), 2.26 (m, 2H), 1.64 (m, 2H), 1.30 (m, 10H), 0.89 (t, J = 7.0 Hz, 3H). 13C NMR (125 MHz, Acetic acid-d4): 175.0, 174.3, 66.3, 55.0, 32.7, 30.9, 30.11, 30.08, 29.3, 26.7, 26.3, 23.4, 14.4.
Synthesis of 5-octyl D-Glu ((R)-2-amino-5-(octyloxy)-5-oxopentanoic acid)
The synthesis of the opposite enantiomer, i.e., 5-octyl D-Glu, was carried out by the exact same procedure starting with D-glutamic acid. The spectroscopic data was identical to that of the enantiomeric compound.
Synthesis of 5-octyl α-KG (5-(Octyloxy)-2,5-dioxopentanoic acid) 1-Benzyl 5-octyl 2-oxopentanedioate
To a solution of 5-octyl L-Glu (0.249 g) in H2O (6.0 mL) and acetic acid (2.0 mL) cooled to 0 °C was added slowly a solution of aqueous sodium nitrite (0.207 g, 3.0 mmol in 4 mL H2O). The reaction mixture was allowed to warm slowly to room temperature and was stirred overnight. The mixture was concentrated. The resulting residue was dissolved in DMF (10 mL) and NaHCO3 (0.42 g, 5.0 mmol) and benzyl bromide (0.242 mL, 2.0 mmol) were added to the mixture. The mixture was stirred at 21 °C overnight and then extracted with ethyl acetate (3 × 30 mL). The combined organic phase was washed with water and brine and dried over anhydrous MgSO4. Flash column chromatography on silica gel eluting with 7/1 hexanes/ethyl acetate gave the mixed diester 1-benzyl 5-octyl (S)-2-hydroxypentanedioate as a colorless oil. To this oil dissolved in dichloromethane (10.0 mL), were added NaHCO3 (0.42 g, 5.0 mmol) and Dess-Martin periodinane (0.509 g, 1.2 mmol) and the mixture was stirred at room temperature for 1 h and then extracted with ethyl acetate (3 × 30 mL). The combined organic phase was washed with water and brine and dried over anhydrous MgSO4. Flash column chromatography on silica gel eluting with 5/1 hexanes/ethyl acetate gave the desired 1-benzyl 5-octyl 2-oxopentanedioate (0.22 g, 66%) as a white solid. 1H NMR (500 MHz, CDCl3): 7.38 (m, 5H), 5.27 (s, 2H), 4.05 (t, J = 6.5 Hz, 2H), 3.14 (t, J = 6.5 Hz, 2H), 2.64 (t, J = 6.5 Hz, 2H), 1.59 (m, 2H), 1.28 (m, 10H), 0.87 (t, J = 7.0 Hz, 3H). 13C NMR (125 MHz, CDCl3): 192.2, 171.9, 160.1, 134.3, 128.7, 128.6, 128.5, 67.9, 65.0, 34.2, 31.7, 29.07, 29.05, 28.4, 27.5, 25.7, 22.5, 14.0.
5-octyl α-KG (5-(Octyloxy)-2,5-dioxopentanoic acid)
To a solution of 1-benzyl 5-octyl 2-oxopentanedioate (0.12 g, 0.344 mmol) in ethyl acetate (15 mL) was added 5% Pd/C (80 mg). Over the mixture was passed a stream of argon and then the argon was replaced with hydrogen gas and the mixture was stirred vigorously for 15 min. The mixture was filtered through a thick pad of Celite to give the desired product 5-octyl α-KG (0.088 g, 99%) as white solid. 1H NMR (500 MHz, CDCl3): 8.16 (br s, 1H), 4.06 (t, J = 6.5 Hz, 2H), 3.18 (t, J = 6.5 Hz, 2H), 2.69 (t, J = 6.0 Hz, 2H), 1.59 (m, 2H), 1.26 (m, 10H), 0.85 (t, J = 7.0 Hz, 3H). 13C NMR (125 MHz, CDCl3): 193.8, 172.7, 160.5, 65.5, 33.0, 31.7, 29.08, 29.06, 28.4, 27.8, 25.8, 22.5, 14.0.
Extended Data
Extended Data Figure 1
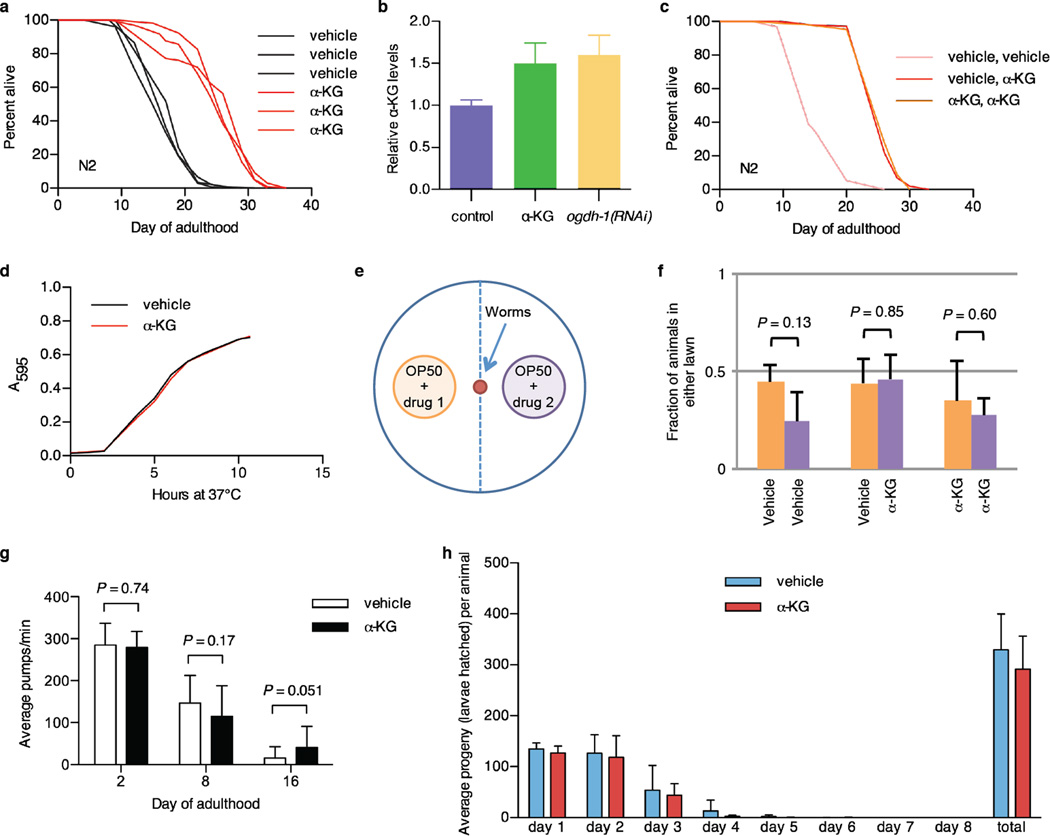
a, Robust lifespan extension in adult C. elegans by α-KG. 8 mM α-KG increased the mean lifespan of N2 by an average of 47.3% in three independent experiments (P < 0.0001 for every experiment, by log-rank test). Expt. #1, mveh = 18.9 (n = 87), mα-KG = 25.8 (n = 96); Expt. #2, mveh = 17.5 (n = 119), mα-KG = 25.4 (n = 97); Expt. #3, mveh = 16.3 (n = 100), mα-KG = 26.1 (n = 104). m, mean lifespan (days of adulthood); n, number of animals tested. b, Worms supplemented with 8 mM α-KG and worms with RNAi knockdown of α-KGDH (encoded by ogdh-1) have increased α-KG levels. Young adult worms were placed on treatment plates seeded with control HT115 E. coli or HT115 expressing ogdh-1 dsRNA, and α-KG content was assayed after 24 h (see Methods). c, α-KG treatment beginning at the egg stage and that beginning in adulthood produced identical lifespan increases. Light red, treatment with vehicle control throughout larval and adult stages (m = 15.6, n = 95); dark red, treatment with vehicle during larval stages and with 8 mM α-KG at adulthood (m = 26.3, n = 102), P < 0.0001 (log-rank test); orange, treatment with 8 mM α-KG throughout larval and adult stages (m = 26.3, n = 102), P < 0.0001 (log-rank test). m, mean lifespan (days of adulthood); n, number of animals tested. d, α-KG does not alter the growth rate of the OP50 E. coli, which is the standard laboratory food source for nematodes. α-KG (8 mM) or vehicle (H2O) was added to standard LB media and the pH was adjusted to 6.6 by the addition of NaOH. Bacterial cells from the same overnight OP50 culture were added to the LB ± α-KG mixture at a 1:40 dilution, and then placed in the 37 °C incubator shaker at 300 rpm. The absorbance at 595 nm was read at 1 h time intervals to generate the growth curve. e, Schematic representation of food preference assay. f, N2 worms show no preference between OP50 E. coli food treated with vehicle or α-KG (P = 0.85, by t-test, two-tailed, two-sample unequal variance), nor preference between identically treated OP50 E. coli. g, Pharyngeal pumping rate of C. elegans on 8 mM α-KG is not significantly altered (by t-test, two-tailed, two-sample unequal variance). h, Brood size of C. elegans treated with 8 mM α-KG. Brood size analysis was conducted at 20 °C. 10 L4 wildtype worms were each singly placed onto an NGM plate containing vehicle or 8 mM α-KG. Worms were transferred one per plate onto a new plate every day, and the eggs laid were allowed to hatch and develop on the previous plate. Hatchlings were counted as a vacuum was used to remove them from the plate. Animals on 8 mM α-KG showed no significant difference in brood size compared with animals on vehicle plates (P = 0.223, by t-test, two-tailed, two-sample unequal variance). Mean ± s.d is plotted in all cases.
Extended Data Figure 2
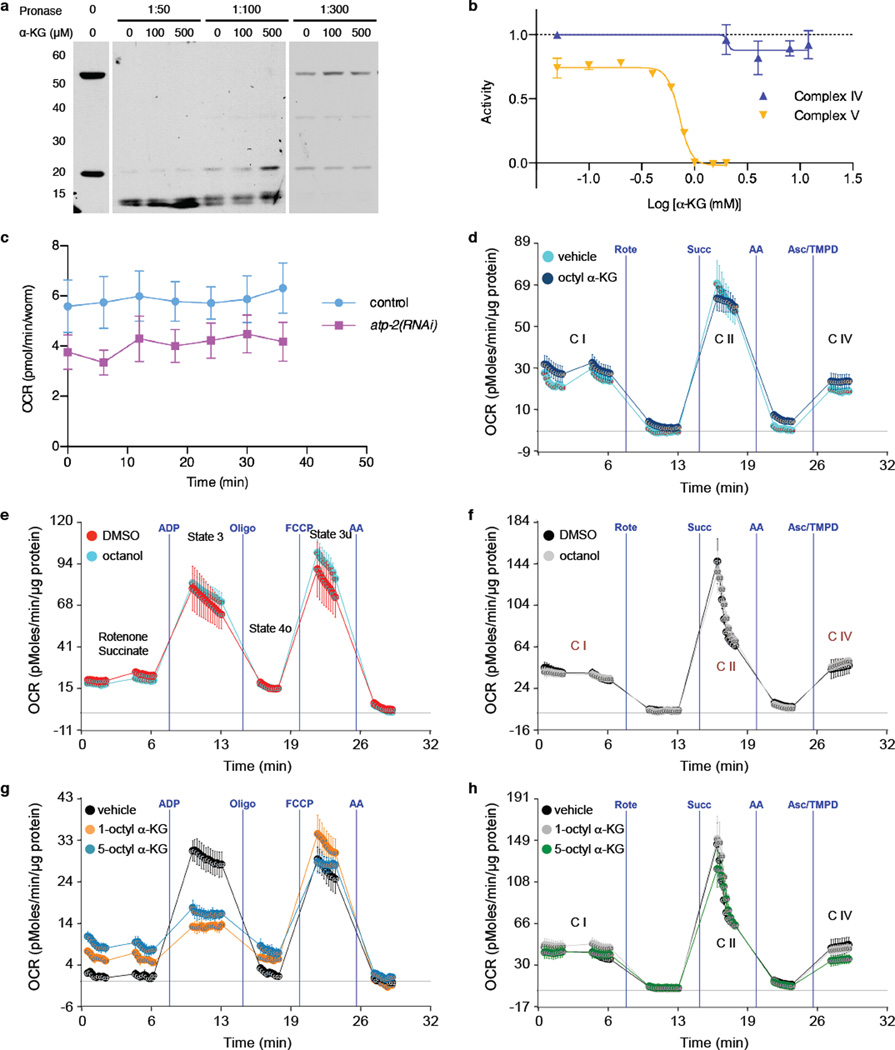
a, Western blot showing protection of the ATP-2 protein from Pronase digestion upon α-KG binding in the DARTS assay. The antibody for human ATP5B (Sigma, AV48185) recognizes the epitope 144IMNVIGEPIDERGPIKTKQFAPIHAEAPEFMEMSVEQEILVTGIKVVDLL193 that has 90% identity to the C. elegans ATP-2. The lower molecular weight band near 20 kDa is a proteolytic fragment of the full-length protein corresponding to the domain directly bound by α-KG. b, α-KG does not affect Complex IV activity. Complex IV activity was assayed using the MitoTox OXPHOS Complex IV Activity Kit (Abcam, ab109906). Relative complex IV activity was compared to vehicle (H2O) controls. Potassium cyanide (Sigma, 60178) was used as a positive control for the assay. Complex V activity was assayed using the MitoTox Complex V OXPHOS Activity Microplate Assay (Abcam, ab109907). c, atp-2(RNAi) worms have lower oxygen consumption compared to control (gfp in RNAi vector), P < 0.0001 (t-test, two-tailed, two-sample unequal variance) for the entire time series (2 independent experiments); similar to α-KG treated worms shown in figure 2g. d, α-KG does not affect the electron flow through the electron transport chain. OCR from isolated mouse liver mitochondria at basal (pyruvate and malate as Complex I substrate and Complex II inhibitor, respectively, in presence of FCCP) and in response to sequential injection of rotenone (Rote; Complex I inhibitor), succinate (Succ; Complex II substrate), antimycin A (AA; complex III inhibitor), ascorbate / tetramethylphenylenediamine (Asc/TMPD; cytochrome c (Complex IV) substrate). No difference in Complex I (C I), Complex II (C II), or Complex IV (C IV) respiration was observed after 30 min treatment with 800 µM of octyl α-KG, whereas Complex V was inhibited (see Fig. 2h) by the same treatment (2 independent experiments). e-f, No significant difference in coupling (e) or electron flow (f) was observed with either octanol or DMSO vehicle control. g-h, Treatment with 1-octyl α-KG or 5-octyl α-KG gave identical results in coupling (g) or electron flow (h) assays. Mean ± s.d. is plotted in all cases.
Extended Data Figure 3
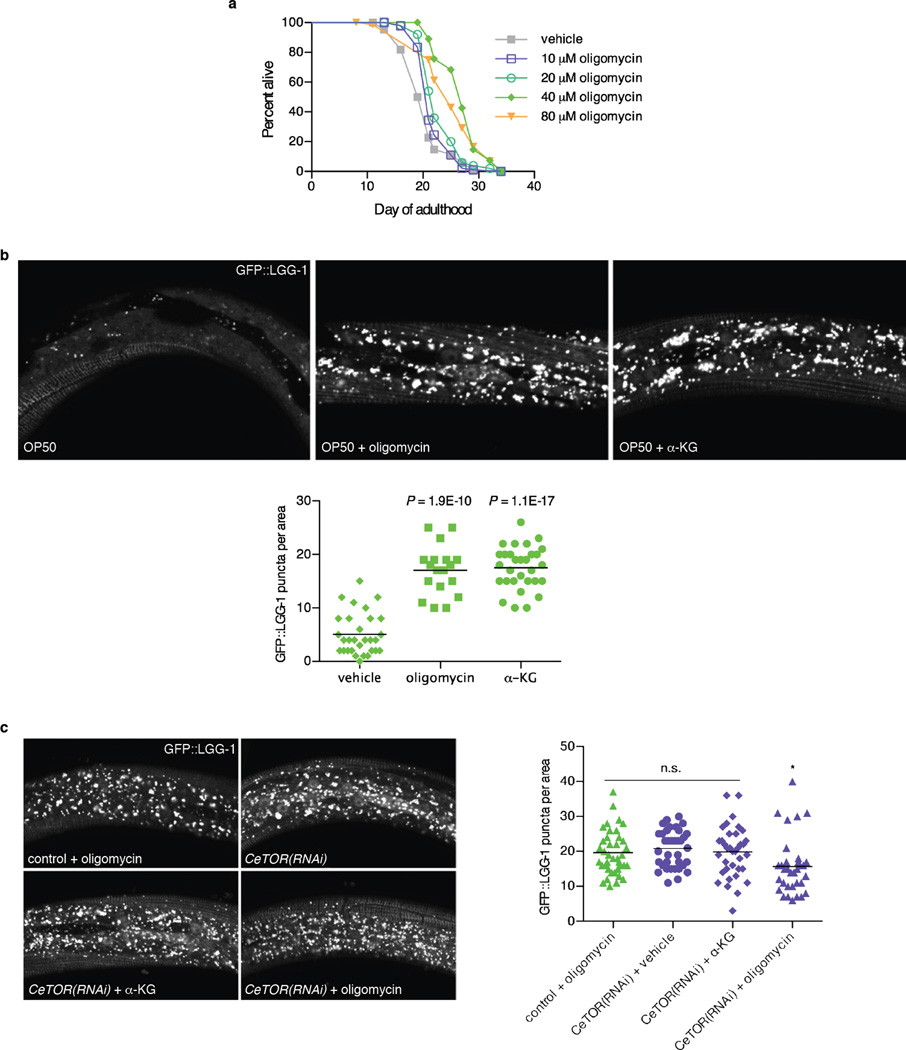
a, Oligomycin extends the lifespan of adult C. elegans in a concentration dependent manner. Treatment with oligomycin began at the young adult stage. 40 µM oligomycin increased the mean lifespan of N2 worms by 32.3% (P < 0.0001, by log-rank test); see Extended Data Table 2 for details. b, Confocal images of GFP::LGG-1 puncta in L3 epidermis of C. elegans with vehicle, oligomycin (40 µM), or α-KG (8 mM), and number of GFP::LGG-1 containing puncta quantitated using ImageJ. Bars indicate the mean. Autophagy in C. elegans treated with oligomycin or α-KG is significantly higher than in vehicle-treated control animals (t-test, two-tailed, two-sample unequal variance). c, There is no significant difference (n.s.) between control worms treated with oligomycin and CeTOR(RNAi) worms treated with vehicle, nor between vehicle and α-KG treated CeTOR(RNAi) worms, consistent with independent experiments in Fig. 4b-c; also, oligomycin does not augment autophagy in CeTOR(RNAi) worms (if anything, there may be a small decrease*); by t-test, two-tailed, twosample unequal variance. Bars indicate the mean.
Extended Data Figure 4
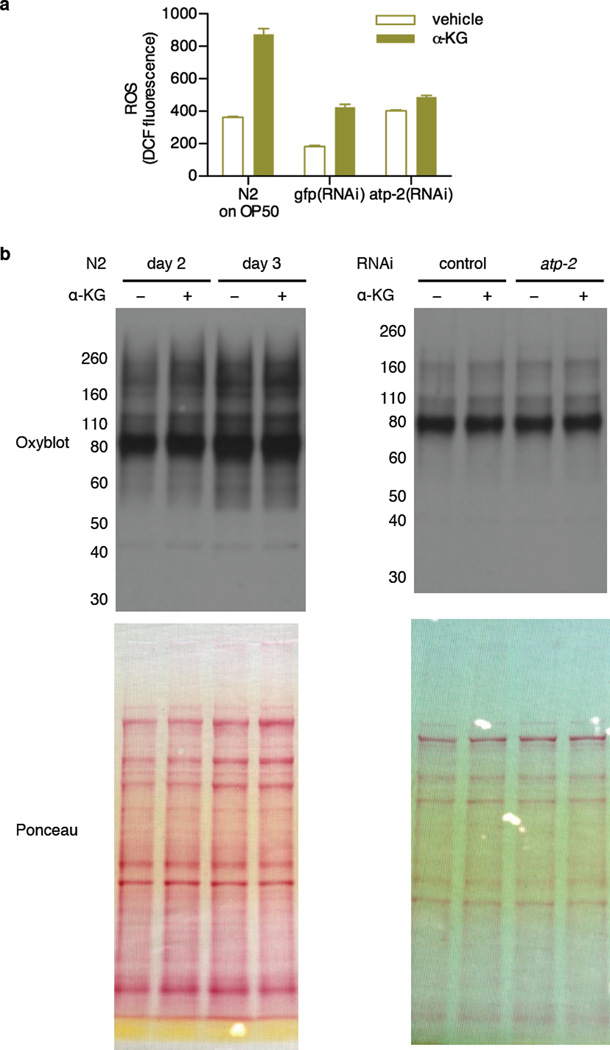
a, The atp-2(RNAi) worms have higher levels of DCF fluorescence than gfp control worms (P < 0.0001, by t-test, two-tailed, two-sample unequal variance). Supplementation with α-KG also leads to higher DCF fluorescence, in both HT115 (for RNAi) and OP50 fed worms (P = 0.0007, and P = 0.0012, respectively). ROS levels were measured using 2',7'-dichlorodihydrofluorescein diacetate (H2DCF-DA). Since whole worm lysates were used, total cellular oxidative stress was measured here. H2DCF-DA (Molecular Probes, D399) was dissolved in ethanol to a stock concentration of 1.5 mg/mL. Fresh stock was prepared every time prior to use. For measuring ROS in worm lysates, a working concentration of H2DCF-DA at 30 ng/mL was hydrolyzed by 0.1 M NaOH at room temperature for 30 min to generate 2’, 7’-dichlorodihydrofluorescein (DCFH) before mixing with whole worm lysates in a black 96-well plate (Greiner Bio-One). Oxidation of DCFH by ROS yields the highly fluorescent 2', 7'-dichlorofluorescein (DCF). DCF fluorescence was read at excitation / emission of 485 / 530 nm using SpectraMax MS (Molecular Devices). H2O2 was used as positive control (not shown). To prepare the worm lysates, synchronized young adult animals were cultivated on plates containing vehicle or 8 mM α-KG and OP50 or HT115 E. coli for 1 day, and then collected and lysed as described in “Assay for ATP levels in C. elegans” (see Methods). Mean ± s.d. is plotted. b, There was no significant change in protein oxidation upon α-KG treatment or atp-2(RNAi). Oxidized protein levels were determined by the OxyBlot. Synchronized young adult N2 animals were placed onto plates containing vehicle or 8 mM α-KG, and seeded with OP50 or HT115 bacteria that expressed control or atp-2 dsRNA. Adult day 2 and day 3 worms were collected and washed 4 times with M9 buffer, and then stored at −80 °C for at least 24 h. Laemmli buffer (Biorad, 161-0737) was added to every sample and animals were lysed by alternate boil/freeze cycles. Lysed animals were centrifuged at 14,000 rpm for 10 min at 4 °C to pellet worm debris, and supernatant was collected for oxyblot analysis. Protein concentration of samples was determined by the 660 nm Protein Assay (Thermo Scientific, 1861426) and normalized for all samples. Carbonylation of proteins in each sample was detected using the OxyBlot Protein Oxidation Detection Kit (Millipore, S7150).
Extended Data Figure 5
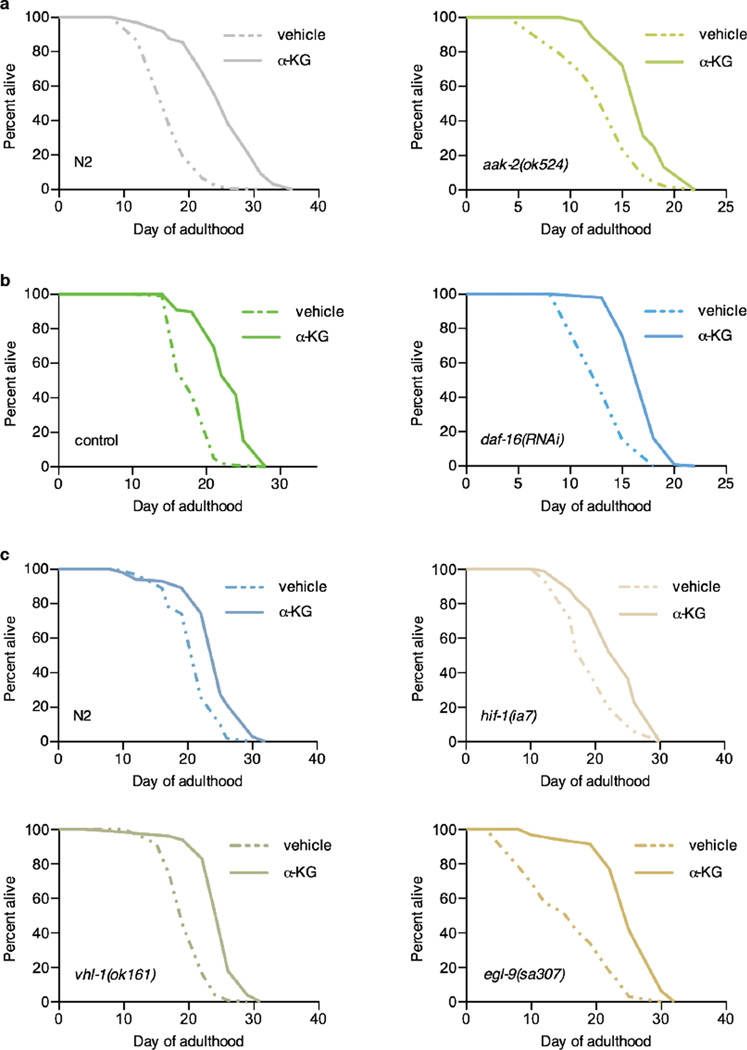
Lifespans of α-KG supplemented a, N2 worms, mveh = 17.5 (n = 119), mα-KG = 25.4 (n = 97), P < 0.0001; or aak-2(ok524) mutants, mveh = 13.7 (n = 85), mα-KG = 17.1 (n = 83), P < 0.0001. b, N2 worms fed gfp RNAi control, mveh = 18.5 (n = 101), mα-KG = 23.1 (n = 98), P < 0.0001; or daf-16 RNAi, mveh = 14.3 (n = 99), mα-KG = 17.6 (n = 99), P < 0.0001. c, N2 worms, mveh = 21.5 (n = 101), mα-KG = 24.6 (n = 102), P < 0.0001; hif-1(ia7) mutants, mveh = 19.6 (n = 102), mα-KG = 23.6 (n = 101), P < 0.0001; vhl-1(ok161) mutants, mveh = 20.0 (n = 98), mα-KG = 24.9 (n = 100), P < 0.0001; or egl-9(sa307) mutants, mveh = 16.2 (n = 97), mα-KG = 25.6 (n = 96), P < 0.0001. m, mean lifespan (days of adulthood); n, number of animals tested. P-values were determined by the log-rank test. Number of independent experiments: N2 (8), hif-1 (5), vhl-1 (1), and egl-9 (2); see Extended Data Table 2 for details. Two different hif-1 mutant alleles 27 have been used: ia4 (shown in Fig. 3g) is a deletion over several introns and exons; ia7 (shown above) is an early stop codon, causing a truncated protein. Both alleles have the same effect on lifespan 27. We tested both alleles for α-KG longevity and obtained the same results.
Extended Data Figure 6
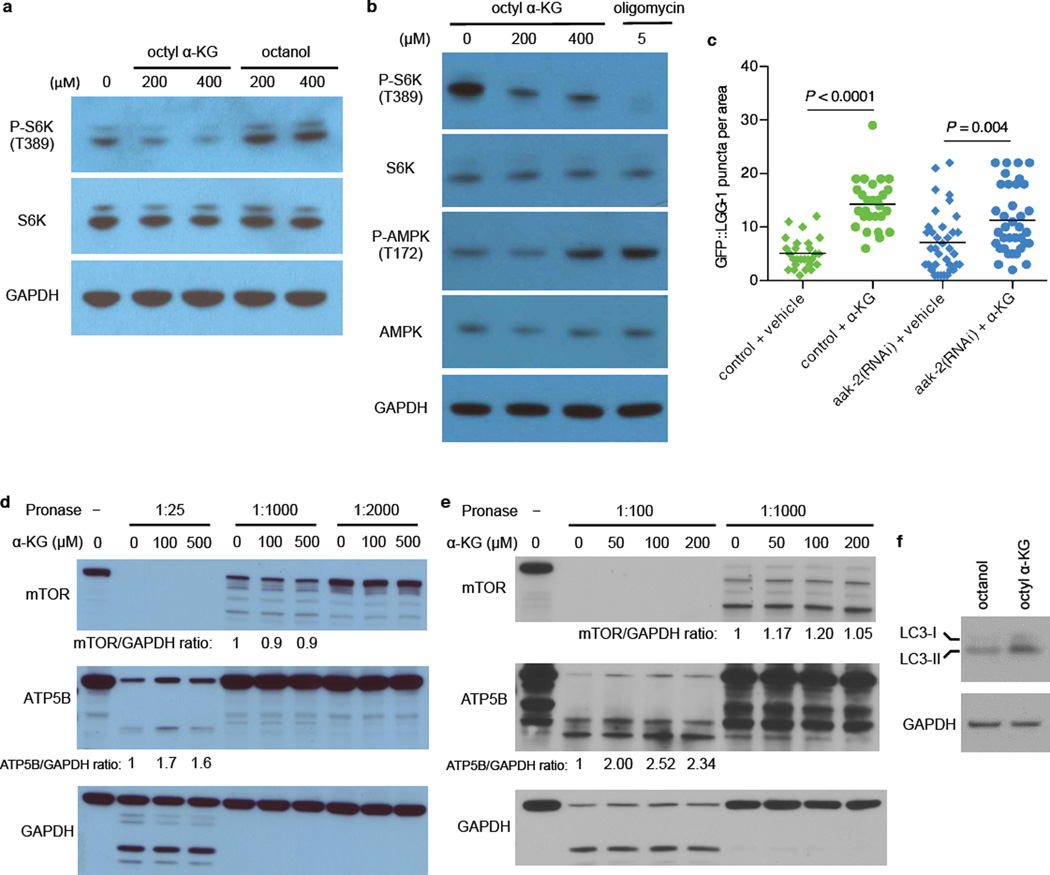
a, Phosphorylation of S6K (T389) was decreased in U87 cells treated with octyl α-KG, but not in cells treated with octanol control. Same results were obtained using HEK-293 and MEF cells. b, Phosphorylation of AMPK (T172) is upregulated in WI-38 cells upon Complex V inhibition by α-KG, consistent with decreased ATP content in α-KG treated cells and animals. However, this activation of AMPK appears to require more severe Complex V inhibition than the inactivation of mTOR, as either oligomycin or a higher concentration of octyl α-KG was required for increasing P-AMPK whereas concentrations of octyl α-KG comparable to those that decreased cellular ATP content (Fig. 2d) or oxygen consumption (Fig. 2f) were also sufficient for decreasing P-S6K. Same results were obtained using U87 cells. Western blotted with specific antibodies against P-AMPK T172 (Cell Signaling, 2535S) and AMPK (Cell Signaling, 2603S). c, α-KG still induces autophagy in aak-2(RNAi) worms; **P < 0.01 (t-test, two-tailed, two-sample unequal variance). Number of GFP::LGG-1 containing puncta was quantitated using ImageJ. Bars indicate the mean. d-e, α-KG does not bind to TOR directly as determined by DARTS. HEK-293 (d) or HeLa (e) cells were lysed in M-PER buffer (Thermo Scientific, 78501) with the addition of protease inhibitors (Roche, 11836153001) and phosphatase inhibitors (50 mM NaF, 10 mM β-glycerophosphate, 5 mM sodium pyrophosphate, 2 mM Na3VO4). Protein concentration of the lysate was measured by BCA Protein Assay kit (Pierce, 23227). Chilled TNC buffer (50 mM Tris-HCl pH 8.0, 50 mM NaCl, 10 mM CaCl2) was added to the protein lysate, and the protein lysate was then incubated with vehicle control (DMSO) or varying concentrations of α-KG for 1 h (for d) or 3 h (for e) at room temperature. Pronase (Roche, 10165921001) digestions were performed for 20 min at room temperature, and stopped by adding SDS loading buffer and immediately heating at 95 °C for 5 min (for d) or 70 °C for 10 min (for e). Samples were subjected to SDS-PAGE on 4–12% Bis-Tris gradient gel (Invitrogen, NP0322BOX) and Western blotted with specific antibodies against ATP5B (Santa Cruz, sc58618), mTOR (Cell Signaling, 2972), or GAPDH (Ambion, AM4300). ImageJ was used to quantify the mTOR/GAPDH and ATP5B/GAPDH ratios. Susceptibility of the mTOR protein to Pronase digestion is unchanged in the presence of α-KG, whereas, as expected, Pronase resistance in the presence of α-KG is increased for ATP5B, which we identified as a new binding target of α-KG. f, Increased autophagy in HEK-293 cells treated with octyl α-KG was confirmed by Western blot analysis of MAP1 LC3 (Novus, NB100-2220), consistent with decreased phosphorylation of the autophagy initiating kinase ULK1 (Fig. 4a).
Extended Data Figure 7
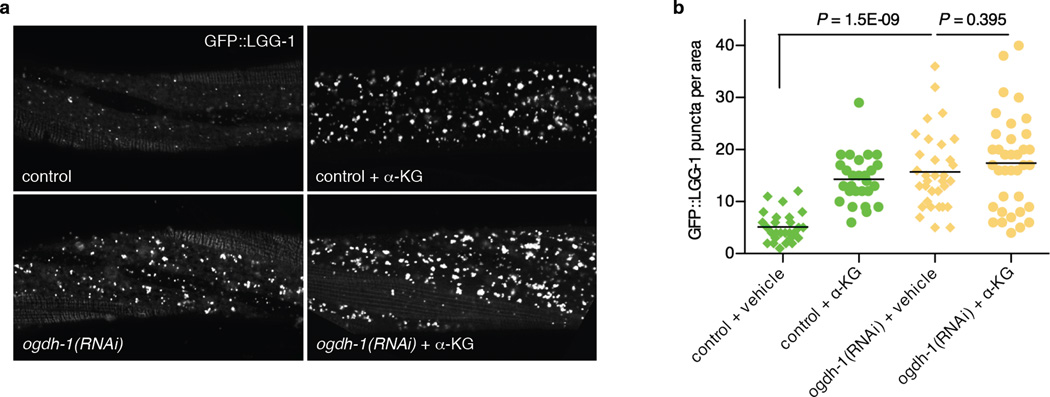
a, Confocal images of GFP::LGG-1 puncta in the epidermis of mid L3 stage, control or ogdh-1 knockdown, C. elegans treated with vehicle or α-KG (8 mM). b, Number of GFP::LGG-1 puncta quantitated using ImageJ. Bars indicate the mean. ogdh-1(RNAi) worms have significantly higher autophagy levels, and α-KG does not significantly augment autophagy in ogdh-1(RNAi) worms (t-test, two-tailed, two-sample unequal variance).
Extended Data Table 1
Enriched proteins in the α-KG DARTS sample.
| Protein Symbol | Protein Name | Score | Control sample | α-KG sample | Enrichment | ||
|---|---|---|---|---|---|---|---|
| Spectra | Peptides | Spectra | Peptides | ||||
| ATP5B | ATP synthase subunit beta | 4088 | 23 | 9 | 121 | 15 | 5.3 |
| HSPD1 | 60 kDa heat shock protein | 2352 | 31 | 11 | 138 | 29 | 4.5 |
| PKM2 | Pyruvate kinase isozymes M1/M2 | 2203 | 56 | 7 | |||
| LCP1 | Plastin-2 | 1865 | 14 | 8 | 76 | 13 | 5.4 |
| ATP5A1 | ATP synthase subunit alpha | 1616 | 41 | 9 | 61 | 12 | 1.5 |
| SHMT2 | Serine hydroxymethyltransferase | 1060 | 7 | 5 | 33 | 10 | 4.7 |
| HSP90AA1 | Heat shock protein HSP 90-alpha | 952 | 29 | 8 | 44 | 8 | 1.5 |
| EEF2 | Elongation factor 2 | 943 | 4 | 2 | 37 | 9 | 9.3 |
| DDX5 | Probable ATP-dependent RNA helicase DDX5 | 652 | 7 | 3 | 33 | 10 | 4.7 |
| HSPA8 | Heat shock cognate 71 kDa protein | 615 | 4 | 2 | 35 | 10 | 8.8 |
Only showing those proteins with at least 15 spectra in α-KG sample and enriched at least 1.5 fold.
Extended Data Table 2
Summary of lifespan data
| Strain | m (mean lifespan, days) | % difference | p-value | n (number of animals) | |||
|---|---|---|---|---|---|---|---|
| vehicle | α-KG | vehicle | α-KG | ||||
| N2 | 18.9 | 25.8 | 36.3 | < 0.0001 | 87 | 96 | |
| N2 | 17.5 | 25.4 | 45.6 | < 0.0001 | 119 | 97 | |
| N2 | 16.3 | 26.1 | 60.2 | < 0.0001 | 100 | 104 | |
| eat-2(ad1116) | 22.8 | 22.9 | 0.5 | 0.79 | 59 | 40 | |
| daf-16(mu86) | 16.3 | 18.8 | 15.1 | < 0.0001 | 106 | 105 | |
| eat-2(ad1116) | 21.1 | 24.0 | 13.4 | 0.23 | 39 | 59 | |
| daf-2(e1370) | 38.0 | 47.6 | 25.1 | < 0.0001 | 72 | 69 | |
| N2 | 13.2 | 22.3 | 69.8 | < 0.0001 | 100 | 104 | |
| daf-16(mu86) | 13.4 | 17.4 | 29.5 | < 0.0001 | 71 | 72 | |
| daf-16(RNAi) | 14.3 | 17.6 | 22.9 | < 0.0001 | 99 | 99 | |
| N2 | 16.1 | 19.1 | 19.3 | 0.0003 | 97 | 96 | |
| daf-2(e1370) | 38.3 | 43.9 | 14.6 | < 0.0001 | 109 | 101 | |
| aak-2(ok524) | 13.7 | 17.1 | 24.3 | < 0.0001 | 85 | 83 | |
| aak-2(ok524) | 16.4 | 17.5 | 6.7 | < 0.0001 | 97 | 97 | |
| aak-2(RNAi) | 16.2 | 19.9 | 23.3 | < 0.0001 | 93 | 92 | |
| N2 | 15.6 | 26.3 | 68.8 | < 0.0001 | 95 | 102 | |
| N2 | 15.6 | 26.3 | 68.5 | < 0.0001 | 95 | 102 | |
| egl-9(sa307) | 16.2 | 25.6 | 58.6 | < 0.0001 | 97 | 96 | |
| egl-9(sa307) | 19.5 | 27.3 | 40.3 | < 0.0001 | 95 | 101 | |
| N2 | 14.7 | 21.6 | 46.9 | < 0.0001 | 100 | 88 | |
| N2 | 14.0 | 20.7 | 47.9 | < 0.0001 | 112 | 114 | |
| N2 | 21.5 | 24.6 | 14.6 | < 0.0001 | 101 | 102 | |
| hif-1(ia4) | 20.5 | 26.0 | 26.5 | < 0.0001 | 85 | 71 | |
| hif-1(ia7) | 19.6 | 23.6 | 20.4 | < 0.0001 | 102 | 101 | |
| hif-1(ia4) | 21.5 | 24.7 | 14.7 | < 0.0001 | 88 | 87 | |
| N2 | 16.7 | 23.4 | 39.7 | < 0.0001 | 104 | 103 | |
| N2 | 15.8 | 22.2 | 40.5 | < 0.0001 | 104 | 94 | |
| N2 | 18.4 | 24.6 | 33.4 | < 0.0001 | 99 | 89 | |
| vhl-1(ok161) | 20.0 | 25.0 | 24.9 | < 0.0001 | 98 | 100 | |
| hif-1(ia7) | 12.4 | 17.3 | 38.9 | < 0.0001 | 97 | 90 | |
| hif-1(ia7) | 17.9 | 23.7 | 32.0 | < 0.0001 | 58 | 55 | |
| N2 | 16.8 | 22.4 | 32.7 | < 0.0001 | 104 | 101 | |
| N2 | 15.7 | 21.6 | 37.6 | < 0.0001 | 85 | 99 | |
| smg-1(cc546ts) | 18.4 | 23.8 | 29.5 | < 0.0001 | 110 | 87 | |
| smg-1(cc546ts);pha-4(zu225) | 14.2 | 13.5 | −4.9 | 0.5482 | 94 | 109 | |
| smg-1(cc546ts);pha-4(zu225) | 17.6 | 15.2 | −14.0 | 0.0877 | 28 | 34 | |
| N2 | 13.6 | 20.7 | 51.8 | < 0.0001 | 103 | 104 | |
| smg-1(cc546ts) | 16.2 | 23.0 | 42.2 | < 0.0001 | 114 | 121 | |
| smg-1(cc546ts);pha-4(zu225) | 13.8 | 15.2 | 10.2 | 0.254 | 45 | 45 | |
| EV RNAi control | 18.6 | 23.4 | 26.1 | < 0.0001 | 94 | 91 | |
| atp-2(RNAi) | 22.8 | 22.5 | −1.3 | 0.3471 | 97 | 94 | |
| EV RNAi control | 18.8 | 22.7 | 20.6 | < 0.0001 | 97 | 94 | |
| gfp RNAi control | 18.5 | 23.1 | 25.3 | < 0.0001 | 101 | 98 | |
| α-kgdh(RNAi) | 21.2 | 21.1 | −0.7 | 0.65 | 98 | 100 | |
| CeTOR(RNAi) | 22.1 | 23.6 | 6.8 | 0.02 | 94 | 95 | |
| gfp RNAi control | 20.2 | 27.7 | 37.4 | < 0.0001 | 99 | 81 | |
| CeTOR(RNAi) | 25.1 | 25.7 | 2.1 | 0.9511 | 96 | 74 | |
| EV RNAi control | 22.8 | 27.2 | 21.6 | <0.0001 | 70 | 72 | |
| CeTOR(RNAi) | 27.4 | 27.2 | −0.8 | 0.7239 | 64 | 80 | |
| EV RNAi control | 19.7 | 24.3 | 23.8 | < 0.0001 | 93 | 84 | |
| atp-2(RNAi) | 25.3 | 23.4 | −7.4 | < 0.0001 | 87 | 63 | |
| Strain | m | % difference | p-value | n | [oligomycin] | ||
| vehicle | oligomycin | vehicle | oligomycin | ||||
| N2 | 20.4 | 25.5 | 25.2 | < 0.0001 | 88 | 72 | 80 µM |
| N2 | 27.0 | 32.3 | < 0.0001 | 82 | 40 µM | ||
| N2 | 23.1 | 13.2 | 0.0005 | 50 | 20 µM | ||
| N2 | 22.0 | 7.9 | 0.0106 | 90 | 10 µM | ||
| Strain | m | % difference | p-value | n | treatment | ||
| vehicle | treatment | vehicle | treatment | ||||
| N2 | 14.5 | 16.9 | 16.8 | 0.0005 | 73 | 71 | octyl α-KG (500 µM) |
| N2 | 14.5 | 17.0 | 16.8 | < 0.0001 | 73 | 60 | α-KG |
| N2 | 14.0 | 18.8 | 33.9 | < 0.0001 | 112 | 114 | dimethyl α-KG |
| N2 | 14.0 | 20.7 | 47.8 | < 0.0001 | 112 | 114 | α-KG |
| N2 | 15.7 | 21.6 | 37.6 | < 0.0001 | 85 | 99 | disodium α-KG |
| Strain | m | % difference | p-value | n | food source | ||
| vehicle | α-KG | vehicle | α-KG | ||||
| N2 | 17.4 | 21.2 | 21.6 | 0.0001 | 108 | 55 | live OP50 |
| N2 | 19.0 | 23.0 | 21.0 | 0.0003 | 88 | 46 | dead OP50 (γ-irradiated) |
Acknowledgments
We thank S. Lee, M. Hansen, B. Lemire, A. van der Bliek, S. Clarke, T. K. Blackwell, R. Johnson, J. E. Walker, A. G. W. Leslie, K. N. Houk, B. Martin, J. Lusis, J. Gober, Y. Wang, H. Sun, and anonymous referees for advice and discussions; J. Avruch for CeTOR RNAi vector; J. Powell-Coffman for strains and advice; and K. Yan for technical assistance. Worm strains were provided by the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We thank the U.S. National Institutes of Health for traineeship support of R.M.C. (T32 GM007104), M.Y.P. (T32 GM007185), B.L. (T32 GM008496), and M.N. (T32 CA009120). X.F. is a recipient of the China Scholarship Council Scholarship. G.C.M. was supported by Ford Foundation and National Science Foundation Graduate Research Fellowships.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions Lifespan assays were performed by R.M.C., M.P., and E.H.; DARTS-MS by S.D. and B.L.; DARTS-Western by M.Y.P., H.H., and R.M.C.; mammalian cell experiments by X.F. and H.H.; mitochondrial respiration study design and analyses by L.V. and K.R.; enzyme kinetics and analyses by R.M.C. and J.H.; confocal microscopy by V.S.M., G.C.M., and A.R.F.; UHPLC-ESI/MS/MS by J.X.W. and S.A.T.; compound syntheses by G.D. and M.E.J.; other analyses by H.H., X.F., M.Y.P., D.B., R.M.C., E.H., G.J., G.M.S., C.K., and A.Q. S.A.W., F.F., M.N., A.S.K., H.A.G., H.R.C., K.F.F., F.G., M.J., S.A.T., A.S., D.B., H.R.C., C.F.C., M.A.T., M.E.J., L.V., K.R., A.R.F., and M.P. provided guidance, specialized reagents, and expertise. J.H. conceived the study. R.M.C. and J.H. wrote the paper. R.M.C., X.F., and J.H. analysed data. All authors discussed the results, commented on the studies, and contributed to aspects of preparing the manuscript.
The authors declare no competing financial interests.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/nature13264
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4263271
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/nature13264
Article citations
Tauroursodeoxycholic acid targets HSP90 to promote protein homeostasis and extends healthy lifespan.
Sci China Life Sci, 24 Sep 2024
Cited by: 0 articles | PMID: 39327392
Nesfatin-1 enhances vascular smooth muscle calcification through facilitating BMP-2 osteogenic signaling.
Cell Commun Signal, 22(1):488, 11 Oct 2024
Cited by: 0 articles | PMID: 39394127 | PMCID: PMC11468037
DOR activation in mature oligodendrocytes regulates α-ketoglutarate metabolism leading to enhanced remyelination in aged mice.
Nat Neurosci, 27(11):2073-2085, 12 Sep 2024
Cited by: 0 articles | PMID: 39266660
Anti-aging Metabolite-Based Polymeric Microparticles for Intracellular Drug Delivery and Bone Regeneration.
Small Sci, 4(10):2400201, 03 Sep 2024
Cited by: 0 articles | PMID: 39386061
A new paradigm in intracellular immunology: Mitochondria emerging as leading immune organelles.
Redox Biol, 76:103331, 29 Aug 2024
Cited by: 0 articles | PMID: 39216270 | PMCID: PMC11402145
Review Free full text in Europe PMC
Go to all (322) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mitobolites: the elixir of life.
Cell Metab, 20(1):8-9, 01 Jul 2014
Cited by: 7 articles | PMID: 24988457 | PMCID: PMC4103626
A mitochondrial ATP synthase subunit interacts with TOR signaling to modulate protein homeostasis and lifespan in Drosophila.
Cell Rep, 8(6):1781-1792, 15 Sep 2014
Cited by: 30 articles | PMID: 25220459 | PMCID: PMC4177368
Genetic inhibition of an ATP synthase subunit extends lifespan in C. elegans.
Sci Rep, 8(1):14836, 04 Oct 2018
Cited by: 13 articles | PMID: 30287841 | PMCID: PMC6172204
Is Rapamycin a Dietary Restriction Mimetic?
J Gerontol A Biol Sci Med Sci, 75(1):4-13, 01 Jan 2020
Cited by: 18 articles | PMID: 30854544 | PMCID: PMC6909904
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: T32 CA009120
NHLBI NIH HHS (2)
Grant ID: P01 HL028481
Grant ID: P01 HL090553
NIGMS NIH HHS (3)
Grant ID: T32 GM008496
Grant ID: T32 GM007104
Grant ID: T32 GM007185
NIH HHS (2)
Grant ID: P40 OD010440
Grant ID: DP2 OD008398





