Abstract
Free full text

Reference Range of Cerebrospinal Fluid Opening Pressure in Children: Historical Overview and Current Data
Abstract
The lumbar puncture and cerebrospinal fluid (CSF) opening pressure (OP) in children remains an essential diagnostic test for children with suspected elevated intracranial pressure. Recent prospective data have revised the normative CSF OP values and described how clinical variables such as age, depth of sedation, and obesity may influence the measurements. In addition, the new normative data are now reflected in revised diagnostic criteria for idiopathic intracranial hypertension/pseudotumor cerebri syndrome. This review highlights the recently published data and provides guidance on how it may impact clinical management.
Case 1
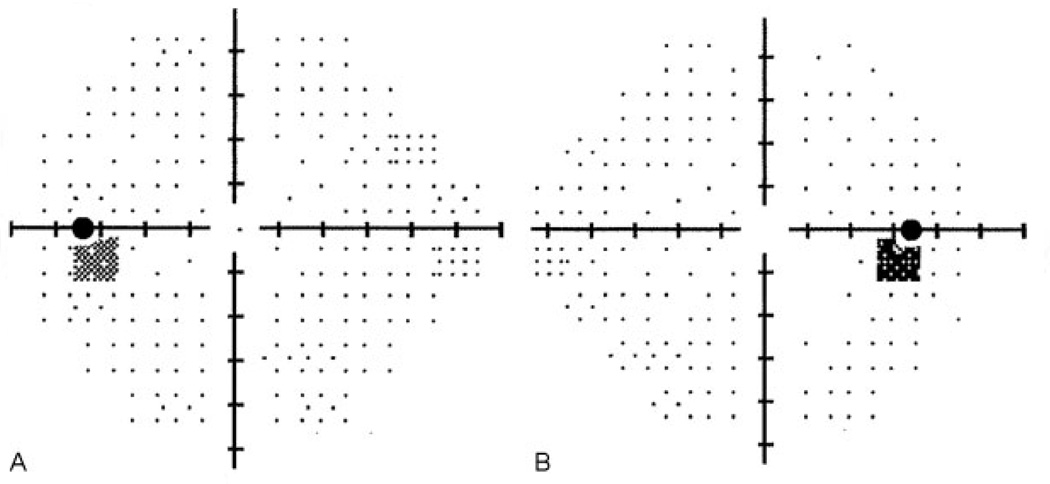
A 14-year-old female patient presented with a 4-week history of unremitting holocephalic headache. Her medical history was significant for irregular menses and she denied taking any medications. Her neurologic examination was normal except for elevated optic nerves (see ►Fig. 1). Her visual acuity was normal, although her visual fields were suspicious for an enlarged blind spot (see ►Fig. 2). Brain magnetic resonance imaging (MRI) with contrast was normal. A lumbar puncture (LP) was performed while awake in the flexed lateral recumbent position and demonstrated an opening pressure (OP) of 25 cm H2O. Her cerebrospinal fluid (CSF) contents were all normal. On the basis of these results, does this child have idiopathic intracranial hypertension (IIH, also known as pseudotumor cerebri)? Is the diagnosis of IIH supported by her OP results?
Case 2
An 8-year-old obese male patient presented with a 2-month history of headache and blurry vision. His medical history was significant for attention deficit disorder, dental anomalies, mild developmental delay, and a benign arachnoid cyst in the posterior fossa. He was taking stimulant medication for the past 4 months. His neurologic examination was normal except for elevated optic nerves (see ►Fig. 3). His visual acuity was 20/50 in each eye and his visual fields demonstrated a mildly enlarged blind spot OU and mild field restriction on the left, although the patient had difficulty cooperating due to his attention deficit disorder. Brain MRI with contrast was normal except for the posterior fossa arachnoid cyst. An LP was performed while the patient was deeply sedated in the flexed lateral recumbent position and demonstrated an OP of 37 cm H2O. His CSF contents were all normal. There was no relief in symptoms after the LP and the patient was placed on acetazolamide. On the basis of these results, does this child have IIH? Is the diagnosis of IIH supported by his OP results?
Introduction
The first LP and subsequent measurement of CSF OP was performed by Dr. Quincke in the late 1800s, providing an indirect, although invaluable, measure of intracranial pressure (ICP).1 The LP with accompanying OP is a relatively noninvasive, easily accessible, and rapid procedure with few risks and adverse effects compared with invasive ICP monitoring. As with any testing modality, establishing normative values to determine normal and abnormal values is central to using a diagnostic test in clinical care.
Like many physicians of his era, Dr. Quincke struggled when treating patients with hydrocephalus. His experience with injecting fluid into the lumbar subarachnoid space of rabbits and dogs prompted him to perform the same procedure in an adult human. As the CSF drained, he counted the drops of fluid and noted changes in speed that were related to the patient’s respiratory rate. This first patient spontaneously improved which lead to a second and third LP.1 During the third LP, Dr. Quincke attached a cannula and measured the first OP as 13 to 15 cm H2O. In this seminal publication, Dr. Quincke described his experience performing an LP on a 5-year-old boy with chronic headache and macrocephaly.1 The results from this one child have been cited by numerous authors which suggested that the OP in children was 4 to 6 cm H2O.2
Twentieth Century Data
In the 20th century, physician’s interest in pediatric ICP blossomed. Welch summarized the current data in 1980 and suggested the OP for infants and children ranged from 1 cm to upward of 10 cm H2O.2 Unfortunately, many of these studies, some of which never listed actual patient data, included children who were extremely ill or had hydrocephalus—clearly limiting their ability to determine a threshold for an abnormal OP. Soon thereafter, Corbett and Mehta published a well-designed study suggesting some adults may have an OP up to 25 cm H2O that could still be considered normal. It is unclear when the pediatric neurology community adopted 20 cm H2O as the upper limit of normal, but numerous general pediatric medicine and pediatric neurology textbooks continue to publish chapters supporting this threshold. In their expert review of pediatric IIH, Rangwala and Liu3 discovered that many of these pediatric textbooks cited the articles from the early 1920s or in fact, crossed referenced other pediatric textbooks which provided no citation for their determination of a normal OP.
In 1994, Ellis was the first to suggest the range of a normal OP in children was higher than previously reported.4 He examined 33 children undergoing LP as part of their oncologic care and concluded a normal OP ranged from 10 to 28 cm based on a novel OP measurement technique—counting the number of CSF drops for 21 seconds.4 In adults, Whiteley et al also suggested the range of normal OP in adults could sometimes reach 28 cm H2O and was weakly related to BMI.5
Since the early recommendations of a normal CSF, OP were not based on systematic studies, it was reasonable to consider that other clinical factors believed to influence OP measures could also be incorrect. For example, many, if not all, neurologists have been taught to straighten the patient’s legs when measuring the OP. Does body position truly affect the OP? Do younger children have a different OP than older children? Will deep sedation provide a more accurate and reliable OP measure compared with a non-sedated child? Are anesthesia type or needle size relevant variables to consider when interpreting OP results? In an effort to address these questions and the methodological flaws of the previously mentioned OP studies, colleagues from six different academic departments at the Children’s Hospital of Philadelphia collaborated to develop a prospective study of CSF OP.6
Current Data
The study by Avery et al6 prospectively recruited children between 1 and 18 years over a 2-year period who were undergoing LP as part of their clinical care. The OP was the primary study outcome measure and the influence of age, body mass index (BMI), depth of sedation, sedation medication used, and LP needle size on the primary outcome were examined. An a priori decision was made to determine the threshold for an abnormal OP as the 90th percentile. A total of 1,066 children were screened and 491 were deemed eligible for enrollment (for the complete listing of enrollment and exclusion criteria see online supplementary material in the study by Avery et al6). Of the 472 subjects enrolled, 58% of subjects were eliminated from the reference group as they had medical conditions, LP features/results or were taking medications that could potentially alter their OP. The OP of the remaining 197 subjects had a relatively normal distribution with a mean of 19.6 cm H2O and the 10th/90th percentile being 11.5 and 28 cm H2O, respectively (see ►Fig. 4A). Our mean OP and its distribution were similar to a smaller retrospective study later published by Lee and Vedanarayanan.7
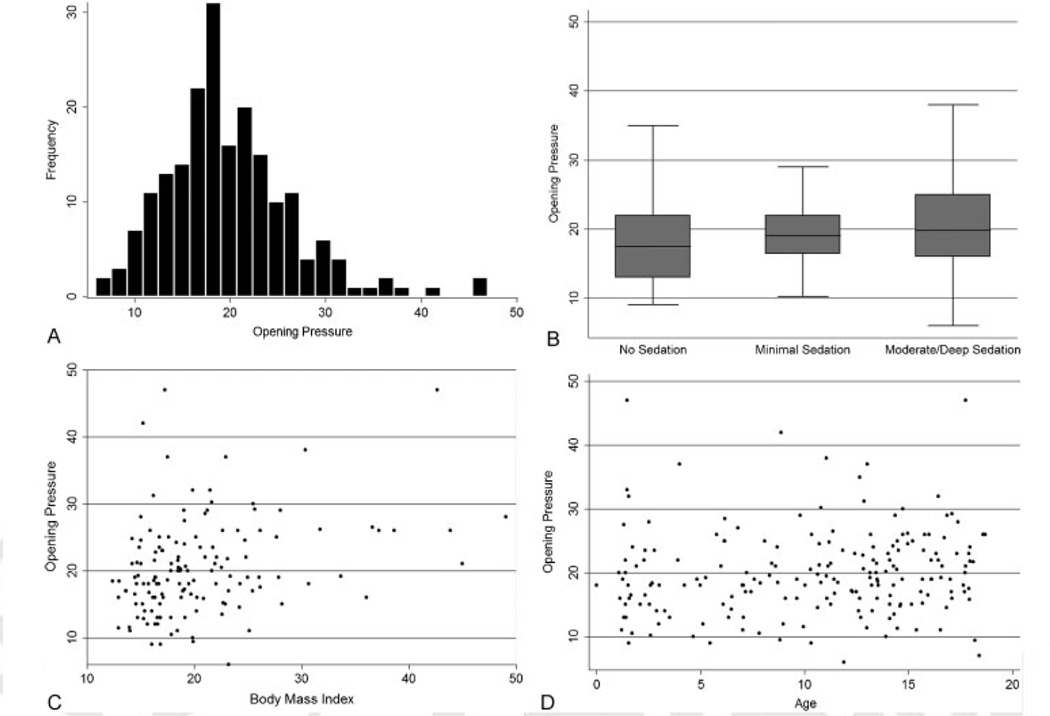
Distribution of cerebrospinal fluid (CSF) opening pressure and effects of sedation, body mass index, and age. (A) The distribution of the CSF opening pressure for 197 study subjects who met the inclusion criteria for the reference group. The mean (± SD) CSF opening pressure was 19.8 ± 6.8 cm of water. The values ranged from6 to 47 cm of water and were similar in subjects 10 years of age or older and those younger than 10 years. The threshold for an abnormally elevated opening pressure, which was based on subjects with opening pressures in the 90th percentile or higher, was 28 cm of water. The threshold for an abnormally low opening pressure, based on subjects in the 10th percentile, was 11.5 cm of water. (B) The results of an unadjusted linear regression analysis of the relationship between opening pressure and sedation (β = 3.459, p = 0.005). (C) The relationship between opening pressure and body mass index (BMI, the weight in kilograms divided by the square of the height in meters) (β = 0.138, p = 0.12) and (D) that between opening pressure and age (β = 0.328, p < 0.001). Statistically significant associations between opening pressure and moderate-to-deep sedation (β = 4.990, p = 0.002) and opening pressure and BMI (β = 0.313, p = 0.002) remained in multivariable analysis adjusted for age. In a multivariable linear regression model, no significant association was found between age (β = 0.097, p = 0.42) and opening pressure, even when age was categorized as 10 years or older or as younger than 10 years (β = 1.141, p = 0.30). No significant association was found between opening pressure and sedation with ketamine (β = 3.080; 95% confidence interval, −0.551 to 6.712; p = 0.10). Reprinted with permission from Avery et al.6
Sedation
Much to the investigators surprise, subjects who were under moderate-to-deep sedation during their LP had an OP nearly 3.5 cm H2O higher than those without sedation (see ►Fig. 4B).6 The difference in OP between these groups increased to nearly 5 cm H2O when adjusting for age and BMI. There was no statistical difference (p = 0.104) between those who did not receive sedation medication and those receiving minimal sedation (i.e., anxiolysis). This elevation in OP is believed to be caused by a mild hypercapnia.8–10 Lim and Lin reported on four children with elevated PCO2 while undergoing LP for suspected IIH. In treating their hypercapnia with hand ventilation, they calculated that for every 1 kpa increase in PCO2, that the OP would be elevated by 3.5 to 12 cm H2O.9 Eidlitz-Markus et al recorded OP at the beginning of sedation with propofol and after the patient regained consciousness (termed end-measurement pressure).10 Interestingly, nearly all of the subjects demonstrated a lower OP after regaining consciousness.10 While the use of end tidal PCO2 monitors could potentially elucidate the magnitude of influence depth of sedation has on OP, they are notoriously inaccurate and tend to underestimate the true arterial PCO2.8,9
Medications, especially ketamine, have long been suspected to contribute to a rise in ICP with few studies specifically targeting OP.11,12 Our secondary analysis examined the influence of sedation medication on OP, although our interpretation was limited due to variety of sedation protocols and medications.6 A majority of subjects received a benzodiazepine (n = 112, 57%) for sedation, followed by opiates (n = 10, 5%), and ketamine (n = 15, 8%).6 Although limited due to few subjects, no statistically significant relationship was found between ketamine use and OP in this population (p = 0.096). Our findings were not consistent with two studies reporting an increase in OP when using ketamine.11,13
The standardization of sedation protocols, including medication dosage and assessment of the depth of sedation, are all key factors that need to be elucidated in future research projects of pediatric OP.
Body Mass Index
We observed a small, but statistically significant increase in OP in subjects based on their BMI (►Fig. 4C). Specifically, regression analysis predicted that for every increase of 10 BMI units, the OP increases 3 cm H2O.6 Others have also reported that those with greater BMI also have higher OP, but the difference is unlikely clinically significant.5
Age
Age-based normative values have varied among experts and were likely influenced by the previously mentioned publications that included medically complicated patients to determine these thresholds.2 Analyzing age as a continuous variable, there was a small trend toward a higher OP in the older compared with the younger children, however, this did not reach statistical significance (►Fig. 4D).6 Even when age was dichotomized to older or younger 10 years, the influence of age on OP did not reach statistical significance.6
How could age not impact OP? If OP is related to blood pressure (BP), there is only a 10 to 15% difference in BP between 1- and 10-year-old children. Therefore, is it reasonable to assume this same 10 to 15% difference could exist for OP. Thus, a 10-year age difference would results in a difference of 2 to 3 cm H2O, likely clinically insignificant and within the normal variability of the OP measurement.
Leg Position
Many textbooks and physicians recommend moving subjects from the flexed position to the extended lateral recumbent position to avoid a false elevation in the OP secondary to increased intra-abdominal pressure.14 While this seems logical as subjects actively performing a valsalva maneuver can falsely elevate their OP,15 this change in position may not be necessary—especially in sedated children. Two studies, one in adults and another in children demonstrated that repositioning the patient from the flexed to the extended position had little effect on the OP pressure measure.16,17 Keeping the subject in the flexed position has the benefit of decreasing the chance of LP needle displacement during repositioning and avoiding unnecessary subject arousal.
Equipment
It is reasonable to suspect that differences in equipment used to perform the LP and OP may influence the measured values. We found that subjects who used a longer needle (i.e., 3.5 inch) had statistically significant decrease in their OP compared with those using the shortest needle (i.e., 1.5 in), but not compared with the average length needle (i.e., 2.5 in; see supplementary online data).6 The relationship between needle size and gauge certainly requires further study. In addition, some clinicians have proposed that the tubing which attaches the needle and manometer may impact the “true” or “actual” OP measurement. As the tube contains a relatively small amount of CSF and remains part of the enclosed system measuring the OP (i.e., manometer), it is doubtful that the OP is significantly altered, although this topic has not been subjected to formal study.
Limitations of the Current Data
All of the aforementioned studies investigating OP values in children contain methodological flaws. No patient undergoing an LP for clinical purposes can be considered a true “healthy control” as it would be unethical to enroll children who did not need to undergo this procedure. While the lack of a true “healthy control” is concerning for some investigators, it can be argued that children undergoing an LP for clinical purposes are the ideal reference group. The physician’s diagnostic dilemma is determining which child’s LP is abnormal when comparing to other children.
It is difficult to confirm that a specific medication causes an increase in OP due to the between groups analysis and inability to control for confounding factors. Many obstacles exist when attempting to perform research on sedation medications in the pediatric population. Most notably, LPs are typically performed in urgent situations with high parental anxiety, thereby reducing the time needed to perform an appropriate informed consent. In addition, without clinical equipoise, testing medications may not be ethical.
Lastly, many physicians with diverse levels of training performed the LPs in our study. While this undoubtedly introduced some variability, our results can be generalized to other tertiary care hospitals and are not restricted to one physician’s technique.
Application of New Opening Pressure Reference Range
How should the new OP reference range data be used in clinical practice? It is important to remember that the prospective study analyzed data from a heterogeneous group of children.6 There is no doubt the influence of contributory variables (i.e., depth of sedation, medications, BMI, etc.) on OP differs among individual subjects. Keeping this in mind, an OP value should never be interpreted in isolation. The clinical history, physical examination, and imaging studies are all equally important in caring for the child with suspected elevated ICP. An OP below 28 cm H2O in a child is reassuring and should be considered “normal.” However, if signs or symptoms of elevated ICP persist, close observation is recommended as ICP is known to fluctuate.18 On the contrary, classifying an OP above 28 H2O as “normal” or “abnormal” should be determined in the context of the entire clinical picture. If the child does not have any other signs or symptoms of elevated ICP, the impact of other variables should be considered. For example, was the child moving/agitated during the LP? Were they deeply sedated for the procedure? If there is a suspicion for elevated ICP in the setting of an OP above 28 cm H2O, serial dilated eye exams should be performed to monitor for developing papilledema. An OP above 28 cm H2O could be classified as “abnormal” when accompanied by objective findings indicative of elevated ICP (i.e., abducens nerve palsy, papilledema, and hydrocephalus). In our prospective cohort, most children with optic nerve head elevation had an OP above 28 cm H2O,19 yet some children from our reference group without optic nerve head elevation also had OP values in the 30 to 40 cm H2O range.
The OP measurement is central to the diagnosis of IIH, also described using the more encompassing term pseudotumor cerebri syndrome (PTCS).20 Recently, revised diagnostic criteria for PTCS in children have included an OP > 28 cm H2O as one of the five required criteria.20 In patients with suspected IIH/PTCS that do not manifest papilledema, a diagnosis of PTCS without papilledema can be achieved by the presence of an objective clinical exam findings (i.e., 6th nerve palsy) or three of four imaging criteria.20 Therefore, patients with an elevated OP and other signs of elevated ICP that do not manifest papilledema should be closely monitored with serial eye exams in case optic nerve swelling develops.
Future Directions
There is no doubt that additional research is needed to validate and further elucidate the findings from our large prospective study.6 Ideally, future studies would standardize the sedation protocols/medications and obtain systemic vital signs to better understand the influence of sedation on OP. A universal method of interpreting pediatric OP measures would certainly improve patients care by reducing unnecessary medical treatment and diagnostic testing.
Case 1
On the basis of the appearance of her optic nerves (►Fig. 1) and OP result, B-scan ultrasonography was ordered which revealed bilateral optic nerve head drusen (►Fig. 5). The patient was diagnosed with new daily tension-type headache and treated with amitriptyline. Her functional and optic nerve exam remained unchanged for over 1 year and her headaches did not return after discontinuing the amitriptyline.
Case 2
The patient’s headaches persisted despite maximal dosing of acetazolamide. Repeat visual field testing remained unreliable over 6 months. B-scan ultrasonography revealed bilateral optic nerve head drusen similar to Case 1. The patient was switched to an alternative stimulant medication and the headaches resolved. Acetazolamide was discontinued and his optic nerve exam remained unchanged over the past 8 months. The elevated OP was attributed to deep sedation. Of note, optic nerve swelling secondary to elevated ICP can coexist in children with optic nerve head drusen.21
Acknowledgments
The article was presented in part at the Gesellschaft für Neuropaediatrie, Muenster, Germany on April 19, 2012.
Funding/Support
Dr. Avery is supported by grant K23 EY022673–01 from the National Institutes of Health/National Eye Institute, Pediatric Research Loan repayment program from the National Institutes of Health/National Eye Institute and the Gilbert Family Neurofibromatosis Institute.
References
Full text links
Read article at publisher's site: https://doi.org/10.1055/s-0034-1376202
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4445377?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1055/s-0034-1376202
Article citations
Increased Intracranial Pressure in Pediatric Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease.
Neurol Neuroimmunol Neuroinflamm, 11(1):e200174, 02 Nov 2023
Cited by: 1 article | PMID: 37918972 | PMCID: PMC10621892
Idiopathic intracranial hypertension: a step change in understanding the disease mechanisms.
Nat Rev Neurol, 19(12):769-785, 13 Nov 2023
Cited by: 8 articles | PMID: 37957260
Review
Dilated Optic Nerve Sheath in Mucopolysaccharidosis I: Common and Not Necessarily High Intracranial Pressure.
AJNR Am J Neuroradiol, 44(1):91-94, 29 Dec 2022
Cited by: 1 article | PMID: 36581456 | PMCID: PMC9835902
The Influence of Movement on the Cerebrospinal Fluid Pressure of the American Alligator (Alligator mississippiensis).
Biology (Basel), 11(12):1702, 25 Nov 2022
Cited by: 3 articles | PMID: 36552212 | PMCID: PMC9774609
Case report: KPTN gene-related syndrome associated with a spectrum of neurodevelopmental anomalies including severe epilepsy.
Front Neurol, 13:1113811, 10 Jan 2023
Cited by: 0 articles | PMID: 36703628 | PMCID: PMC9871926
Go to all (23) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
How Much Cerebrospinal Fluid Should We Remove Prior to Measuring a Closing Pressure?
J Child Neurol, 32(4):356-359, 08 Dec 2016
Cited by: 11 articles | PMID: 27932598
Cerebrospinal fluid total protein in idiopathic intracranial hypertension.
J Neurol Sci, 381:226-229, 01 Sep 2017
Cited by: 4 articles | PMID: 28991687
Sedation of children during measurement of CSF opening pressure: lack of standardisation in German children with pseudotumor cerebri.
Klin Padiatr, 224(1):40-42, 14 Dec 2011
Cited by: 1 article | PMID: 22170166
Idiopathic intracranial hypertension.
J Neuroophthalmol, 24(2):138-145, 01 Jun 2004
Cited by: 102 articles | PMID: 15179068
Review
Funding
Funders who supported this work.
NEI NIH HHS (2)
Grant ID: K23 EY022673
Grant ID: K23 EY022673-01