Abstract
Free full text

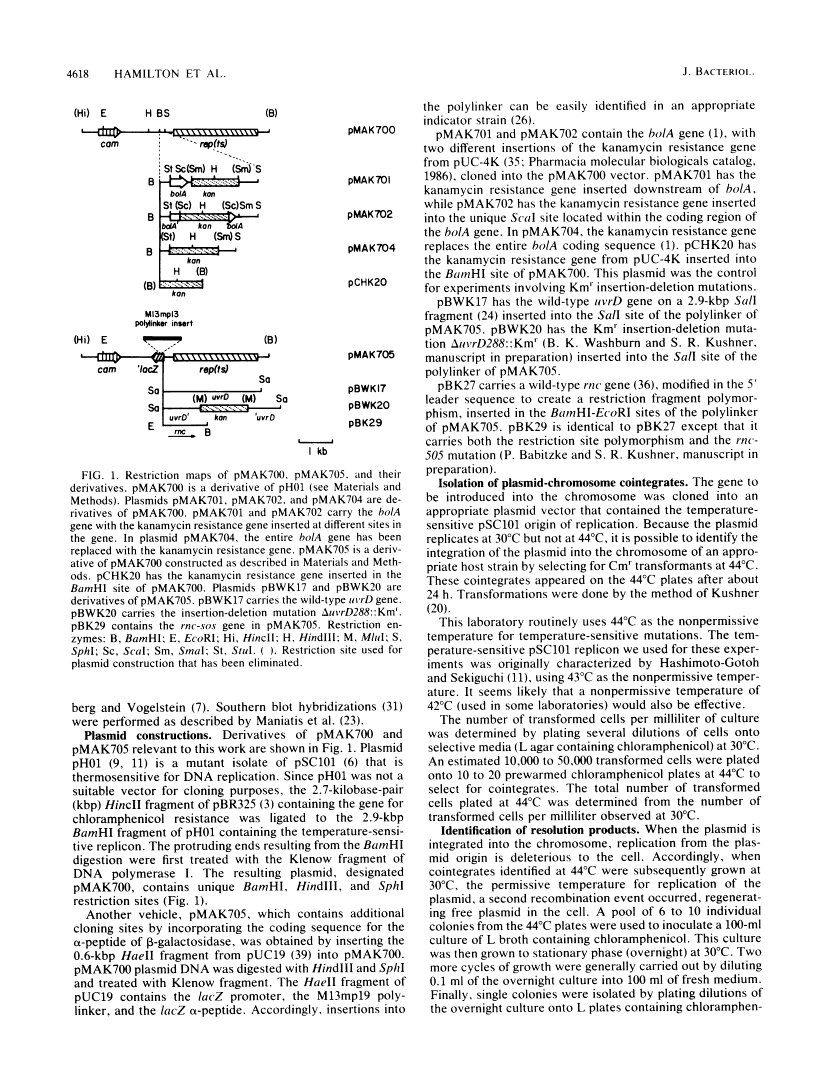
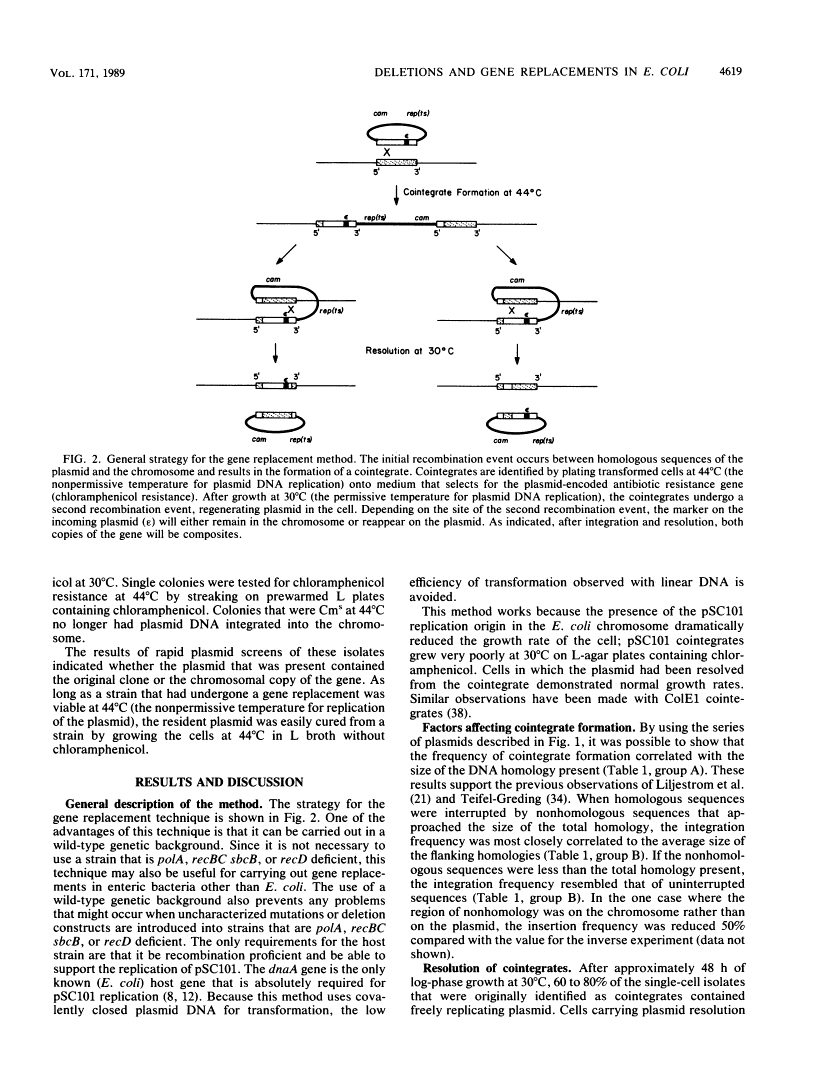
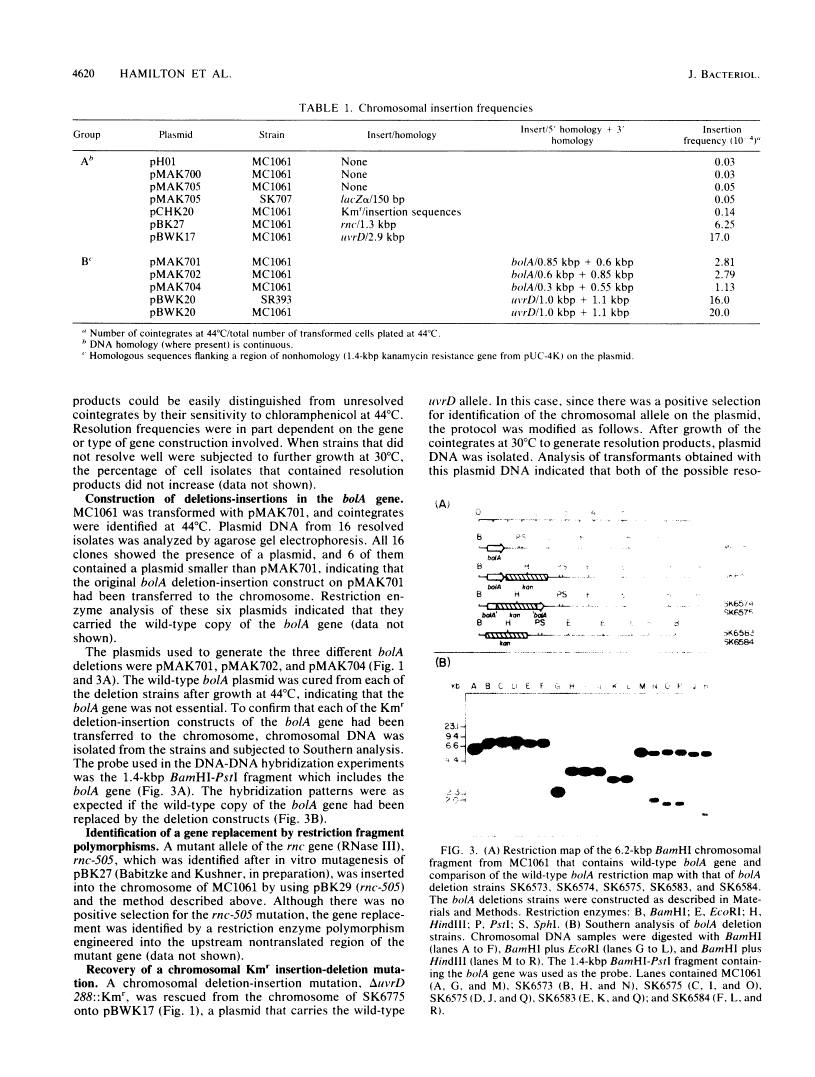
New method for generating deletions and gene replacements in Escherichia coli.
Abstract
We describe a method for generating gene replacements and deletions in Escherichia coli. The technique is simple and rapid and can be applied to most genes, even those that are essential. What makes this method unique and particularly effective is the use of a temperature-sensitive pSC101 replicon to facilitate the gene replacement. The method proceeds by homologous recombination between a gene on the chromosome and homologous sequences carried on a plasmid temperature sensitive for DNA replication. Thus, after transformation of the plasmid into an appropriate host, it is possible to select for integration of the plasmid into the chromosome at 44 degrees C. Subsequent growth of these cointegrates at 30 degrees C leads to a second recombination event, resulting in their resolution. Depending on where the second recombination event takes place, the chromosome will either have undergone a gene replacement or retain the original copy of the gene. The procedure can also be used to effect the transfer of an allele from a plasmid to the chromosome or to rescue a chromosomal allele onto a plasmid. Since the resolved plasmid can be maintained by selection, this technique can be used to generate deletions of essential genes.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldea M, Hernández-Chico C, de la Campa AG, Kushner SR, Vicente M. Identification, cloning, and expression of bolA, an ftsZ-dependent morphogene of Escherichia coli. J Bacteriol. 1988 Nov;170(11):5169–5176. [Europe PMC free article] [Abstract] [Google Scholar]
- Balakrishnan R, Backman K. Controllable alteration of cell genotype in bacterial cultures using an excision vector. Gene. 1988 Jul 15;67(1):97–103. [Abstract] [Google Scholar]
- Balbás P, Soberón X, Merino E, Zurita M, Lomeli H, Valle F, Flores N, Bolivar F. Plasmid vector pBR322 and its special-purpose derivatives--a review. Gene. 1986;50(1-3):3–40. [Abstract] [Google Scholar]
- Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. [Europe PMC free article] [Abstract] [Google Scholar]
- Bochner BR, Huang HC, Schieven GL, Ames BN. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. [Europe PMC free article] [Abstract] [Google Scholar]
- Cohen SN, Chang AC. Revised interpretation of the origin of the pSC101 plasmid. J Bacteriol. 1977 Nov;132(2):734–737. [Europe PMC free article] [Abstract] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. [Abstract] [Google Scholar]
- Frey J, Chandler M, Caro L. The effects of an Escherichia coli dnaAts mutation on the replication of the plasmids colE1 pSC101, R100.1 and RTF-TC. Mol Gen Genet. 1979 Jul 13;174(2):117–126. [Abstract] [Google Scholar]
- Gamas P, Burger AC, Churchward G, Caro L, Galas D, Chandler M. Replication of pSC101: effects of mutations in the E. coli DNA binding protein IHF. Mol Gen Genet. 1986 Jul;204(1):85–89. [Abstract] [Google Scholar]
- Gutterson NI, Koshland DE., Jr Replacement and amplification of bacterial genes with sequences altered in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4894–4898. [Europe PMC free article] [Abstract] [Google Scholar]
- Hashimoto-Gotoh T, Sekiguchi M. Mutations of temperature sensitivity in R plasmid pSC101. J Bacteriol. 1977 Aug;131(2):405–412. [Europe PMC free article] [Abstract] [Google Scholar]
- Hasunuma K, Sekiguchi M. Replication of plasmid pSC101 in Escherichia coli K12: requirement for dnaA function. Mol Gen Genet. 1977 Sep 9;154(3):225–230. [Abstract] [Google Scholar]
- Ish-Horowicz D, Burke JF. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. [Europe PMC free article] [Abstract] [Google Scholar]
- Jasin M, Schimmel P. Deletion of an essential gene in Escherichia coli by site-specific recombination with linear DNA fragments. J Bacteriol. 1984 Aug;159(2):783–786. [Europe PMC free article] [Abstract] [Google Scholar]
- Jones IM, Primrose SB, Robinson A, Ellwood DC. Maintenance of some ColE1-type plasmids in chemostat culture. Mol Gen Genet. 1980;180(3):579–584. [Abstract] [Google Scholar]
- Joyce CM, Grindley ND. Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J Bacteriol. 1984 May;158(2):636–643. [Europe PMC free article] [Abstract] [Google Scholar]
- Kiel JA, Vossen JP, Venema G. A general method for the construction of Escherichia coli mutants by homologous recombination and plasmid segregation. Mol Gen Genet. 1987 May;207(2-3):294–301. [Abstract] [Google Scholar]
- Kleckner N, Roth J, Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. [Abstract] [Google Scholar]
- Kushner SR. In vivo studies of temperature-sensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1213–1218. [Europe PMC free article] [Abstract] [Google Scholar]
- Liljeström P, Pirhonen M, Palva ET. In vivo transfer of chromosomal mutations onto multicopy plasmids by transduction with bacteriophage P1. Gene. 1985;40(2-3):241–246. [Abstract] [Google Scholar]
- Maloy SR, Nunn WD. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. [Europe PMC free article] [Abstract] [Google Scholar]
- Maples VF, Kushner SR. DNA repair in Escherichia coli: identification of the uvrD gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5616–5620. [Europe PMC free article] [Abstract] [Google Scholar]
- Matsuyama S, Mizushima S. Construction and characterization of a deletion mutant lacking micF, a proposed regulatory gene for OmpF synthesis in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1196–1202. [Europe PMC free article] [Abstract] [Google Scholar]
- Parker B, Marinus MG. A simple and rapid method to obtain substitution mutations in Escherichia coli: isolation of a dam deletion/insertion mutation. Gene. 1988 Dec 20;73(2):531–535. [Abstract] [Google Scholar]
- Shevell DE, Abou-Zamzam AM, Demple B, Walker GC. Construction of an Escherichia coli K-12 ada deletion by gene replacement in a recD strain reveals a second methyltransferase that repairs alkylated DNA. J Bacteriol. 1988 Jul;170(7):3294–3296. [Europe PMC free article] [Abstract] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. [Abstract] [Google Scholar]
- Summers DK, Sherratt DJ. Multimerization of high copy number plasmids causes instability: CoIE1 encodes a determinant essential for plasmid monomerization and stability. Cell. 1984 Apr;36(4):1097–1103. [Abstract] [Google Scholar]
- Sunshine MG, Kelly B. Extent of host deletions associated with bacteriophage P2-mediated eduction. J Bacteriol. 1971 Nov;108(2):695–704. [Europe PMC free article] [Abstract] [Google Scholar]
- Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. [Abstract] [Google Scholar]
- Watson N, Apirion D. Molecular cloning of the gene for the RNA-processing enzyme RNase III of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Feb;82(3):849–853. [Europe PMC free article] [Abstract] [Google Scholar]
- Winans SC, Elledge SJ, Krueger JH, Walker GC. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. [Europe PMC free article] [Abstract] [Google Scholar]
- Yamaguchi K, Tomizawa J. Establishment of Escherichia coli cells with an integrated high copy number plasmid. Mol Gen Genet. 1980;178(3):525–533. [Abstract] [Google Scholar]
- Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. [Abstract] [Google Scholar]
- Zieg J, Maples VF, Kushner SR. Recombinant levels of Escherichia coli K-12 mutants deficient in various replication, recombination, or repair genes. J Bacteriol. 1978 Jun;134(3):958–966. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.171.9.4617-4622.1989
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc210259?pdf=render
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/171/9/4617
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/171/9/4617
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Comprehensive structural, infrared spectroscopic and kinetic investigations of the roles of the active-site arginine in bidirectional hydrogen activation by the [NiFe]-hydrogenase 'Hyd-2' from Escherichia coli.
Chem Sci, 14(32):8531-8551, 25 Jul 2023
Cited by: 2 articles | PMID: 37592998 | PMCID: PMC10430524
Systematic strategies for developing phage resistant Escherichia coli strains.
Nat Commun, 13(1):4491, 02 Aug 2022
Cited by: 11 articles | PMID: 35918338 | PMCID: PMC9345386
The Autonomous Glycyl Radical Protein GrcA Restores Activity to Inactive Full-Length Pyruvate Formate-Lyase In Vivo.
J Bacteriol, 204(5):e0007022, 04 Apr 2022
Cited by: 3 articles | PMID: 35377165 | PMCID: PMC9112899
A single amino acid exchange converts FocA into a unidirectional efflux channel for formate.
Microbiology (Reading), 168(1), 01 Jan 2022
Cited by: 4 articles | PMID: 35084298 | PMCID: PMC8914244
Versatile selective evolutionary pressure using synthetic defect in universal metabolism.
Nat Commun, 12(1):6859, 25 Nov 2021
Cited by: 9 articles | PMID: 34824282 | PMCID: PMC8616928
Go to all (486) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Deletion of an essential gene in Escherichia coli by site-specific recombination with linear DNA fragments.
J Bacteriol, 159(2):783-786, 01 Aug 1984
Cited by: 91 articles | PMID: 6086588 | PMCID: PMC215717
Replacement of the folC gene, encoding folylpolyglutamate synthetase-dihydrofolate synthetase in Escherichia coli, with genes mutagenized in vitro.
J Bacteriol, 174(6):1750-1759, 01 Mar 1992
Cited by: 17 articles | PMID: 1548226 | PMCID: PMC205775
Transposon vectors for stable chromosomal integration of cloned genes in rhizosphere bacteria.
Gene, 100:201-205, 01 Apr 1991
Cited by: 8 articles | PMID: 1647354
Bacterial plasmids: their extraordinary contribution to molecular genetics.
Gene, 135(1-2):67-76, 01 Dec 1993
Cited by: 14 articles | PMID: 8276280
Review
Funding
Funders who supported this work.
NIGMS NIH HHS (2)
Grant ID: GM07103
Grant ID: GM27997