Abstract
Free full text

Flavocoxid, a Nutraceutical Approach to Blunt Inflammatory Conditions
Abstract
Flavonoids, from Scutellaria baicalensis (Chinese skullcap) and Acacia catechu (black catechu), have been shown to exert a variety of therapeutic effects, including anti-inflammatory, antiviral, antibacterial, and anticancer activities. Flavocoxid is a mixed extract containing baicalin and catechin and it acts as a dual balanced inhibitor of cyclooxygenase-1 (COX-1) and COX-2 peroxidase enzyme activities with a significant inhibition of 5-lipoxygenase (5-LOX) enzyme activity in vitro. Flavocoxid downregulates gene or protein expression of several inflammatory markers and exerts also strong antioxidant activity in several experimental models. Controlled clinical trials and a postmarketing study have clearly shown that flavocoxid is as effective as naproxen in managing the signs and symptoms of osteoarthritis of the knee and it has better upper gastrointestinal, renal, and respiratory safety profile than naproxen. Flavocoxid may therefore provide a potential therapeutic approach to the treatment of chronic inflammatory conditions.
1. Introduction
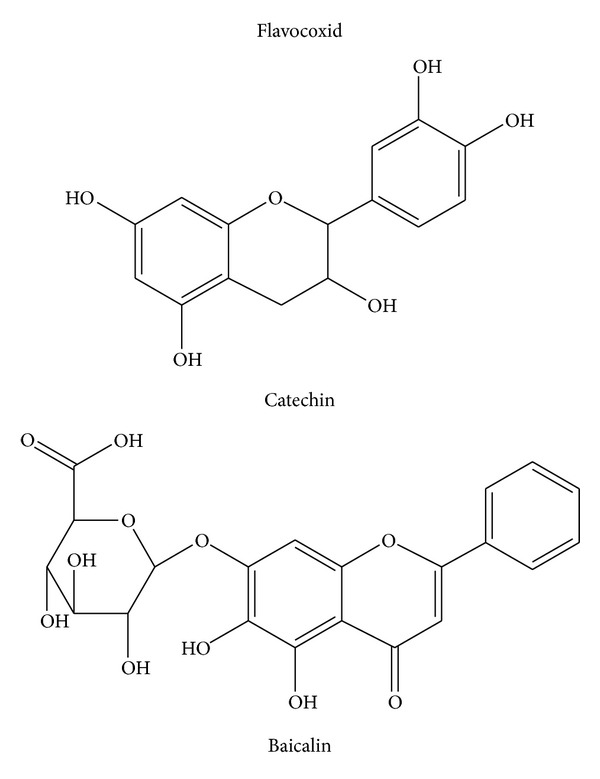
Inflammation is considered the main response of the body evoked to deal with injuries and its hallmarks include swelling, redness, pain, and fever [1]. Several mediators regulate the events of acute inflammation, influence vascular changes, and provoke inflammatory cell recruitment [1–3]. The inflammatory response is a complex self-limiting process precisely regulated to prevent extensive damage of the host. When the self-limiting nature of this protective mechanism is inappropriately regulated, it is transformed to a detrimental chronic state of inflammation. Several diseases are associated with chronic inflammation, including osteoarthritis, atherosclerosis, diabetes, obesity, Crohn's disease, and cancer [1–3]. Although the today used steroidal anti-inflammatory drugs (SAID) and nonsteroidal anti-inflammatory drugs (NSAIDs) effectively manage the acute inflammatory reaction, in chronic inflammatory states the long-term treatment is followed by severe adverse effects. This justifies the search for innovative and safe anti-inflammatory agents. Attractive research candidates are plant constituents. Recently, there has been interest in the potential of flavonoids to modulate the chronic inflammatory reaction. Flavonoids are major constituents of fruits, vegetables, and beverages, such as wine, tea, cocoa, and fruit juices. Flavonoids have a similar structure consisting of two aromatic rings (A and B), which are bound together by three carbon atoms, forming an oxygenated heterocycle (ring C; Figure 1). According to changes in the chemical structure, flavonoids are divided into seven classes: flavonols, flavones, flavanones, flavanonols, flavanols, anthocyanidins, and isoflavones [4]. The flavanols, also referred to as flavan-3-ols, are mainly present in green and black tea, red wine, chocolate, and fruits such as apples, grapes, and strawberries. Typical dietary flavanols include catechin, epicatechin, epigallocatechin (EGC), and epigallocatechin gallate (EGCG). Flavones, such as apigenin and luteolin, are found in parsley, chives, artichoke, and celery. Dietary flavanones include naringenin, hesperetin, and taxifolin and are found mainly in citrus fruit and tomatoes. Finally, isoflavones such as genistein and daidzein are a subclass of the flavonoids family found in soy and soy products. They have a large structural variability and more than 600 isoflavones have been identified to date and are classified according to oxidation level of the central pyran ring (Figure 1). Acacia catechu, also called black catechu, is a traditional medicinal plant commonly used in Indian subcontinent and Southeast Asia, with antipyretic, antidiarrheal, hypoglycaemic, and hepatoprotective activities [5, 6]. The major chemical components in an Acacia catechu extract are catechin and epicatechin-3-O-gallate. Catechin reduces inflammation and blunts the production of proinflammatory cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α), as demonstrated in rats with experimental arthritis [7]. Scutellaria baicalensis, a traditional Chinese herbal medicine, also called Chinese skullcap, has been used to treat respiratory inflammatory diseases [8–12], viral infections [13–15], cardiovascular diseases [16–18], and cancer [19, 20]. Baicalin, extracted by Scutellaria baicalensis, inhibits cyclooxygenase COX-1 and COX-2 peroxidase and 5-lipoxygenase (5-LOX) enzyme activities, decreases production of proinflammatory eicosanoids, and attenuates edema in an in vivo model of inflammation [21]. The anti-inflammatory activity of baicalin was also associated with a binding inhibition of chemokines, such as macrophage inflammatory protein (MIP)-1β, monocyte chemotactic protein-2 (MCP-2), causing a reduced capacity of the chemokines to induce cell migration [21]. The flavonoids, baicalin and catechin, modulate the activities of arachidonic acid, metabolizing enzymes involved in this pathway, and iNOS (NO producing enzyme). Flavocoxid is a mixture of the flavonoid molecules catechin, from Acacia catechu, and baicalin, extracted from Scutellaria baicalensis, concentrated to greater than 90% purity (Figure 2). Thanks to its components flavocoxid, in addition to anti-inflammatory properties, may also act as an antioxidant, reducing reactive oxygen species including hydroxyl radical, superoxide anion radical, and hydrogen peroxide. The combined effect of the 2 flavonoids is greater than the isolated molecules, as shown in a recent paper [22]. As a consequence of NF-κB modulation, flavocoxid reduces COX-2, 5-LOX, iNOS, and TNFα production, and it also blunts the formation of COX-2, 5-LOX, and iNOS metabolites, as PGE2, LTB4, and nitrates [22–24]. These effects provide a rationale for the use of a dual inhibitor in acute and chronic inflammatory conditions. Flavocoxid, marketed as Limbrel in the USA, is a USFDA-regulated prescription, and it is expected to have significant therapeutic efficacy in the managing of chronic inflammation. This review will focus on the preclinical pharmacology, toxicology, and clinical pharmacology of this new attractive compound.
2. Preparation of Flavocoxid
Flavocoxid is prepared from roots of Scutellaria baicalensis and Acacia catechu (US patent number 7,514,469). The roots of Scutellaria baicalensis are extracted with 70% ethanol and then recrystallized with an ethanol/water solvent [23]. The Scutellaria baicalensis extract contains baicalin as the major component and additional minor free-B-ring flavonoids. In the roots of Acacia catechu, (+)-catechin is the major component at a content of 80%. Minor amount of its enantiomer epicatechin and other minor amounts of flavans are present [23]. Analysis of the extracts is carried out separately by high performance liquid chromatography with photodiode array detector (HPLC/PDA) and liquid chromatography-mass spectrometry (LC/MS) and indicates a major compound, baicalin, from Scutellaria baicalensis extract and (+)-catechin from Acacia catechu extract by comparison with known standards. The presence of these compounds is then confirmed by carbon nuclear magnetic resonance (13C-NMR) and proton nuclear magnetic resonance (1H-NMR) analysis, respectively. The final flavocoxid formulation (Figure 2) is a mixture of >90% purified baicalin and catechin with the remainder being excipient (5-6%) and water (3%). Confirmation of the combined flavonoids content can be obtained by HPLC analysis. Both flavonoids are detected using UV detector at 275 nm and identified based on retention time by comparison with known flavonoids standards [23]. These ingredients are generally recognized as safe (GRAS). For an ingredient to be recognized as GRAS by the US Food and Drug Administration (FDA), it requires technical demonstration of nontoxicity and safety, general recognition of safety through widespread usage, and agreement of that safety by experts in the field.
nm and identified based on retention time by comparison with known flavonoids standards [23]. These ingredients are generally recognized as safe (GRAS). For an ingredient to be recognized as GRAS by the US Food and Drug Administration (FDA), it requires technical demonstration of nontoxicity and safety, general recognition of safety through widespread usage, and agreement of that safety by experts in the field.
3. Effects of Flavocoxid on Arachidonic Acid Formation and Metabolism
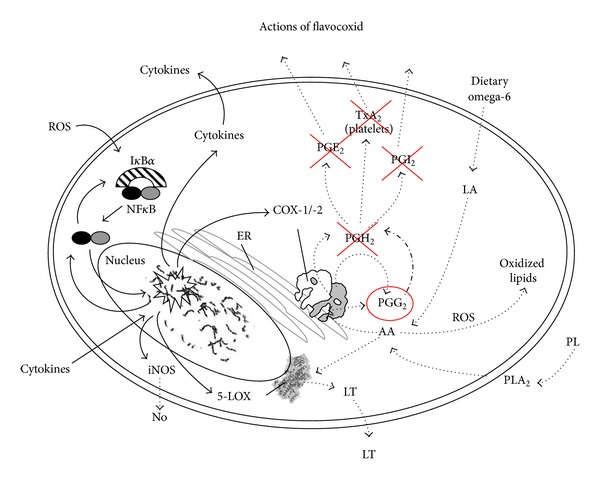
A series of in vitro and in vivo experiments have been carried out in order to dissect out the exact mechanism of action of flavocoxid. First of all, flavocoxid was tested in peritoneal macrophages (MΦ) stimulated with lipopolysaccharide (LPS) to investigate a possible effect on phospholipase A2 (PLA2) activity [24]. Flavocoxid did not significantly modify cell viability at 200 and 500 μg/mL, but it markedly inhibited PLA2 activity (IC50 = 60
μg/mL, but it markedly inhibited PLA2 activity (IC50 = 60 μg/mL) at doses of 50, 100, 200, and 500
μg/mL) at doses of 50, 100, 200, and 500 μg/mL [24]. This finding indicates that flavocoxid modulates the generation of AA from membrane phospholipids caused by tissue damage during chronic inflammation. The effects of flavocoxid on COX-1 and COX-2 enzyme activities were also investigated in dedicated in vitro enzyme assays [24]. COX proteins have two different enzymatic moieties for AA metabolism: the cyclooxygenase (CO) one and the peroxidase one (PO). The CO activity converts AA to PGG2 and the PO activity transforms PGG into PGH2. Finally, cell synthases and isomerases convert PGH2 to thromboxanes (TXB), prostaglandins (PG), and prostacyclin (PGI). Experiments were carried out to investigate the specific inhibitor effects of flavocoxid on CO and PO enzyme moieties of either COX-1 or COX-2. The compound had no significant anti-CO COX-2 activity up to 50
μg/mL [24]. This finding indicates that flavocoxid modulates the generation of AA from membrane phospholipids caused by tissue damage during chronic inflammation. The effects of flavocoxid on COX-1 and COX-2 enzyme activities were also investigated in dedicated in vitro enzyme assays [24]. COX proteins have two different enzymatic moieties for AA metabolism: the cyclooxygenase (CO) one and the peroxidase one (PO). The CO activity converts AA to PGG2 and the PO activity transforms PGG into PGH2. Finally, cell synthases and isomerases convert PGH2 to thromboxanes (TXB), prostaglandins (PG), and prostacyclin (PGI). Experiments were carried out to investigate the specific inhibitor effects of flavocoxid on CO and PO enzyme moieties of either COX-1 or COX-2. The compound had no significant anti-CO COX-2 activity up to 50 μg/mL. In addition, flavocoxid showed CO COX-1 IC50 of 25
μg/mL. In addition, flavocoxid showed CO COX-1 IC50 of 25 μg/mL, while indomethacin has a CO COX-1 IC50 of 0.012
μg/mL, while indomethacin has a CO COX-1 IC50 of 0.012 μg/mL, thus suggesting that flavocoxid has little anti-CO activity on both COX enzymes compared to well-known anti-inflammatory agents. Imbalances in COX-2 versus COX-1 inhibition caused by selective COX-2 lead to production of several AA metabolites responsible for an enhanced risk of edema, hypertension, and myocardial infarction [25]. Flavocoxid produced a balanced inhibition of both COX-1 and COX-2 PO activities, with IC50 of 12.3 and 11.3
μg/mL, thus suggesting that flavocoxid has little anti-CO activity on both COX enzymes compared to well-known anti-inflammatory agents. Imbalances in COX-2 versus COX-1 inhibition caused by selective COX-2 lead to production of several AA metabolites responsible for an enhanced risk of edema, hypertension, and myocardial infarction [25]. Flavocoxid produced a balanced inhibition of both COX-1 and COX-2 PO activities, with IC50 of 12.3 and 11.3 μg/mL, respectively. This clearly indicates that flavocoxid exerts its activity via modulation of the PO activity of these enzymes. Currently available NSAIDs and COX-2 inhibitors do not influence the 5-LOX pathway and therefore do not block the production of the dangerous leukotrienes (LTs) that cause vasoconstriction and leukocyte attraction and accumulation. Furthermore COX-1 and COX-2 blockade by NSAIDs or selective COX-2 inhibitors causes a shunting of the AA metabolism towards the 5-LOX pathway, thus inducing an overproduction of these fatty acid mediators which can cause multiple organ damage [26]. Enhanced levels of LTs are also measured in a variety of pathological conditions such as asthma, gastric ulceration, renal insufficiency, and cardiovascular complications [27, 28]. Flavocoxid was incubated along with purified 5-LOX enzyme in the presence of an oxygen sensing chromagen in vitro to study the formation of unstable hydroperoxyeicosatetraenoic acids (HPETEs) intermediates in the synthesis of LTs. Flavocoxid inhibited the 5-LOX enzyme showing an IC50 of 110
μg/mL, respectively. This clearly indicates that flavocoxid exerts its activity via modulation of the PO activity of these enzymes. Currently available NSAIDs and COX-2 inhibitors do not influence the 5-LOX pathway and therefore do not block the production of the dangerous leukotrienes (LTs) that cause vasoconstriction and leukocyte attraction and accumulation. Furthermore COX-1 and COX-2 blockade by NSAIDs or selective COX-2 inhibitors causes a shunting of the AA metabolism towards the 5-LOX pathway, thus inducing an overproduction of these fatty acid mediators which can cause multiple organ damage [26]. Enhanced levels of LTs are also measured in a variety of pathological conditions such as asthma, gastric ulceration, renal insufficiency, and cardiovascular complications [27, 28]. Flavocoxid was incubated along with purified 5-LOX enzyme in the presence of an oxygen sensing chromagen in vitro to study the formation of unstable hydroperoxyeicosatetraenoic acids (HPETEs) intermediates in the synthesis of LTs. Flavocoxid inhibited the 5-LOX enzyme showing an IC50 of 110 μg/mL. Phenidone, a well-known 5-LOX inhibitor used as a positive control in theses assays, had an IC50 of 1.3
μg/mL. Phenidone, a well-known 5-LOX inhibitor used as a positive control in theses assays, had an IC50 of 1.3 μg/mL. No other NSAIDs or selective COX-2 inhibitors, including rofecoxib, valdecoxib, diclofenac, meloxicam, and aspirin, displayed an anti-5-LOX activity. All these findings, taken together, indicate that flavocoxid also reduces the production of LTBs from 5-LOX and may avoid the deleterious accumulation of these lipid mediators caused by the NSAIDs-induced 5-LOX shunt. Nonenzymatic lipid peroxidation is another important pathway of the AA metabolism. Reactive oxygen species (ROS) may react with exaggerated AA levels leading to the production of F2-isoprostanes and 4-hydroxynonenal (HNE) together with enhanced malondialdehyde levels. All these markers of oxidative stress are elevated during chronic inflammation and contribute to the pathological cascade leading to organ damage and dysfunction. Therefore, it is relevant for an anti-inflammatory agent to possess an antioxidant effect. Experiments were carried out to analyze this issue, and the in vitro antioxidant activity of flavocoxid was evaluated using oxygen radical absorbance capacity (ORAC) procedures and was compared with that of well-known antioxidants such as vitamin C and vitamin E. The ORAC analysis provides a measure of the scavenging capacity of antioxidants against the peroxyl radical. Trolox, a water-soluble vitamin E analog, is used as the calibration standard, and the ORAC result is expressed as μmolTE/g dry weight. The ORACtotal for flavocoxid (3719
μg/mL. No other NSAIDs or selective COX-2 inhibitors, including rofecoxib, valdecoxib, diclofenac, meloxicam, and aspirin, displayed an anti-5-LOX activity. All these findings, taken together, indicate that flavocoxid also reduces the production of LTBs from 5-LOX and may avoid the deleterious accumulation of these lipid mediators caused by the NSAIDs-induced 5-LOX shunt. Nonenzymatic lipid peroxidation is another important pathway of the AA metabolism. Reactive oxygen species (ROS) may react with exaggerated AA levels leading to the production of F2-isoprostanes and 4-hydroxynonenal (HNE) together with enhanced malondialdehyde levels. All these markers of oxidative stress are elevated during chronic inflammation and contribute to the pathological cascade leading to organ damage and dysfunction. Therefore, it is relevant for an anti-inflammatory agent to possess an antioxidant effect. Experiments were carried out to analyze this issue, and the in vitro antioxidant activity of flavocoxid was evaluated using oxygen radical absorbance capacity (ORAC) procedures and was compared with that of well-known antioxidants such as vitamin C and vitamin E. The ORAC analysis provides a measure of the scavenging capacity of antioxidants against the peroxyl radical. Trolox, a water-soluble vitamin E analog, is used as the calibration standard, and the ORAC result is expressed as μmolTE/g dry weight. The ORACtotal for flavocoxid (3719 μmolTE/g) was significantly greater than that of either vitamin C (2000
μmolTE/g) was significantly greater than that of either vitamin C (2000 μmolTE/g) or vitamin E (1100
μmolTE/g) or vitamin E (1100 μmolTE/g). Flavocoxid showed also high values for the ferric reducing/antioxidant power (FRAP), the peroxynitrite radical averting capacity (NORAC), and the Trolox equivalent antioxidant capacity (TEAC), thus clearly showing that this compound exerts a strong antioxidant activity [24].
μmolTE/g). Flavocoxid showed also high values for the ferric reducing/antioxidant power (FRAP), the peroxynitrite radical averting capacity (NORAC), and the Trolox equivalent antioxidant capacity (TEAC), thus clearly showing that this compound exerts a strong antioxidant activity [24].
4. Effects of Flavocoxid on Proinflammatory Gene Expression
The effects of flavocoxid on gene and protein expression of inflammatory markers were studied in rat peritoneal macrophages stimulated with Salmonella enteritidis LPS [29]. In fact it is worthy of interest to identify for an anti-inflammatory agent a mechanism of action at the level of inflammatory gene and protein expression. Peritoneal macrophages had a constitutive expression of COX-1 and LPS did not modify it. By contrast LPS stimulation of peritoneal macrophages resulted in a marked increase in both COX-2 and 5-LOX expression [29]. Flavocoxid attenuated, in a concentration dependent manner, the increase in COX-2 and 5-LOX expression [29]. Together with LPS-induced COX-2 and 5-LOX activation, the generation of PGE2 and LTB4 was markedly augmented. Flavocoxid significantly reduced the increase in PGE2 and LTB4. In addition, LPS-primed macrophages had an enhanced mRNA expression for iNOS and augmented nitrate content; flavocoxid significantly blunted, in a concentration dependent manner, the increase in iNOS and nitrate production. These results [22, 24, 29] suggest that flavocoxid inhibits COX-2, 5-LOX, and iNOS gene activation and abrogate the biosynthesis of related inflammatory mediators. Among these mediators an important role is played by TNF-α, a pleiotropic proinflammatory cytokine [30], markedly induced in peritoneal macrophages stimulated with LPS. Flavocoxid caused a significant and concentration dependent reduction in the levels of TNF-α mRNA and in the formation of the mature protein. Nuclear factor kappa-B (NF-κB) plays a prominent role in the inflammatory cascade [31]; it is an important transcription factor complex that regulates the expression of several genes involved in immune and inflammatory responses during chronic human diseases [32, 33]. In unstimulated cells, NF-κB is constitutively localized in the cytosol as a heterodimer by physical association with an inhibitory protein, called IκBα. Following activation, the NF-κB heterodimer is rapidly translocated to the nucleus where it activates the transcription of target genes, including the genes encoding for proinflammatory cytokines, adhesion molecules, chemokines, and inducible enzymes (such as COX-2, 5-LOX, and iNOS). Flavocoxid was shown to reduce IκBα loss from the cytoplasm and to blunt NF-κB binding to DNA in LPS-stimulated macrophages [29]. Therefore flavocoxid, acts at gene and protein expression level through NF-κB activity inhibition, blocking the auto-amplifying loop during the inflammatory response.
5. Effects of Flavocoxid on Experimental Models of Inflammation
Flavocoxid was studied in animal experimental models of inflammation aimed to test its efficacy. In a first experiment, collagen-induced arthritis (CIA) was induced in DBA/1 mice by an intradermal injection of an emulsion containing bovine type II collagen in complete Freund's adjuvant. CIA animals were then randomized to receive vehicle or flavocoxid (20 mg/kg) and the treatment lasted 45 days (Figure 3, data on file). Flavocoxid reduced PGE2 and LTB4 levels, significantly ameliorated the clinical signs of arthritis, improved the histological damage, decreased the cartilage expression and the circulating levels of several markers of severity disease including TNF-α, IL-6, high mobility group box-1 (HMBG-1), and also caused an enhanced expression of the anti-inflammatory cytokine IL-10. Interestingly, flavocoxid positively modulated the balance between receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG), a cytokine system involved in bone and cartilage remodeling. Collectively these experimental findings demonstrate that flavocoxid is a valuable therapeutic agent in the treatment of inflammatory conditions, including arthritis and osteoarthritis.
mg/kg) and the treatment lasted 45 days (Figure 3, data on file). Flavocoxid reduced PGE2 and LTB4 levels, significantly ameliorated the clinical signs of arthritis, improved the histological damage, decreased the cartilage expression and the circulating levels of several markers of severity disease including TNF-α, IL-6, high mobility group box-1 (HMBG-1), and also caused an enhanced expression of the anti-inflammatory cytokine IL-10. Interestingly, flavocoxid positively modulated the balance between receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG), a cytokine system involved in bone and cartilage remodeling. Collectively these experimental findings demonstrate that flavocoxid is a valuable therapeutic agent in the treatment of inflammatory conditions, including arthritis and osteoarthritis.

Effects of flavocoxid in a collagen-induced arthritis model. # P < 0.001 versus ctrl; *P < 0.005 versus CIA + vehicle.
The dual inhibitor was also tested in acute inflammatory diseases such as acute pancreatitis, an autodigestive inflammatory disease, that causes acinar cell damage and finally culminates in hemorrhagic necrosis of the pancreas and eventually multiple organ failure [34]. A large body of evidence suggests that upregulation of inflammatory mediators, including COX-2, 5-LOX, cytokines, and chemokines, orchestrates this pathological process [35]. Acute pancreatitis can be induced in rats by injection of cerulein, a secretagogue agent. Flavocoxid was investigated for its effects in cerulein-induced pancreatitis [36] at a dose of 20 mg/kg; it inhibited COX-2 and 5-LOX expression and reduced serum levels of lipase and amylase and the degree of pancreatic edema. Administration of flavocoxid also blunted the increased pancreatic TNF-α mRNA expression, serum LTB4 and PGE2 levels, and protected against histological damage in terms of vacuolization and leukocyte infiltration. These interesting findings may provide a potential therapeutic approach to the treatment of patients at high risk of developing this life-threatening condition.
mg/kg; it inhibited COX-2 and 5-LOX expression and reduced serum levels of lipase and amylase and the degree of pancreatic edema. Administration of flavocoxid also blunted the increased pancreatic TNF-α mRNA expression, serum LTB4 and PGE2 levels, and protected against histological damage in terms of vacuolization and leukocyte infiltration. These interesting findings may provide a potential therapeutic approach to the treatment of patients at high risk of developing this life-threatening condition.
Flavocoxid was also tested in a degenerative chronic disease, Duchenne muscle dystrophy (DMD), a progressive muscle-wasting disease leading to death, usually in early adulthood [37]. The disease results from absence of the protein dystrophin, which is an essential component of the dystrophin-glycoprotein complex that maintains membrane integrity of muscle fibers by linking cytoskeleton to extracellular matrix. Muscle degeneration in muscle dystrophy is exacerbated by the endogenous inflammatory response and increased oxidative stress, and NF-κB plays a pivotal role in orchestrating this inflammatory cascade [38, 39]. The effects of flavocoxid were studied in a comparison study with methylprednisolone, the gold standard treatment for DMD patients, using the mdx mice, the murine model of DMD [37]. Five-week-old mdx mice were treated for 5 weeks with flavocoxid, methylprednisolone, or vehicle. Flavocoxid was more effective than methylprednisolone in ameliorating functional properties both in vivo and in vitro; in reducing serum creatine kinase (CK), a marker of muscle necrosis; in blunting the expression of oxidative stress markers and inflammatory mediators. Furthermore more efficiently than methylprednisolone, flavocoxid inhibited NF-κB and mitogen-activated protein kinases (MAPKs) signal pathways, decreased muscle necrosis, and augmented muscle regeneration [37]. This experiment suggests that flavocoxid counteracts the chronic inflammatory cascade that contributes to muscle necrosis and degeneration in DMD showing a degree of activity higher than that of methylprednisolone. Since methylprednisolone possesses also important side effects, flavocoxid with a better safety-efficacy profile might represent a valuable alternative in the treatment of DMD. However this hypothesis deserves to be confirmed in a clinical setting.
In another experimental model of chronic inflammation, benign prostatic hyperplasia (BPH), we tested the effects of flavocoxid since COX and 5-LOX are significantly elevated in the overgrowing prostate [40]. Therefore a “dual inhibitor” of the COX and 5-LOX enzymes might be of benefit in this disease. Rats were treated, daily, with testosterone propionate (3 mg/kg/sc) or its vehicle for 14 days. Testosterone administered animals were randomized to receive vehicle (1
mg/kg/sc) or its vehicle for 14 days. Testosterone administered animals were randomized to receive vehicle (1 mL/kg, ip) or flavocoxid (20
mL/kg, ip) or flavocoxid (20 mg/kg, ip) for 14 days. Flavocoxid reduced prostate weight and hyperplasia, blunted the augmented expression of COX-2 and 5-LOX as well as the increased production of PGE2 and LTB4, enhanced the proapoptotic Bax and caspase-9, and decreased the antiapoptotic Bcl-2 mRNA. Flavocoxid reduced also the epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) expression. The data obtained from this study would indicate that a “dual inhibitor” of the COX and 5-LOX enzymes, such as flavocoxid, might represent a rationale therapeutic approach to reduce benign prostate growth [40].
mg/kg, ip) for 14 days. Flavocoxid reduced prostate weight and hyperplasia, blunted the augmented expression of COX-2 and 5-LOX as well as the increased production of PGE2 and LTB4, enhanced the proapoptotic Bax and caspase-9, and decreased the antiapoptotic Bcl-2 mRNA. Flavocoxid reduced also the epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) expression. The data obtained from this study would indicate that a “dual inhibitor” of the COX and 5-LOX enzymes, such as flavocoxid, might represent a rationale therapeutic approach to reduce benign prostate growth [40].
6. Toxicity Studies on Flavocoxid
A toxicological testing of the relative pure combination of baicalin and (+)-catechin has been performed in vitro and in experimental animals [41, 42]. THP-1 cells, a human immortalized monocyte cell line, were used to assess cytotoxicity in an LDH assay. When cells were grown to confluent monolayer and then exposed to increasing concentration of several NSAIDs, celecoxib, and the baicalin/(+)-catechin combination, only indomethacin and celecoxib showed significant cytotoxicity above 50 μg/mL. Flavocoxid showed low cytotoxicity at the highest test concentration (100
μg/mL. Flavocoxid showed low cytotoxicity at the highest test concentration (100 μg/mL) [41]. Acute (2000
μg/mL) [41]. Acute (2000 mg/kg/day for 14 consecutive days) and subchronic (50, 250, and 500
mg/kg/day for 14 consecutive days) and subchronic (50, 250, and 500 mg/kg/day) toxicity study in mice revealed no abnormalities in any toxicological end points examined including animal body weight, gross organ pathology and tissue histology, and blood chemistry or serology [42]. Flavocoxid was also administered in Fisher 344 rats, a model for gastric toxicity of NSAIDs, and showed no sign of ulceration [41]. The baicalin/(+)-catechin combination was tested at a 10
mg/kg/day) toxicity study in mice revealed no abnormalities in any toxicological end points examined including animal body weight, gross organ pathology and tissue histology, and blood chemistry or serology [42]. Flavocoxid was also administered in Fisher 344 rats, a model for gastric toxicity of NSAIDs, and showed no sign of ulceration [41]. The baicalin/(+)-catechin combination was tested at a 10 μM concentration in a liver microsomal assay with endoplasmic reticulum fractions using spectrophotometric quantization of 7-benzyloxy-4-(trifluoromethyl)-coumarin as the substrate for CYP450 profiling. Flavocoxid showed only moderate inhibition of CYP1A2 (23%) at a 10
μM concentration in a liver microsomal assay with endoplasmic reticulum fractions using spectrophotometric quantization of 7-benzyloxy-4-(trifluoromethyl)-coumarin as the substrate for CYP450 profiling. Flavocoxid showed only moderate inhibition of CYP1A2 (23%) at a 10 μM concentration and low inhibition for all other CYP isoforms (11/16%). The combined extract also showed no mutagenicity in the AMES test [41]. An additional 90-day oral toxicity study was carried out in Hsd
μM concentration and low inhibition for all other CYP isoforms (11/16%). The combined extract also showed no mutagenicity in the AMES test [41]. An additional 90-day oral toxicity study was carried out in Hsd :
: SD rats to determine the potential of the baicalin/(+)-catechin combination to produce gastric toxicity [42]. Flavocoxid was administered at the dose levels of 250, 500, and 1000
SD rats to determine the potential of the baicalin/(+)-catechin combination to produce gastric toxicity [42]. Flavocoxid was administered at the dose levels of 250, 500, and 1000 mg/kg/day. There were no flavocoxid-related adverse events, including mortality, changes in body weight and food consumption, neurological effects (assessed by the Functional Observational Battery and motor activity), organ weight changes, or histopathological alterations. In addition flavocoxid caused no change in sperm count and comparable estrus aging [42]. This study identified a dose of 1000
mg/kg/day. There were no flavocoxid-related adverse events, including mortality, changes in body weight and food consumption, neurological effects (assessed by the Functional Observational Battery and motor activity), organ weight changes, or histopathological alterations. In addition flavocoxid caused no change in sperm count and comparable estrus aging [42]. This study identified a dose of 1000 mg/kg/day/as the no-observed-adverse-effect level (NOAEL). This promising preclinical safety profile encourages the use of flavocoxid in humans.
mg/kg/day/as the no-observed-adverse-effect level (NOAEL). This promising preclinical safety profile encourages the use of flavocoxid in humans.
7. Clinical Evidence Supporting Flavocoxid Efficacy and Safety
A study was designed to compare the effectiveness and safety of flavocoxid to naproxen in subjects with moderate to severe osteoarthritis (OA) of the knee [43, 44]. This was a randomized, multicenter, and double-blind study involving 220 subjects with OA of the knee that were randomized to receive either flavocoxid (500 mg twice daily) or naproxen (500
mg twice daily) or naproxen (500 mg twice daily for 12 weeks). Primary outcome measures included the Western Ontario and McMaster Universities Osteoarthritis index (WOMAC) and subscales and timed walk. More than 90% of the subjects in both groups had significant reduction in the signs and symptoms of OA. No statistical significant difference in efficacy between flavocoxid and naproxen was observed [43, 44]. Flavocoxid treated patients had also significantly fewer upper gastrointestinal, renal, and respiratory adverse events (AEs). These results indicate that flavocoxid is effective as naproxen in the management of OA of the knee and shows a better safety profile than naproxen. An additional study in healthy volunteers found that flavocoxid does not affect the primary or extrinsic pathway of hemostasis and, by not inhibiting the anticoagulation effects of aspirin, may have utility in cardiovascular patients with chronic inflammation [45]. An open-label, postmarketing study (GOAL: Gauging Osteoarthritis [OA] with Limber) was performed to determine the overall efficacy and gastrointestinal tolerability of flavocoxid [46]. A total of 1067 patients at 41 rheumatology practices were enrolled and prescribed flavocoxid 500
mg twice daily for 12 weeks). Primary outcome measures included the Western Ontario and McMaster Universities Osteoarthritis index (WOMAC) and subscales and timed walk. More than 90% of the subjects in both groups had significant reduction in the signs and symptoms of OA. No statistical significant difference in efficacy between flavocoxid and naproxen was observed [43, 44]. Flavocoxid treated patients had also significantly fewer upper gastrointestinal, renal, and respiratory adverse events (AEs). These results indicate that flavocoxid is effective as naproxen in the management of OA of the knee and shows a better safety profile than naproxen. An additional study in healthy volunteers found that flavocoxid does not affect the primary or extrinsic pathway of hemostasis and, by not inhibiting the anticoagulation effects of aspirin, may have utility in cardiovascular patients with chronic inflammation [45]. An open-label, postmarketing study (GOAL: Gauging Osteoarthritis [OA] with Limber) was performed to determine the overall efficacy and gastrointestinal tolerability of flavocoxid [46]. A total of 1067 patients at 41 rheumatology practices were enrolled and prescribed flavocoxid 500 mg b.i.d. for 60 days. The Physician Global Assessment of Disease (PGAD) visual analog scale (VAS) was used as a global measure to assess the signs and the symptoms of OA including joint discomfort, functional stiffness, functional mobility, and quality of life. Furthermore both overall tolerability and upper GI tolerability were assessed by individual questions scored on a 5-part Likert scale. Physicians were also asked to monitor any interruption in or cessation of use of flavocoxid due to the GI symptom as well as changes in the use of gastroprotective medications. A close monitoring of adverse events (AEs) was also carried out. In the 1005 patients who completed all the follow-up visits there was a significant improvement in VAS score. The most important improvement was observed in patients with moderate to severe OA and in those subjects that were historically nonresponders to NSAIDs. A low incidence of o AEs was also observed with a good overall and GI tolerability. In addition the use of flavocoxid resulted in a >30% reduction in or cessation of the gastroprotective medications including proton pump inhibitors or histamine-2 receptor antagonists [46]. Overall this study, even if it was open label and not rigorously controlled, shows that flavocoxid possesses a significant efficacy in the management of OA with a good safety profile [46].
mg b.i.d. for 60 days. The Physician Global Assessment of Disease (PGAD) visual analog scale (VAS) was used as a global measure to assess the signs and the symptoms of OA including joint discomfort, functional stiffness, functional mobility, and quality of life. Furthermore both overall tolerability and upper GI tolerability were assessed by individual questions scored on a 5-part Likert scale. Physicians were also asked to monitor any interruption in or cessation of use of flavocoxid due to the GI symptom as well as changes in the use of gastroprotective medications. A close monitoring of adverse events (AEs) was also carried out. In the 1005 patients who completed all the follow-up visits there was a significant improvement in VAS score. The most important improvement was observed in patients with moderate to severe OA and in those subjects that were historically nonresponders to NSAIDs. A low incidence of o AEs was also observed with a good overall and GI tolerability. In addition the use of flavocoxid resulted in a >30% reduction in or cessation of the gastroprotective medications including proton pump inhibitors or histamine-2 receptor antagonists [46]. Overall this study, even if it was open label and not rigorously controlled, shows that flavocoxid possesses a significant efficacy in the management of OA with a good safety profile [46].
8. Conclusions
Steroidal anti-inflammatory drugs and nonsteroidal anti-inflammatory drugs are effective in the management of the acute inflammatory reaction, but they do not influence successfully chronic inflammatory states and possess severe adverse effects. Flavocoxid is a mixture of the flavonoids molecules catechin, from Acacia catechu, and baicalin, extracted from Scutellaria baicalensis, concentrated to greater than 90% purity. Flavocoxid hind PLA2 causes a balanced inhibition of the COX-1 and COX-2 peroxidase moieties and decreases the generation of LTBs from 5-LOX, avoiding the deleterious accumulation of these lipid mediators caused by the NSAIDs-induced 5-LOX shunt. It has also a strong antioxidant activity, and through NF-κB activity inhibition blocks the amplifying loop of the inflammatory response and acts at the level of gene and protein expression (Figure 4). It exerts beneficial effects in several experimental models of inflammation. In vitro toxicity testing and acute and subchronic toxicity animal studies indicate for flavocoxid an optimal preclinical safety profile. Finally, clinical trials and a postmarketing study show that flavocoxid has a significant efficacy in management of OA and a good overall and GI tolerability. Flavocoxid may therefore provide a potential therapeutic approach to the treatment of acute and chronic inflammatory conditions.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
Articles from Mediators of Inflammation are provided here courtesy of Wiley
Full text links
Read article at publisher's site: https://doi.org/10.1155/2014/790851
Read article for free, from open access legal sources, via Unpaywall:
https://downloads.hindawi.com/journals/mi/2014/790851.pdf
Citations & impact
Impact metrics
Article citations
Molecular mechanisms underlying cyclophosphamide-induced cognitive impairment and strategies for neuroprotection in preclinical models.
Mol Cell Biochem, 479(8):1873-1893, 31 Jul 2023
Cited by: 4 articles | PMID: 37522975 | PMCID: PMC11339103
Review Free full text in Europe PMC
Targeting leukotriene biosynthesis to prevent atherosclerotic cardiovascular disease.
Cond Med, 6(2):33-41, 01 Apr 2023
Cited by: 0 articles | PMID: 38800614 | PMCID: PMC11126214
Potential Utility of Natural Products against Oxidative Stress in Animal Models of Multiple Sclerosis.
Antioxidants (Basel), 11(8):1495, 29 Jul 2022
Cited by: 6 articles | PMID: 36009214 | PMCID: PMC9404913
Review Free full text in Europe PMC
Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects.
Molecules, 27(1):233, 30 Dec 2021
Cited by: 186 articles | PMID: 35011465 | PMCID: PMC8746501
Review Free full text in Europe PMC
A Phase 1/2 Study of Flavocoxid, an Oral NF-κB Inhibitor, in Duchenne Muscular Dystrophy.
Brain Sci, 11(1):115, 16 Jan 2021
Cited by: 7 articles | PMID: 33467104 | PMCID: PMC7830560
Go to all (23) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation.
J Med Food, 10(3):442-451, 01 Sep 2007
Cited by: 85 articles | PMID: 17887937
Flavocoxid, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, blunts pro-inflammatory phenotype activation in endotoxin-stimulated macrophages.
Br J Pharmacol, 157(8):1410-1418, 01 Aug 2009
Cited by: 60 articles | PMID: 19681869 | PMCID: PMC2765324
Flavocoxid is as effective as naproxen for managing the signs and symptoms of osteoarthritis of the knee in humans: a short-term randomized, double-blind pilot study.
Nutr Res, 29(5):298-304, 01 May 2009
Cited by: 28 articles | PMID: 19555810
The metabolic effects of inhibitors of 5-lipoxygenase and of cyclooxygenase 1 and 2 are an advancement in the efficacy and safety of anti-inflammatory therapy.
Prostaglandins Other Lipid Mediat, 71(3-4):147-162, 01 Jul 2003
Cited by: 29 articles | PMID: 14518558
Review