Abstract
Free full text

MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats
Abstract
Dysregulation of the nociceptin (N/OFQ) system has been implicated in alcohol abuse and alcoholism and growing evidence suggests that targeting this system may be beneficial for treating alcoholism. To further explore the treatment target potential of the N/OFQ system, the novel non-peptide, small-molecule N/OFQ (NOP) agonist MT-7716, (R)-2-{3-[1-(Acenaphthen-1-yl)piperidin-4-yl]-2-oxo-2,3-dihydro-1H-benzimidazol-1-yl}-N-methylacetamide hydrochloride hydrate, was examined for its effects on ethanol self-administration and stress induced reinstatement of alcohol seeking in non-dependent and post-dependent rats. Male Wistar rats were trained to self-administer ethanol and then made ethanol dependent via repeated intragastric ethanol intubation. The effects of MT-7716 (0.3 and 1 mg/kg; PO) on alcohol self-administration were determined two weeks following dependence induction, when baseline self-administration was restored. Effects of MT-7716 on stress-induced reinstatement were tested in separate cohorts of rats, one and three weeks post-withdrawal. MT-7716 reduced alcohol self-administration and stress-induced reinstatement of alcohol seeking in post-dependent rats, but was ineffective in non-dependent animals. Moreover, the prevention of stress-induced reinstatement by MT-7716 was more pronounced at 3 weeks post-dependence. The results further confirm treatment target potential for the NOP receptor and identify non-peptide NOP agonists as promising potential treatment drugs for alcohol abuse and relapse prevention. The findings also support dysregulation of the N/OFQ system as a factor in alcohol seeking and reinforcement.
Introduction
The nociceptin/orphanin FQ (N/OFQ) receptor (NOP) also referred to as opioid receptor-like 1 (ORL1), was identified as the fourth member of the opioid receptor family (Bunzow et al., 1994; Mollereau et al., 1994). N/OFQ is a heptadecapeptide that binds with high affinity its receptor, but does not bind to mu (MOP), delta (DOP) and or kappa (KOP) opioid receptors. N/OFQ and NOP are present in the cortex, amygdala, bed nucleus of the stria terminalis (Neal et al., 1999), areas that regulate the motivating actions of drugs of abuse (Eckardt et al., 1998; Everitt and Wolf, 2002; Martin-Fardon et al., 2010; Wise, 1998). Dysregulation of the nociception system has been widely implicated as a factor in high alcohol intake (Economidou et al., 2008; Martin-Fardon et al., 2010). For example, higher expression of N/OFQ and NOP receptor mRNAs in the central nucleus of the amygdala (CeA) contributes to excessive alcohol intake in Marchigian Sardinian Alcohol Preferring (msP) rats in which genetic selection for high ethanol preference is correlated with anxiety and hypersensitivity to stress (Ciccocioppo et al., 2006; Economidou et al., 2008). In addition to the role of increased NOP receptor messenger RNA expression and binding in predisposition to high alcohol intake, up-regulation of this system by chronic ethanol may be a factor in the perseveration of alcohol seeking and use. In support of this hypothesis, Wistar rats with a history of ethanol dependence show increased expression of NOPs that is associated with both increased alcohol intake and anxiety-like behavior (Roberto and Siggins, 2006). Moreover, in Wistar rats, intracerebroventricular administration of N/OFQ reduces ethanol intake following a history of ethanol dependence but not in the nondependent state, and produces greater anxiolytic effects in the post-dependent than in the non-dependent state (Martin-Fardon et al., 2010).
Consistent with a role of the nociception system in alcohol abuse, pharmacological findings implicate the N/OFQ system as a highly promising target for treating alcohol addiction. N/OFQ reduces alcohol intake and alcohol self-administration in alcohol preferring rats (Ciccocioppo et al., 2004; Ciccocioppo et al., 1999), attenuates alcohol-induced conditioned place preference, and reduces reinstatement of alcohol seeking induced by stress or alcohol-related cues (Ciccocioppo et al., 2004; Martin-Fardon et al., 2000). N/OFQ also has anxiolytic-like and anti-stress actions (Aujla et al., 2013) and attenuates protracted alcohol withdrawal symptoms in rats (Economidou et al., 2011).
Recently, a blood brain barrier penetrating NOP receptor agonist MT-7716, hydrochloride of W-212393 (Teshima et al., 2005) has become available, providing a suitable pharmacological tool to study the treatment target potential of the nociception system with direct translational implications. MT-7716 exhibits high affinity for human NOP receptors expressed in HEK293 cells (Kallupi et al., 2014). The affinity of MT-7716 for the NOP is similar to that of the endogenous agonist N/OFQ and is in the nM range (Kallupi et al., 2014). The NOP agonistic activity of MT-7716 was evaluated by GTPγ35S binding to human NOP expressed in HEK293 cells. In this preparation MT-7716 demonstrated full agonism at NOP receptor (Kallupi et al., 2014). Here, the effects of this NOP agonist were examined on two processes relevant for alcohol addiction: ethanol self-administration and stress induced reinstatement of ethanol seeking in rats with or without a history of ethanol dependence. Since time-dependent changes in nociception and NOP gene expression following ethanol withdrawal have recently been reported (Aujla et al., 2013), a secondary goal was to establish the effects of MT-7716 on ethanol self-administration and reinstatement at different time points following withdrawal.
Materials and Methods
Animals
Male Wistar rats (Charles River, Wilmington, MA, USA). Rats (N = 156) were housed two per cage on a reverse 12:12 h light/dark cycle (lights off at 8:00 a.m.) in a temperature (20–22°C) and humidity (45–55%) controlled vivarium with ad libitum access to tap water and food pellets (PJ Noyes Company, Inc, Lancaster, NH). All procedures were conducted in strict adherence to the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. At the time of testing, the rats’ body weight ranged between 350 and 400 g.
Drugs
MT-7716 was synthesized at Mitsubishi Tanabe Pharma Corporation (Japan) and dissolved in distilled water by sonication in a heated bath (45–60°C). Alcohol drinking solution 10% (w/v) was prepared by dilution of ethanol 95 % (w/v) in water.
Equipment
All training and testing was conducted in standard operant conditioning chambers (Med Associate, St Albans, VT) located in sound-attenuating, ventilated environmental cubicles. Each chamber was equipped with a drinking reservoir (volume capacity: 0.15 ml) positioned 4 cm above the grid floor in the center of the front panel of the chamber, and two retractable levers located 3 cm to the right or the left of the drinking receptacle. Visual stimuli were presented via a cue light located on the front panel. A microcomputer controlled the delivery of fluids, presentation of visual stimuli, and recording of the behavioral data.
Ethanol Self-administration training
Rats were trained to self-administer 10% (w/v) alcohol in 30-min daily sessions on a fixed-ratio 1 (FR 1) schedule of reinforcement, where each response resulted in delivery of 0.1 ml fluid (Weiss et al., 1993). Responses at the right, active lever were initially reinforced by 0.1 ml of saccharin (0.2% w/v) on a FR 1 schedule. After acquisition, ethanol (5% w/v) was added to the saccharin solution. During subsequent training, ethanol concentrations were gradually raised to 10% (w/v), while saccharin was slowly eliminated. A second but inactive lever then was introduced at which responses were recorded but had no programmed consequences.
Ethanol dependence induction
Once stable 10% (w/v) ethanol self-administration was obtained, three groups of rats were made ethanol dependent via repeated intragastric ethanol intubation as previously described (Braconi et al., 2010) with modifications. Briefly, ethanol solution (final concentration 25% w/v) was made up in a dietary supplement (Boost®, Nestlé, Florham Park, NJ) and intubated four times per day for 5 consecutive days (11 g/kg of alcohol per day), with each daily dose administered at 4 h intervals (Figure 1A). Non-dependent controls received intragastric administration of Boost® vehicle at volumes and times identical to those in ethanol-treated rats. Blood alcohol levels (BALs) were determined from tail blood samples (200 μl) taken 1 h after the first (Bleed 1) and second (Bleed 2) intragastric administration on day 4. Behavioral signs of withdrawal were measured on day 5, 12 h after the final ethanol intubation on the previous day, before the first alcohol (or vehicle) administration, using a rating scale adapted from (Macey et al., 1996). Withdrawal signs scored included: ventromedial limb retraction (VLR), irritability to touch (vocalization), tail rigidity, abnormal gait and body tremors. Each sign was assigned a score of 0–2, based on the following severity scale: 0 = no sign, 1 = moderate, 2 = severe. The sum of the four observation scores (0–8) was used as a quantitative measure of withdrawal severity.
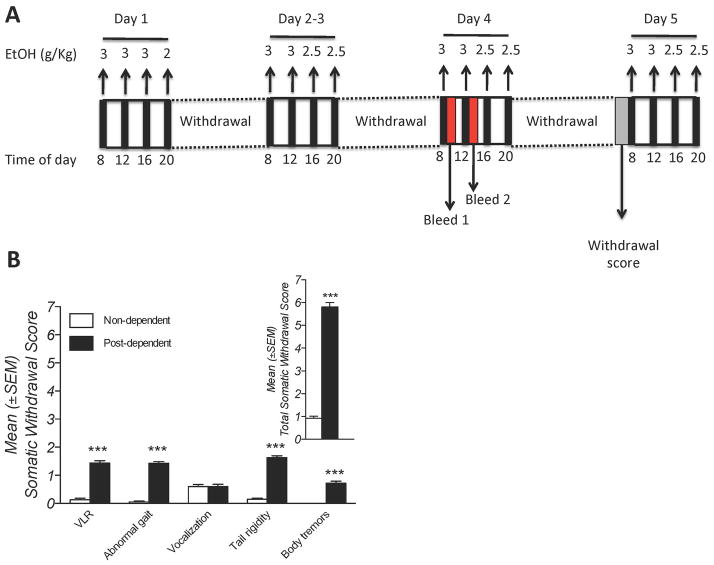
A: Experimental protocol for ethanol dependence induction. Ethanol solution (final concentration 25% w/v) was intubated four times per day for 5 consecutive days in a total volume of 22 ml/kg/day. B: somatic withdrawal signs measured at the beginning of treatment day 5. Insert: Overall withdrawal severity (sum of somatic withdrawal scores across the five behavioural signs of ethanol withdrawal). ***p < 0.001 versus non-dependent. VLR, ventromedial limb retraction.
Effect of MT-7716 on alcohol self administration in post-dependent and non-dependent rats
Wistar rats (N=19) were trained to self-administer 10% (w/v) alcohol until stable self-administration (± 10% over the last three sessions) was established. The following day, rats were divided into two groups for dependence induction (N=9; Fig. 1A) vs. vehicle treatment (N=10; Fig. 1A). Two days after completion of dependence induction (or control) procedures, daily ethanol self-administration sessions resumed until stable levels of intake were re-established. Animals were injected by gavage with MT-7716 (0.0, 0.3 or 1.0 mg/kg), 1 hour before the test session. Each rat was tested with all MT-7716 doses according to a Latin square design, with 2–3 baseline EtOH self-administration sessions between tests.
Effect of MT-7716 on stress-induced reinstatement in post-dependent and non-dependent rats
Wistar rats were trained to self-administer 10% (w/v) alcohol until stable responding was established, followed by ethanol dependence induction. Rats then were divided into two groups for testing following 1 or 3 weeks of ethanol withdrawal. Rats in the 1-week post-withdrawal group were subjected to 30-min extinction sessions for 4 consecutive days (2 sessions per day) beginning forty-eight hours after completion of ethanol dependence induction. Extinction sessions were identical to the EtOH self-administration sessions, except that responses at the active lever activated the syringe pump but did not result in any EtOH delivery.
For rats in the 3-week post-withdrawal group, extinction sessions began 2 weeks after completion of ethanol dependence induction. One day after the final extinction session, nondependent and post-dependent rats were divided into three groups for testing the effects of MT-7716 (0.0, 0.3 and 1.0 mg/kg, PO) on reinstatement of ethanol seeking induced by footshock stress (15-min, variable intermittent electric foot shock, 0.5 mA; duration, 0.5 sec; mean shock interval, 40 sec; range, 10–70 sec), administered through the grid floor of the operant chambers. Each animal was tested with only one dose of MT-7716 or vehicle (administered 1 hour before session onset) according to a between-subjects design. Two minutes following termination of foot-shock, levers were extended and responses were recorded for 30 minutes.
Statistical Analysis
The effect of MT-7716 on alcohol self-administration was analyzed by two-way ANOVA with one factor within (treatment) and one factor between (intoxication condition). Reinstatement data were analyzed by three-way Anova with time (1 week vs 3 weeks), dependence (non-dependent vs post-dependent) as between factors and condition (extinction vs relapse) as within factor. The effect of MT-7716 on stress-induced reinstatement of alcohol seeking was analyzed by three-way ANOVA with three factors between (treatment, time and intoxication condition). Where appropriate, ANOVAs were followed by Protected Least Significant Difference (PLSD) post hoc tests. Withdrawal signs were analyzed by the nonparametric Mann-Whitney U statistic.
Results
Blood alcohol levels and withdrawal ratings
Mean (± SEM) blood alcohol levels (BALs) measured on day 4 of dependence induction were elevated (Bleed 1: 256.3 ± 12.51 mg/100 ml, Bleed 2: 351.1 ± 10.9 mg/100 ml). In the morning of day 5, post-dependent rats showed overt withdrawal signs (Figure 1B) with significant increases in ventromedial limb retraction (VLR; Mann-Whitney, U = 1937.6, p< 0.001), tail rigidity (U = 2143.2; p< 0.001), abnormal gait (U = 787.0; p < 0.001), and body tremors (U = 1224.3; p < 0.001). The sum of the five rating scores revealed the presence of significant overall withdrawal severity (U = 2545.8; p< 0.001; Figure 2 insert).
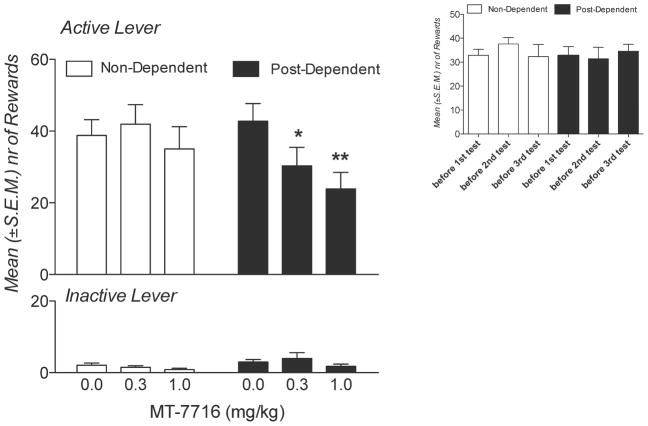
Effect of MT-7716 on alcohol-reinforced responses in ethanol non-dependent and post-dependent Wistar rats. Animals self-administered alcohol on a fixed ratio 1 schedule in 30-minute daily sessions in which each reinforced response resulted in the delivery of 0.1 ml of 10% ethanol. Treatment with MT-7716 reduced operant responses for alcohol preferentially in post-dependent animals at both doses tested (*p<0.05 and ** p<0.01). Insert: re-establishment of alcohol self-administration baseline across the three test days.
Effects of MT-7716 on alcohol self-administration in post-dependent and non-dependent rats
The mean (± SEM) number of responses before drug testing was similar in non-dependent (43.6 ± 5.6) and post-dependent (44.6 ± 5.7) rats. As well, mean (± SEM) of responses following vehicle administration were similar in non-dependent (38.8 ± 3.1) and post-dependent (42.8 ± 3.3) animals. MT-7716 reduced ethanol self-administration selectively in post-dependent rats (Figure 2). This effect was confirmed by the ANOVA with a main effect of dose [F (2,17) = 8.32, p < 0.01] and a dose x groups (non-dependent vs, post-dependent) interaction [F (2,34) = 5.01; p < 0.05]. PLSD post hoc tests verified that MT-7716 significantly reduced alcohol self-administration in post-dependent rats at both 0.3 (p<0.05 vs vehicle) and 1 mg/kg (p<0.001vs vehicle). Inactive lever responses remained negligible and unaltered by MT-7716.
Importantly, baseline alcohol self-administration across the three test days remained stable in both nondependent [F (2,9) = 1.397; NS] and post-dependent [F (2,8) = 0.43; NS] animals (Figure 2, insert).
Effect of MT-7716 on stress-induced reinstatement of alcohol seeking in post-dependent and non-dependent rats
To evaluate the reinstatements, comparison of extinction vs MT-7716 vehicle treated groups were analyzed. A three way Anova with time (1 week vs 3 weeks), dependence (non-dependent vs post-dependent) as between factors and condition (extinction vs reinstatement) as within factor showed main effects of condition [F(1,46)=119.85; p<0.0001] and of dependence [F(1,46)=3.68; p<0.05]. A significant time x dependence interaction was also observed [F(1,46)=5.23; p<0.05]. Compared to extinction, footshock elicited significant (p<0.001) reinstatement of lever pressing in both vehicle treated non-dependent and post-dependent rats (Fig 3A and 3B). Post hoc analysis of time x dependence interaction showed that at 3 weeks, reinstatement of responding in post-dependent rats was significantly higher (p<0.05) than in the non-dependent group (Fig 3B). Inactive lever responses remained negligible and unaltered by footshock stress.
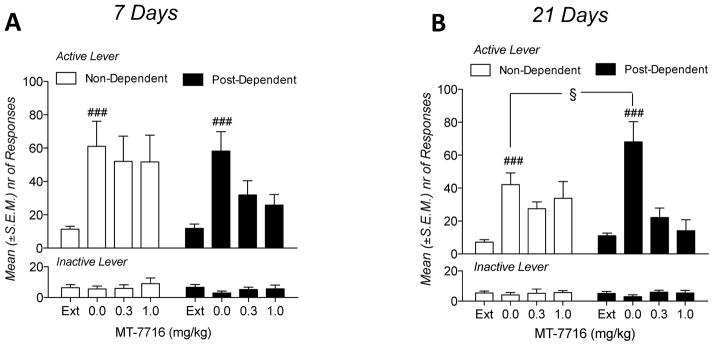
A: Effect of MT-7716 on stress induced reinstatement of alcohol seeking in non-dependent and post-dependent rats 1 week after completion of ethanol dependence induction. Results are expressed as mean (±SEM) responses at the active and the inactive levers. ### p<0.01 0.0 mg/kg vs last 3 days of extinction. B: Effect of MT-7716 on stress induced reinstatement of alcohol seeking in non-dependent and post-dependent rats 3 weeks after completion of ethanol dependence induction. ### p<0.01 0.0 vs last 3 days of extinction; § p<0.05 0.0 mg/kg non-dependent vs 0.0 post-dependent.
Analysis of the effects of MT-7716 by three-way ANOVA revealed a main effect for treatment (MT-7716 doses) [F(2,127)=13,11; p<0.001], time [F(1,127)=6.80; p<0.05] and treatment x dependence interaction [F(2,127)=4.95; p<0.01]. Post hoc analysis of treatment x dependence interaction revealed that administration of MT-7716 significantly reduced footshock stress-induced reinstatement at 1 week and at 3 weeks only in the post-dependent group (p<0.001; Fig 3A and 3B). Inactive lever responses remained negligible and unaltered by MT-7716.
Discussion
The results confirm that activation of NOP receptors by the synthetic agonist MT-7716, like the endogenous agonist N/OFQ (Ciccocioppo et al., 2004; Martin-Fardon et al., 2000), reduces alcohol self-administration and stress-induced reinstatement of alcohol seeking. Both effects were, however, observed only in rats with a history of ethanol dependence. Even though the NOP agonist was behaviorally inert in ethanol nondependent animal in post-dependent rats, MT-7716 prevented footshock-induced reinstatement following one or three weeks of withdrawal similarly. Both observations are suggestive of ethanol-induced neuroadaptive changes in N/OFQ-NOP functioning whereby rats with a history of ethanol dependence become responsive to NOP receptor agonist treatments.
In the alcohol self-administration tests, vehicle-treated postdependent and nondependent rats showed similar levels of ethanol-reinforced responding, in contrast to previous reports of increased alcohol intake in ethanol postdependent rats (Roberts et al., 2000; Rodd et al., 2006; Valdez et al., 2002). However, in these previous studies, rats had been allowed to self-administer ethanol during acute withdrawal. Therefore, the lack of increased self-administration in post-dependent rats in the present study may be attributable to the lack of opportunity to self-administer alcohol during withdrawal. In spite of the absence of differences in alcohol intake, MT-7716 reduced alcohol self-administration only in rats with a history of dependence. This observation is in agreement with previous findings showing that N/OFQ (administered ICV) does not reduce alcohol self-administration in nondependent Wistar rats, although the peptide effectively reduces alcohol self-administration in nondependent Marchigian Sardinian Alcohol-Preferring (msP) rats (Economidou et al., 2008), a line of rats with dysregulated N/OFQ function (Ciccocioppo et al., 2006). Notably, several lines of evidence indicate that the msP rat line has innate characteristics resembling rats with a history of chronic alcohol intoxication and has been proposed as a postdependent phenotype (Ciccocioppo et al., 2006; Gozzi et al., 2013; Hansson et al., 2007; Hansson et al., 2006).
Stress-regulatory systems can undergo time-dependent biphasic functional changes following ethanol withdrawal with corresponding biphasic changes in the behavioral effects of stress challenges (Zhao et al., 2007; Zorrilla et al., 2001).
Similarly, we have previously observed that nociception produces opposite effects on stress-induced experimental anxiety at 7 vs. 21 days following alcohol withdrawal (Aujla et al., 2013). Guided by these findings, the effects of MT-7716 on stress-induced reinstatement in the present study were probed for at the same post-withdrawal time points in order to evaluate possible similar biphasic effects of NOP activation on stress-induced reinstatement of alcohol seeking.
Stress-induced reinstatement in vehicle-treated rats did not differ as a function of ethanol history in the 1-week post-withdrawal test, similar to the absence of differences in ethanol self-administration between nondependent and postdependent rats. At first glance these data seem inconsistent with previous findings (Liu and Weiss, 2002) showing an enhancement of stress-induced reinstatement in post-dependent versus non-dependent rats. However, in the Liu et al. study, reinstatement was tested 2 – 3 weeks after completion of ethanol withdrawal, a time corresponding closely to the 3-weeks time point in the present study. Consistent with this time line, higher reinstatement levels were observed also in the present study 3 weeks post-dependence. Thus, despite differences in dependence induction and extinction training procedures, the present study reproduced the enhanced responsiveness to stress after 3 weeks of abstinence originally reported by Liu et al.
MT-7716 significantly attenuated the effects of footshock at both post-withdrawal time points and, as in the case of self-administration, did so selectively in ethanol postdependent rats.
The selective effects of MT-7716 on ethanol self-administration and reinstatement in postdependent rats are likely to be linked to neuroadaptive changes in the N/OFQ system that develop as a result of ethanol intoxication and withdrawal and convey or enhance sensitivity to NOP activation. Consistent with this hypothesis, are recent findings that have described that NOP gene expression in the bed nucleus of the stria terminalis (BNST) is enhanced in ethanol postdependent rats (Martin-Fardon et al., 2010). The BNST plays an important role in animal models of stress-induced reinstatement of drug seeking (Erb and Stewart, 1999; Silberman and Winder, 2013). Moreover, as determined in slice preparations, ethanol postdependent rats show changes in the effects of N/OFQ on GABA transmission in the central nucleus of the amygdala during the acute withdrawal, characterized by an enhancement in the ability of N/OFQ to decrease the facilitation of GABAA neurotransmission by ethanol (Roberto and Siggins, 2006). Notably, CeA electrophysiological recording also revealed that N/OFQ more potently attenuates CRF-induced facilitation of GABA neurotransmission compared to nondependent controls (Cruz et al., 2012). Although these electrophysiological data were obtained during acute withdrawal whereas the present findings were obtained during protracted withdrawal, these findings document that functional changes develop in the N/OFQ system as a result of ethanol dependence, and that this aspect can be characterized behaviorally (Martin-Fardon et al., 2010)
Overall, the present findings suggest that ethanol dependence leads to neuroadaptive alteration in the N/OFQ-NOP system. Nonetheless, the precise functional changes in this system underlying the effects of MT-7716 here remain unclear. For example, increased NOP gene expression in the BNST has been found in ethanol post-dependent Wistar rats after one and three weeks of ethanol withdrawal (Aujla et al., 2013). However, in the same study, ICV administration of N/OFQ was reported to produce anxiogenic-like actions after 3 weeks of withdrawal, in contrast with the current finding where MT-7716 prevented stress-induced reinstatement in postdependent animals only and at both one and three weeks withdrawal. On one hand, these differences may be explained by the fact that stress-induced reinstatement and anxiety are not necessarily regulated by the same neurocircuitry. On the other hand the discrepant behavioral effects of MT-7716 vs. N/OFQ may be due to a different pharmacological profile of these two NOP agonists (Teshima et al., 2005). This is, however, unlikely because recent findings show that MT-7716 has the same electrophysiological effects as N/OFQ on CeA GABA-ergic neurotransmission (Cruz et al., 2012; Kallupi et al., 2014). Importantly, activation of NOP receptors in this area (and related regulation of GABAergic activity) is critical for the anxiolytic effect of N/OFQ (Ciccocioppo et al., 2014). Consistent with this evidence, we found that MT-7716 (0.3–1 mg/kg; p.o) significantly increased open arm time on the elevated plus maze in a model of hangover-induced anxiety, confirming potent anxiolytic-like actions (Teshima, unpublished observations). Nonetheless, further thorough pharmacological characterization of MT-7716 in comparison with N/OFQ will be required to understand overlap and differences in the behavioral actions of these molecules and to characterize the clinical potential of MT-7716.
The specific inhibition of alcohol drinking in postdependent rats following MT-7716 parallels recent findings showing that administration of corticotropin-releasing factor (CRF) receptor-1 antagonists reduces alcohol consumption in post-dependent Wistar and in msP rats but not in nondependent stock rats (Ayanwuyi et al., 2013; Funk et al., 2007; Hansson et al., 2006; Sommer et al., 2008). Several studies have documented that N/OFQ acts as a functional antagonist of behavior controlled by extra-hypothalamic CRF1 receptors (Ciccocioppo et al., 2003). For example, ICV administration of N/OFQ prevented both the anorexic and anxiogenic effects of stress induced by central CRF administration (Ciccocioppo et al., 2003; Ciccocioppo et al., 2001). Thus, it is possible that, in addition to neuroadaptations of the N/OFQ system produced by dependence-inducing ethanol intoxication, functional interactions between MT-7716 and CRF1 receptors (as described for N/OFQ) may play a role in the differential effects of the MT-7716 in post-dependent vs. non-dependent rats observed here.
In conclusion, the results further confirm that NOP receptors participate in mediating alcohol reinforcement and stress-induced reinstatement of alcohol seeking and that these receptors may represent a potential pharmacotherapeutic target to treat alcoholism. In particular, the “anti-stress” effects of MT-7716 suggest that blood brain barrier (BBB) penetrating NOP agonists may have substantial clinical potential as complements to presently available anti-craving pharmacotherapies (such as naltrexone), that are ineffective in preventing stress-induced alcohol seeking (Le et al., 1999; Liu and Weiss, 2002). Lastly, the findings illustrate the importance of studying NOP receptor function to better understand the neurobiology of alcohol abuse.
Acknowledgments
This is publication number 24061 from The Scripps Research Institute This study was supported by NIH/NIAAA AA014351 (FW). The authors thank Tony Kerr, Belinda Leos and Alfredo Fiorelli for expert technical assistance.
Footnotes
Disclosure/Conflict of Interest
The authors declare no biomedical financial interests or potential conflict of interest.
Authors Contribution
GdG, RMF, RC and FW were responsible for the study concept and design. GdG and RMF performed the experiments and collected all data. GdG and RMF assisted with the data analysis, interpretation of findings and drafted the manuscript. All authors critically reviewed the content and approved the final version for publicationReferences
- Aujla H, Cannarsa R, Romualdi P, Ciccocioppo R, Martin-Fardon R, Weiss F. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol. 2013;18:467–479. [Europe PMC free article] [Abstract] [Google Scholar]
- Ayanwuyi LO, Carvajal F, Lerma-Cabrera JM, Domi E, Bjork K, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R, Cippitelli A. Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats. Frontiers in psychiatry. 2013;4:23. [Europe PMC free article] [Abstract] [Google Scholar]
- Braconi S, Sidhpura N, Aujla H, Martin-Fardon R, Weiss F, Ciccocioppo R. Revisiting intragastric ethanol intubation as a dependence induction method for studies of ethanol reward and motivation in rats. Alcoholism, clinical and experimental research. 2010;34:538–544. [Europe PMC free article] [Abstract] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. [Abstract] [Google Scholar]
- Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, Oleata CS, Heilig M, Roberto M. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:363–372. [Europe PMC free article] [Abstract] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. [Europe PMC free article] [Abstract] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology. 2004;172:170–178. [Europe PMC free article] [Abstract] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:9445–9451. [Europe PMC free article] [Abstract] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F, Massi M. Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 2001;12:1145–1149. [Abstract] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology. 1999;141:220–224. [Abstract] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biological psychiatry. 2012;71:666–676. [Europe PMC free article] [Abstract] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcoholism, clinical and experimental research. 1998;22:998–1040. [Abstract] [Google Scholar]
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, Martin-Fardon R, Weiss F, Massi M, Ciccocioppo R. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcoholism, clinical and experimental research. 2011;35:747–755. [Europe PMC free article] [Abstract] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biological psychiatry. 2008;64:211–218. [Europe PMC free article] [Abstract] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:RC35. [Abstract] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:3312–3320. [Abstract] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biological psychiatry. 2007;61:78–86. [Europe PMC free article] [Abstract] [Google Scholar]
- Gozzi A, Agosta F, Massi M, Ciccocioppo R, Bifone A. Reduced limbic metabolism and fronto-cortical volume in rats vulnerable to alcohol addiction. Neuroimage. 2013;69:112–119. [Europe PMC free article] [Abstract] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–34. [Abstract] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. [Europe PMC free article] [Abstract] [Google Scholar]
- Kallupi M, Oleata C, Luu G, Teshima K, Ciccocioppo R, Roberto M. MT-7716, a novel selective nociceptin receptor agonist, effectively blocks ethanol-induced increase in GABAergic transmission in the central nucleus of the amygdala. Front Integr Neurosci 2014 [Europe PMC free article] [Abstract] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. [Abstract] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:7856–7861. [Abstract] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. [Abstract] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. [Abstract] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res. 2010;1314:145–161. [Europe PMC free article] [Abstract] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. [Abstract] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999;412:563–605. [Abstract] [Google Scholar]
- Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci U S A. 2006;103:9715–9720. [Europe PMC free article] [Abstract] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. [Abstract] [Google Scholar]
- Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, McBride WJ. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behavioural brain research. 2006;171:207–215. [Abstract] [Google Scholar]
- Silberman Y, Winder DG. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Frontiers in psychiatry. 2013;4:42. [Europe PMC free article] [Abstract] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biological psychiatry. 2008;63:139–145. [Abstract] [Google Scholar]
- Teshima K, Minoguchi M, Tounai S, Ashimori A, Eguchi J, Allen CN, Shibata S. Nonphotic entrainment of the circadian body temperature rhythm by the selective ORL1 receptor agonist W-212393 in rats. Br J Pharmacol. 2005;146:33–40. [Europe PMC free article] [Abstract] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcoholism, clinical and experimental research. 2002;26:1494–1501. [Abstract] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [Abstract] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. [Abstract] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcoholism, clinical and experimental research. 2007;31:1505–1515. [Abstract] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology. 2001;158:374–381. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1111/adb.12157
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4268094?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/adb.12157
Article citations
Pharmacological blockage of NOP receptors decreases ventral tegmental area dopamine neuronal activity through GABAB receptor-mediated mechanism.
Neuropharmacology, 248:109866, 15 Feb 2024
Cited by: 0 articles | PMID: 38364970
Voluntary and forced exposure to ethanol vapor produces similar escalation of alcohol drinking but differential recruitment of brain regions related to stress, habit, and reward in male rats.
Neuropharmacology, 222:109309, 02 Nov 2022
Cited by: 6 articles | PMID: 36334765 | PMCID: PMC10022477
Regulation of N-type calcium channels by nociceptin receptors and its possible role in neurological disorders.
Mol Brain, 15(1):95, 24 Nov 2022
Cited by: 2 articles | PMID: 36434658 | PMCID: PMC9700961
Review Free full text in Europe PMC
Subregional Differences in Alcohol Modulation of Central Amygdala Neurocircuitry.
Front Mol Neurosci, 15:888345, 05 Jul 2022
Cited by: 2 articles | PMID: 35866156 | PMCID: PMC9294740
Review Free full text in Europe PMC
A Role for Neuropeptide S in Alcohol and Cocaine Seeking.
Pharmaceuticals (Basel), 15(7):800, 27 Jun 2022
Cited by: 1 article | PMID: 35890099 | PMCID: PMC9317571
Review Free full text in Europe PMC
Go to all (30) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Chronic treatment with novel brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat.
Neuropsychopharmacology, 39(11):2601-2610, 27 Jun 2014
Cited by: 35 articles | PMID: 24863033 | PMCID: PMC4207340
The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence.
Pharmacol Ther, 141(3):283-299, 01 Nov 2013
Cited by: 109 articles | PMID: 24189487 | PMCID: PMC5098338
Review Free full text in Europe PMC
The nociceptin/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: validation in rat models.
Psychopharmacology (Berl), 233(19-20):3553-3563, 11 Aug 2016
Cited by: 16 articles | PMID: 27515665 | PMCID: PMC5021736
A Novel, Orally Bioavailable Nociceptin Receptor Antagonist, LY2940094, Reduces Ethanol Self-Administration and Ethanol Seeking in Animal Models.
Alcohol Clin Exp Res, 40(5):945-954, 16 Apr 2016
Cited by: 36 articles | PMID: 27084498 | PMCID: PMC4844902
Funding
Funders who supported this work.
NIAAA NIH HHS (1)
Grant ID: R01 AA014351
NIH/NIAAA (1)
Grant ID: AA014351
PHS HHS (1)
Grant ID: NIH/NIAAA AA014351





