Abstract
Objective
To document the various clinical manifestations, lab parameters, complications and outcomes of Falciparum Malaria. The above data would be correlated with the parasitic index to deduce whether it would be an effective measure of the same.Methods
This was a prospective study among 183 inpatients aged above 18 from Kasturba Hospital, Manipal from May 2009 to January 2011. Ethical clearance was taken. Statistical analysis was done with the independent paired t test, linear correlation and Chi square test using SPSS 16.Results
In this study 78% cases were males. Most cases occurred during the monsoons. Fever was the major presentation with others being jaundice, vomiting and head ache. 50.8 % had complications, including hepatic dysfunction (40.9%), renal failure (19.13%), shock (7%), altered sensorium (9%), ARDS (3.27%) and severe anemia (1.63%). Hypoglycemia and gram negative sepsis were rare. Parasitic index, renal parameters and death were correlating positively. ESR was significantly related (P<0.003) to complications and not to cerebral malaria. There were 12 mortalities out of which 9 were due to MODS and 3 due to ARDS.Conclusions
50.8% cases conformed to the WHO definition of severe malaria indicating most present with complications. High parasite index and abnormal renal function are predictors of mortality and complications. Early diagnosis, anticipation of complications, close monitoring and combination therapy to over come drug resistance helps to contain the extent of mortality.Free full text

The emerging trends of falciparum malaria: a study from a tertiary centre in an endemic area of India
Abstract
Objective
To document the various clinical manifestations, lab parameters, complications and outcomes of Falciparum Malaria. The above data would be correlated with the parasitic index to deduce whether it would be an effective measure of the same.
Methods
This was a prospective study among 183 inpatients aged above 18 from Kasturba Hospital, Manipal from May 2009 to January 2011. Ethical clearance was taken. Statistical analysis was done with the independent paired t test, linear correlation and Chi square test using SPSS 16.
Results
In this study 78% cases were males. Most cases occurred during the monsoons. Fever was the major presentation with others being jaundice, vomiting and head ache. 50.8 % had complications, including hepatic dysfunction (40.9%), renal failure (19.13%), shock (7%), altered sensorium (9%), ARDS (3.27%) and severe anemia (1.63%). Hypoglycemia and gram negative sepsis were rare. Parasitic index, renal parameters and death were correlating positively. ESR was significantly related (P<0.003) to complications and not to cerebral malaria. There were 12 mortalities out of which 9 were due to MODS and 3 due to ARDS.
Conclusions
50.8% cases conformed to the WHO definition of severe malaria indicating most present with complications. High parasite index and abnormal renal function are predictors of mortality and complications. Early diagnosis, anticipation of complications, close monitoring and combination therapy to over come drug resistance helps to contain the extent of mortality.
1. Introduction
Known since millennia, malaria has played a major role in the history of mankind and has been a problem in India for centuries. It is often said that but for malaria, the history and geographical demarcations of our planet would have been different from what we have today. Malaria continues to wreak havoc on millions, particularly in the poorest parts of our world. Malaria has transmission in 107 countries containing 3 billion people and causing 1–3 million deaths each year. It is widespread in tropical and subtropical regions, including much of Sub-Saharan Africa, Asia and the Americas. Most deaths among the infected are caused by falciparum malaria. Hence it was declared to be the first priority tropical disease by the World Health Organisation (WHO)[1].
The considerable mortality and morbidity in falciparum malaria is due to its protean manifestations, multi organ involvement, delay in diagnosis and failure of administration of treatment promptly and adequately[2]. Added to this are the increasing problems of drug resistance of the parasite and insecticide resistance of the vectors. Although there are promising new control and research initiatives, malaria remains today, as it has been for centuries, a heavy burden on tropical communities, a threat to non endemic countries, and a danger to travelers. It has been especially of burden to the west coast of Karnataka, India especially during the monsoons. Hence an evaluation of the clinical profile of malaria for the emerging trends is appropriate in this context. The objective of this study was to document the various clinical manifestations, lab parameters and outcomes of falciparum malarial infection. Various complications encountered during the disease process were recorded and correlated with the parasitic index as a predictor of outcome and mortality.
2. Materials and methods
A total of 183 inpatients of Kasturba Hospital, Manipal aged above 18 years who were positive for Plasmodium falciparum were included in this study. This was conducted as a prospective study from May 2009 to January 2011 at Kasturba Hospital, Manipal, Karnataka, India. Our hospital, a tertiary referral centre is situated in an endemic area for malaria.
Ethical clearance was taken from the hospital committee and informed consent was taken from the patients or party as the situation demanded.
The methods used for diagnosis were immunofluoroscence (falcivax), quantitative buffy coat (QBC) and peripheral smear. Falcivax is a rapid, qualitative, two site sandwich immunoassay utilizing whole blood for the detection of Plasmodium falciparum specific histidine rich protein-2 (Pf. HRP-2) and Plasmodium vivax specific pLDH. The test can also be used for specific detection and differentiation of vivax and falciparum malaria in areas with high rates of mixed infections[3].
The QBC test is a new method for identifying the malarial parasite in the peripheral blood. It involves staining of the centrifuged and compressed red cell layer with acridine orange and its examination under UV light. It is fast, easy and claimed to be more sensitive than the traditional thick smear examination[4]. Out of the cases 4 people had vivax positivity in addition to falciparum.
Severe falciparum malaria was defined according to the one proposed by a working group convened by the WHO[5].
Cerebral malaria-Unarousable coma not attributable to any other cause in a patient with falciparum malaria. The coma should persist for at least 30 min after a generalised convulsion to make the distinction from transient post ictal coma.
Severe anemia-Normocytic anemia with hematocrit < 15% or hemoglobin <5 mg% in the presence of parasitemia >10 000/µL.
Renal failure-defined as a urine out put of <400 mL in 24 h in adults or 12 mL/kg in 24 h in children, failing to improve after rehydration and serum creatinine of >3 mg%.
Pulmonary edema or acute respiratory distress syndrome (ARDS).
Hypoglycemia-defined as a whole blood glucose concentration of <2.2 mmol/L (40 mg%)
Circulatory collapse or shock-hypotension (systolic blood pressure less than 50 mm Hg in children of 1-5 years or less than 70 mmHg in adults) with cold clammy skin or core-skin temperature difference of >10 °C.
Spontaneous bleeding from gums, nose, and gastrointestinal tract etc. and or substantial laboratory evidence of disseminated intravascular coagulation.
Repeated generalised convulsions more than 2 observed with in 24 h despite cooling of fever.
Acidosis-defined as an arterial pH <7.25 or as a plasma bicarbonate concentration <15 mmol/L.
Macroscopic hemoglobinuria-if definitely associated with acute malarial infection and not merely the result of oxidant anti malarial drugs in patients with erythrocyte enzyme defects e.g., G6PD deficiency.
Postmortem confirmation of diagnosis in fatal cases by histological examination of needle necropsy of the brain.
One or more of the above features in the presence of asexual parasitemia defines severe malaria.
Other manifestations of severe malaria which (according to the WHO document) do not in themselves define the condition in all geographical areas and age groups include the following:
Impairment of consciousness less marked than unarousable coma.
Prostration or weakness, so that the patient cannot sit or walk with no obvious neurological explanation.
Very high parasite densities are associated with increased risk of severe disease. Most authorities would regard a parasitemia of more than 10% as indicating a potentially dangerous infection irrespective of the other features. (hyperparasitemia).
Jaundice which is detected clinically or defined by a serum bilirubin concentration more than 3 mg %.
Hyperpyrexia-rectal temperature more than 40 °C (sustained hyperpyrexia in severe malaria indicates a poor prognosis).
In severe malaria there is often evidence of multiple organ dysfunction and more than one of the above criteria are fulfilled
Statistical analysis was done using SPSS version 16.Tests used were independent paired t test, linear correlation and Chi square test. Results obtained were compared with a few similar studies.
3. Results
A total of 183 cases of Falciparum malaria were included in this study. The male sex group showed an increase in incidence (78%) compared to the female group. Of the 183 patients, 54% cases were between 21 and 50 years of age, with a peak of 22.5% patients in the second decade of life.
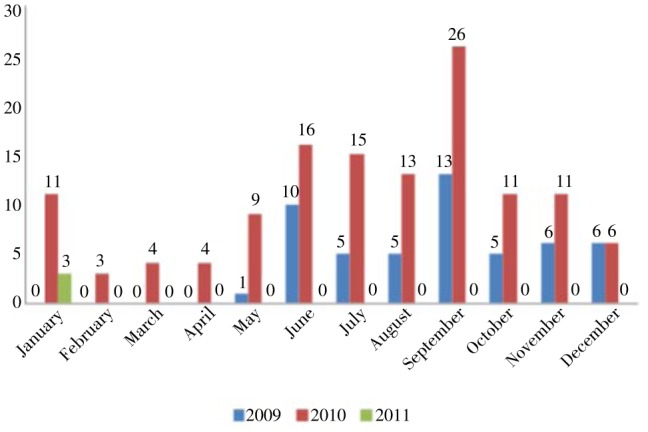
Most of the cases were between June and September corresponding with the monsoon season (Figure 1).This corresponded to two other studies carried out by Wasnik PN et al. in 2012 and Koni MB in 2008 who had around 85% cases occurring during monsoons[6]–[8]. All cases were equally distributed in all classes of society. The nature of work and exposure to vector did not play a significant role. As our area contains a significant student community most of the cases were from the same group. 49.2% cases were from Udupi district, Karnataka in which our hospital is situated. The rest were cases with complications referred to our tertiary centre.
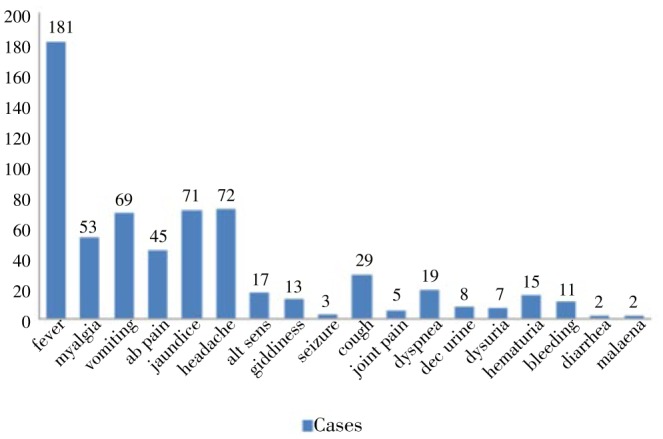
A total of 181 cases presented with fever as the initial symptom. Other major presenting symptoms in this study were jaundice, abdominal pain, vomiting, bleeding manifestations and head ache (Figure 2). A comparison of features with reference to other studies was as below (Table 1). And 48.1% cases were afebrile the day after admission. Average days of fever were one week. The presence of fever post admission was influenced by treatment which the patient had received outside. One case had hyperpyrexia (41.66 °C) which is a noted complication of falciparum malaria.
Table 1
Twenty-seven cases had blood pressure below 100/60 mmHg and were given inotropic support. Out of this 14 cases fell into the WHO definition of shock which is a blood pressure below 70/60 mmHg[5].
Seventeen (9.28%) patients presented with altered sensorium. Out of these 3 persons went into unarousable coma. Altered sensorium and flapping tremors with deranged liver function tests were seen in 6 (3.27%) patients. Levels of ammonia were found to be above the upper range of normal (35 µmol/L) in these patients. Features of cerebral malaria in our study and in a similar study by Koni et al. were as follows. (Table 2)[7].
Table 2
| Koni et al. 2008 [7] | Present study | |
| Incidence | 57.5 | 9.28 |
| Delirium | 52.5 | 9.28 |
| Coma | 7.5 | 3.82 |
| Headache | 82.5 | 39.34 |
| Meningeal signs | 40.0 | 4.00 |
Cough was found in 37.5% of patients. Fifteen percent of the patients had early signs of respiratory insufficiency with tachypnoea, dyspnoea, shallow breathing, acidosis and bilateral chest signs like decreased air entry, crepitations and rhonchi. ARDS was the end result in 6 (3.27%) patients of which 3 (1.64%) were put on ventilator. All three expired eventually.
Three cases had hemoglobin less than 5 g/dL according to the WHO criteria for severe anemia in malaria (All three cases had hemoglobin levels less than 3 g/dL). And 28 cases (33.73%) had counts more than 15 000/µL. And 128 cases (69.9%) had platelet counts less than 1 lakh. Five cases had platelet counts less than 5 000/µL. And 30.05% had anemia according to WHO and 27.23% had deranged bleeding parameters. Twenty-eight cases required packed cell transfusion and 22 cases required platelet transfusion.
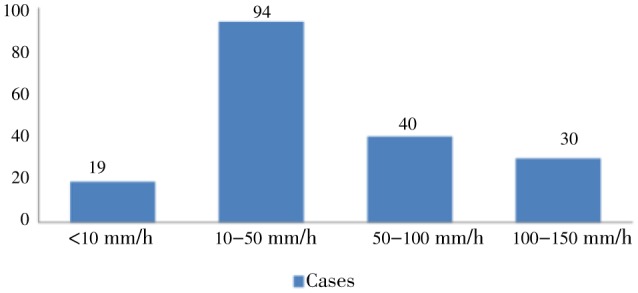
ESR levels were high in most patients (Figure 3). The relationship between WHO severity and ESR was significant with P<0.003. Severe cases had an average ESR of 60.10±42.30 as compared to the rest which had 38.83±31.87. The relationship of ESR and complications in malaria was found to be positive in several other studies[9]. The relationship between ESR and cerebral malaria was found to be not significant with a P value of 0.234.
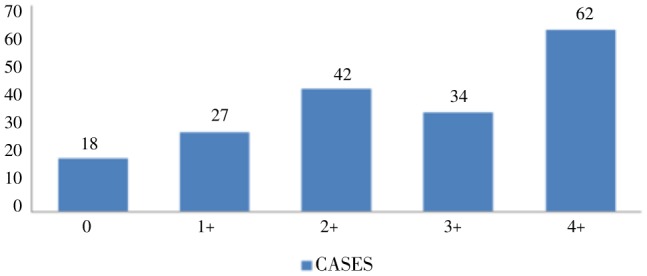
Peripheral smear, QBC method and falcivax were the usual methods of diagnosis of falciparum malaria. Rate of positivity hence sensitivity was more with falcivax where 13 cases were positive in which QBC was negative. The 5 cases of negative peripheral smear were positive by falcivax (Table 3). Sensitivity and specificity was not calculated using McNemar's formula as according to data, a gold standard investigation could not be established. Sixty-two cases (33.8%) had high parasitemia (4+) by QBC method (Figure 4). Distribution of parasitic index was as follows (Table 4).
Table 3
| Falcivax | MP QBC | Peripheral smear | |
| Positive | 161 | 165 | 178 |
| Negative | 10 | 18 | 5 |
| Not available | 12 | 0 | 0 |
Table 4
| Parasitic index | Cases |
| <0.5 | 69 |
| 0.5-1.0 | 13 |
| 1.0-5.0 | 37 |
| 5.0-10.0 | 4 |
| >10.0 | 1 |
A creatinine value more than two is considered as renal failure according to the WHO criteria[5]. Thirty-five cases (19.13%) had renal failure. Eleven cases required dialysis. The relation between creatinine and death was significant with P<0.0001. Parasitic index and death according to Levenes test of equality of variance was significant with P<0.01 (Table 5). And 84 cases (45.9%) had elevated urea levels. In this study correlation between parasitic index and urea and creatinine values was significant according to Pearson's formula with a P value less than 0.000.
Table 5
| Death | Cases | Parasitic index | Creatinine |
| Yes | 12 | 2.9±3.2 | 4.4±4.19 |
| No | 112 | 1.0±1.6 | 1.63±1.39 |
Contrary to popular belief hypoglycemia was not observed in this study during admission. Only 5 cases had random blood sugar values less than 60 mg/dL. None had less than 40 mg/dL according to the WHO severity criteria[5]. Also 15 patients were detected to be hyperglycemic out of who 9 were diabetics. The rest of the patients had been started on dextrose containing IV fluids prior to blood glucose being checked. This occurred probably due to over cautious management of anticipated hypoglycemia.
A total of 74.86% had normal sodium levels with 3.82% having hyponatremia. There was 1 case of hypernatremia. And 83.6% had normal potassium levels with 7.65% having hyperkalemia. All the cases of hyperkalemia were associated with renal failure. A total of 75 cases had a total bilirubin more than 3 g/dL which is taken as per the WHO severity criteria. Around 50% of the cases had deranged liver enzymes.
Out of the 15 cases of hematuria suspected clinically, 5 urine hemoglobin was sent all of which were positive. Out of the 5 cases of severe metabolic acidosis 3 were due to renal failure.
The relationship between age and death was not significant with P=0.216.
A few adverse effects of medications were recorded. There were two documented cases of cinchonism (tinnitus, giddiness) in whom quinine had been used. One case of lumefantrine induced psychosis and nightmares were observed. There was severe hemolysis in a patient induced by primaquine whose glucose six phosphate dehydrogenase (G6PD) level turned out to be normal. Levels of G6PD observed was as follows (Table 6)
Table 6
| G6PD (U/g Hb) | Cases |
| Not available | 95 |
| Normal (6-12 U/g Hb) | 85 |
| Low (<6 U/g Hb) | 3 |
Six blood cultures yielded positive results. There were two incidences of Acinetobacter and two of E. coli sepsis (grew in repeated cultures with evidence of sepsis in the patient). The other two grew Gram negative cocci which were probably contaminants (Table 7).
Table 7
| Culture reports | Cases |
| Sterile | 147 |
| Acinetobacter | 2 |
| E. coli | 2 |
| Gram negative cocci | 2 |
| Not available | 30 |
Fourty-eight cases (26 %) required ICU care (Table 8). The mortality rate observed during the course of this study was 12.7%. The following were the causes of death found in the present study: 9 were due to multi organ dysfunction syndrome, severe metabolic acidosis. Three were due to isolated ARDS. One case of death was in a pregnant lady with high parasitemia due to ARDS and multi organ dysfunction syndrome.
Table 8
| ICU requirement | Cases | |
| Dialysis | 11 | |
| Inotropes | 27 | |
| Ventilation | Venturi | 15 |
| Non invasive ventilation | 5 | |
| Invasive ventilation | 10 | |
| ARDS | 6 | |
| Altered sensorium | 17 | |
| MODS | 9 |
4. Discussion
This study was conducted on a prospective basis from May 2009 to January 2011. A total of 183 cases of Plasmodium falciparum were taken into account. The male group showed an increase in incidence (78%) as compared to the female group. And 54% of the cases were between 21 to 50 years of age, with a peak of 22.5% patients in the 2nd decade of life.
The male sex group showed an increase in incidence (78%) probably due to their occupation and hence proximity to vector contact. The relationship between age and death was not significant with P=0.216. One reason may be that children were not included and geriatric cases who are relatively immunosuppressed were less in number. Sex and age affect the incidence of falciparum malaria infection as they relate to the frequency of exposure and the development of immunity[10].
Falciparum malaria is equally distributed in all classes of society. As our population at Manipal is predominantly a student community a significant number of cases was from the same group. Quite a number of people who work indoors such as housewives were found to contract the disease. The nature of work and exposure to vector from outdoors did not play a significant role in the transmission of disease.
All the 181 cases presented with fever as the initial symptom. In a study conducted in the Royal Liverpool university hospital a majority of patients were afebrile through out the illness and presented with only vague constitutional symptoms and later as complications[11].
Seventeen cases (9.28%) patients presented with altered sensorium. Out of these 3 persons went into unarousable coma. The reported incidence of cerebral malaria in most studies is between 2%–55%. All the patients had fever, headache and altered sensorium of variable intensity during presentation. This hospital being a tertiary referral centre, the high incidence of cerebral malaria is expected. The comparison of the clinical profile of cerebral malaria in various studies showed a slightly lesser incidence[12]. The relationship between ESR and cerebral malaria was not significant (P=0.234) as found in other studies[13].
ARDS was the end result in 6 (3.28%) patients of which 3 (1.64%) were put on ventilator.
Twenty-eight cases (33.73 %) had counts more than 15,000. Gram negative sepsis which is thought to be a common event in complicated falciparum was rare in this study[14],[15]. These infections could have occurred due to over zealous use of antibiotics and treatment in outside hospitals. Perhaps the falciparum malaria also made them more prone for the gram negative infections.
Anemia and abnormal bleeding parameters including deranged PT, APTT and thrombocytopenia were almost similar to other studies[16].
The rate of renal failure in our study was 32.7% which was almost similar to those conducted by Wasnik et al and Koni et al. which was 32.5% and 45% respectively[6],[7]. The correlation between parasitic index and urea and creatinine values was significant according to Pearson's formula with P<0.000. In a similar study conducted in Indonesia, parasitic index was correlating with urea levels (P<0.05) and not correlating with creatinine levels (P>0.05)[17]–[19]. The relation between creatinine and death was significant with P<000.1 [17].
The following main conclusions were obtained from this study. Ninety-three (50.8%) cases had severe malaria according to the WHO criteria indicating most infections present with complications. The incidence of Gram negative sepsis was less. High parasite index and abnormal renal function tests are predictors of mortality and complications of disease.
Early diagnosis, anticipation of complications, close monitoring of vital parameters and combination therapy to over come drug resistance perhaps helps to contain the extent of mortality.
Acknowledgments
In the course of this study I have benefited greatly from many people, without their help this study would not have been possible. I would like to convey my heartfelt gratitude to all of them for their kind support. I take this opportunity to express my feelings of gratitude to my esteemed teachers. To learn and associate with such great people is a matter of honor and privilege.
I would first like to dedicate my work to the late Dr. Nagaraja MV, who was my first teacher at medical school and was a great inspiration to me during my initiation at college.
I would like to express my sincere regards and gratitude to my guide, Dr. Shivashankara KN, for his motivation and guidance during this study. His valuable suggestions were of immense help in conducting my work.
I thank heads of all the units of medicine department for having permitted me to include their patients and also all the patients, patient's informants, who willingly participated in my study. I thank my family, friends and last but not least the almighty for making this possible.
This study was supported by Department of Medicine, KMC Manipal with the college number MCI-251(22)/2010-Med./44235.
Notes
Comments
Background
Malaria plays a major role in the history of mankind and has been a problem in India for centuries. Malaria continues to wreak havoc on millions, particularly in the poorest parts of the world. Malaria has transmission in 107 countries containing 3 billion people and causing 1-3 million deaths each year. It is widespread in tropical and subtropical regions, including much of Sub-Saharan Africa, Asia and the Americas. Most deaths among the infected are caused by falciparum malaria.
Research frontiers
A total of 183 inpatients of Kasturba Hospital, Manipal aged above 18 years who were positive for Plasmodium falciparum were included in this study. This was conducted as a prospective study from May 2009 to January 2011 at Kasturba Hospital, Manipal, Karnataka, India. The hospital is a tertiary referral centre is situated in an endemic area for malaria. The methods used for diagnosis were immunofluoroscence (falcivax), QBC and peripheral smear.
Related reports
In this study, 181 cases presented with fever as the initial symptom. However, in a study conducted in the Royal Liverpool University Hospital, majority of patients were afebrile throughout the illness and presented with only vague constitutional symptoms and later as complications. The comparison of the clinical profile of cerebral malaria in Laman et al. (2013) showed a slightly lesser incidence. The relationship between ESR and cerebral malaria was not significant as found in Ebuehi et al. (2012) study. The rate of renal failure in the present study was 32.7% which was almost similar to those conducted by Wasnik et al. (2012) and Koni et al. (2008) which were 32.5% and 45%, respectively.
Innovations and breakthroughs
This study reported that 50.8% of the cases had severe malaria according to the WHO criteria indicating most infections present with complications. The incidence of Gram negative sepsis was less. High parasite index and abnormal renal function tests are predictors of mortality and complications of disease. Early diagnosis, anticipation of complications, close monitoring of vital parameters and combination therapy to overcome drug resistance perhaps helps to contain the extent of mortality.
Applications
It may be significant to know the various clinical manifestations, lab parameters and outcomes of Plasmodium falciparum infection and correlate with the parasitic index as a predictor of outcome and mortality.
Peer review
This is a well-written manuscript on falciparum malaria in an endemic area of India. The results are interesting and suggested that 93 cases had severe malaria high parasite index and abnormal renal function tests are predictors of mortality and complications of disease.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
Articles from Asian Pacific Journal of Tropical Biomedicine are provided here courtesy of China Humanity Technology Publishing House
Citations & impact
Impact metrics
Article citations
Diagnostic tools used in the evaluation of acute febrile illness in South India: a scoping review.
BMC Infect Dis, 19(1):970, 13 Nov 2019
Cited by: 19 articles | PMID: 31722678 | PMCID: PMC6854686
Review Free full text in Europe PMC
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Study of clinical profile of falciparum malaria in a tertiary referral centre in Central India.
J Assoc Physicians India, 60:33-36, 01 Oct 2012
Cited by: 5 articles | PMID: 23777023
The changing spectrum of severe falciparum malaria: a clinical study from Bikaner (northwest India).
J Vector Borne Dis, 43(3):104-108, 01 Sep 2006
Cited by: 19 articles | PMID: 17024858
Complicated falciparum Malaria in western Maharashtra.
Trop Parasitol, 2(1):49-54, 01 Jan 2012
Cited by: 3 articles | PMID: 23507667 | PMCID: PMC3593511
Management of severe and complicated malaria.
J Postgrad Med, 52(4):281-287, 01 Oct 2006
Cited by: 21 articles | PMID: 17102547
Review