Abstract
Free full text

Immunogenic peptide discovery in cancer genomes
Abstract
As immunotherapies to treat malignancy continue to diversify along with the tumor types amenable to treatment, it will become very important to predict which treatment is most likely to benefit a given patient. Tumor neoantigens, novel peptides resulting from somatic tumor mutations and recognized by the immune system as foreign, are likely to contribute significantly to the efficacy of immunotherapy. Multiple in silico methods have been developed to predict whether peptides, including tumor neoantigens, will be presented by the major histocompatibility complex (MHC) Class I or Class II, and interact with the T cell receptor (TCR). The methods for neoantigen prediction will be reviewed here, along with the most important examples of their use in the field of oncology.
Introduction: why prediction, why now?
When William B. Coley, the ‘father’ of immunotherapy, injected streptococcal organisms into patients with metastatic solid tumors in the 1890s, it was not known what aspects of ‘Coley’s Toxins,’ the patient and/or tumor, were responsible for tumor regression in a subset of patients with metastatic cancer. Nearly a century later, IL-2 was approved for the treatment of metastatic melanoma [1]. Since then, checkpoint blockade therapies and T cell therapies have proliferated, with promising results for both [2,3,4●●]. With this increase in immunotherapies, clinicians will need tools to predict which type of immunotherapy is most likely to benefit a specific patient.
A growing body of literature suggests that response to multiple types of immunotherapy results from the anti-tumor immune response against a critical neoantigen(s), non-self peptides resulting from exonic missense mutations (reviewed in [5●]). Cancer vaccines have historically used tumor-associated antigens which are over-expressed in tumors and have restricted tissue expression. However, these therapies require overcoming central and peripheral tolerance. In contrast, a tumor neoantigen would in theory not be limited by tolerance, with data both in preclinical models and humans supporting this idea [6●,7●]. It is important to note that not all effective immunotherapies target tumor neoantigens, but rather may target viral [8] or other antigens, as with chimeric antigen receptors [9,10] and therapeutic antibodies [11].
The major hurdle to selecting, improving and designing immunotherapies based on neoantigens lies in their accurate prediction, a challenging process in light of the complexities of the immune system. The major histocompatibility complex (MHC) molecules include two classes. MHC Class I (in humans, human leukocyte antigen, or HLA, Class I) molecules bind intracellular antigens of 8–11 amino acids in length and present them to cytotoxic CD8+ T cells. MHC Class II molecules bind extracellular antigens of 11–20 amino acids and present them to T helper CD4+ cells [12]. The HLA alleles are incredibly diverse, with greater than 6000 HLA Class I and Class II alleles described to date [13]. The number of potential peptides processed from a given pathogen is also vast, with a small proportion actually binding MHC Class I or II [14]. These facts make peptide prediction both important and challenging.
An immunogenic peptide fulfills at least two criteria: presentation by an MHC molecule and recognition by a T-cell receptor (Figure 1). In order to be presented by the MHC, a protein may be cleaved, and is typically presented by the antigen presentation machinery. Multiple computational algorithms exist for each step in this process. A fairly exhaustive list of prediction tools is provided (http://cancerimmunity.org/resources/webtools/ and tables in [15–17]), and the bioinformatic aspects of many of these programs has been reviewed [17,18●,19●].
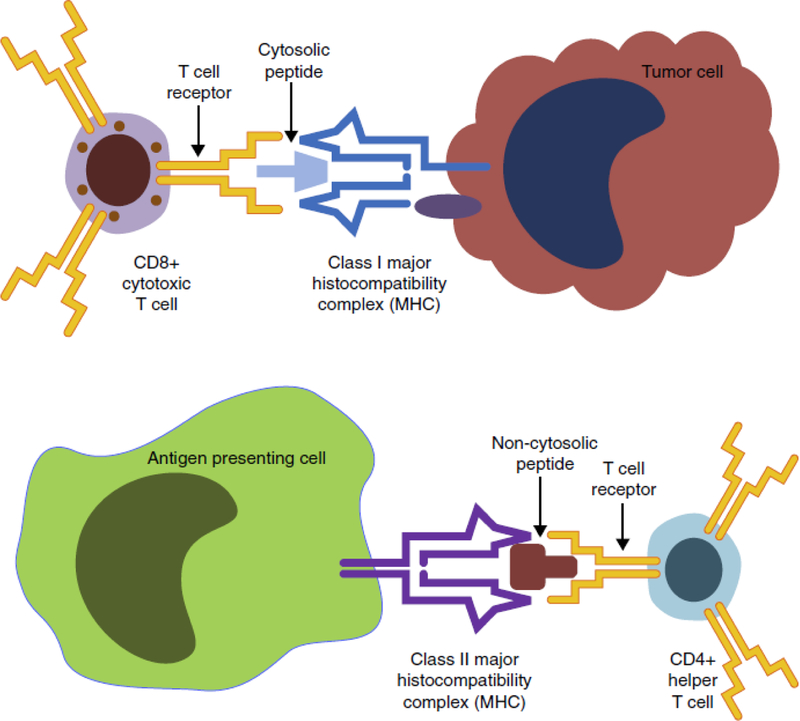
Summary of antigen presentation. A tumor cell (top) loads a peptide onto the Class I major histocompatibility complex (MHC) for presentation to and interaction with a CD8+ cytotoxic T cell. An antigen presenting cell (APC) loads a peptide onto the Class II MHC for presentation to and interaction with a CD4+ helper T cell (right). Additional co-receptor interactions between the APC and each type of T cell are not displayed here. These interactions play an important role in determining the downstream fate of each T cell, but as yet cannot be predicted bioinformatically. Image based on figures from motifolio.com.
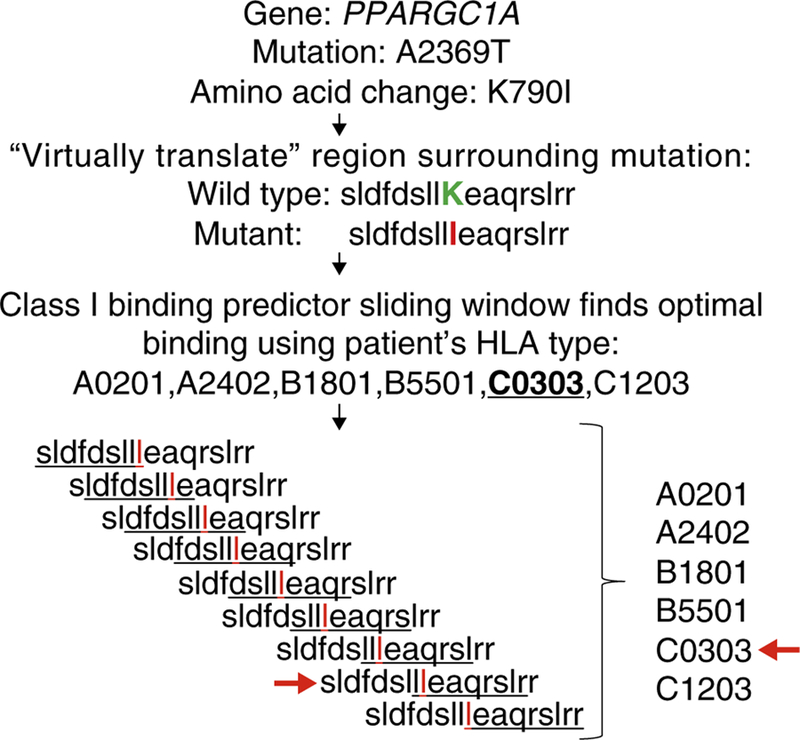
In the absence of data showing which peptides are presented by a given MHC, whole exome sequencing can be used to indirectly predict this. The sequenced DNA can be ‘virtually translated’ into predicted proteins, then the analytic tools described here evaluate which peptides may be presented by a given patient’s HLA repertoire (Figure 2). Here, we will first describe and evaluate the most commonly used MHC Class I, Class II and T cell predictors, highlighting those with data to support their use both in infectious diseases and oncology. We will then describe the most important recent uses of these tools in preclinical and clinical oncologic settings.
MHC Class I prediction
There are many MHC Class I prediction tools (Table 1). Most have been trained on data from the Immune Epitope Database (IEDB) [20]. There are two general categories of prediction tools: allele-specific and pan-specific. Initial programs relied on allele-specific motifs; for example, positions 2 and 9 constitute anchor residues on HLA-A*02:01, commonly occupied by leucine, valine or isoleucine [21]. Other positions are similarly stereotyped [22,23] and unknown input peptides searched for allele-specific motifs. A common problem with these methods is that there are insufficient data from rare alleles to reliably predict peptides which will bind to them. Three-dimensional structural models have been designed, but thus far underperform the models trained on actual data [19●].
Table 1
MHC Class I and Class II prediction tools
| Name | Website | Validated in vitro | Pros | Cons | MHC Class predictor |
|---|---|---|---|---|---|
| BIMAS [31] | http://www-bimas.cit.nih.gov/molbio/hla_bind/ | Yes [32,35] | Used historically | Predictions for selected HLA A, B and C alleles | I |
| RANKPEP [36] | http://imed.med.ucm.es/Tools/rankpep.html | Yes [37,38,62] | Widely validated | Predictions for selected HLA A, B and C, DP, DQ and DR alleles | I, II |
| SYFPEITHI [26] | http://www.syfpeithi.de/ | Yes [27–29, 59–61] | Widely validated | Predictions for selected HLA A, B and C alleles; Class II limited to HLA DRB1 | I, II |
| NetMHC [pan] [24,39] and NetMHCIIpan3.0 [55] | http://www.cbs.dtu.dk/services/NetMHCpan/ | Yes [43●●, 56,57] | Pan-specific; Class I has best in vitro and in vivo data, Class II predictor is considered most accurate [18●] | Uncommon alleles more difficult to predict due to limited in vitro data | I, II |
| IEDB recom-mended | http://tools.immuneepitope.org/mhci/ | No | Combines multiple predictive algorithms including NetMHCpan | Has not yet been validated, although some component programs have been | I, II |
| TEPITOPE [pan] [48] | http://www.biokdd.fudan.edu.cn/Service/TEPITOPEpan/TEPITOPEpan.html | Yes [49,50, 52,53] | Pan-specific; widely validated and user-friendly | May be inferior to NetMHCIIpan2.0 [18●] | II |
| PickPocket [97] | Not publically available | No | Pan-specific | Not publically available | II |
| MULTIPRED2 [98] | http://cvc.dfci.harvard.edu/multipred2/HTML/prediction2.php | N/A | Pan-specific combination of MULTIPRED, PEPVAC, NetMHCpan and NetMHCIIpan | Has not yet been validated, although some component programs have been | II |
| MultiRTA [99] | http://bordnerlab.org/MultiRTA | No | Based exclusively on experimental binding data | Has not yet been validated | II |
To address this problem, pan-specific programs were created to extrapolate from existing data to less common alleles with a paucity of in vitro data. While more useful because of their inclusion of more diverse HLA types, the pan-specific programs do become less accurate as the difference between novel and known HLA allele increases [24]. Prediction tools are generally developed using two steps: model training, then prediction on additional dataset(s). Thus, all of the models have in silico validation by the nature of their development, whereas there are fewer studies validating each in vitro or in vivo.
In the most highly cited paper using bioinformatic software to predict tumor neoantigens [25●●], the authors analyzed 1152 neoepitopes in breast and colorectal cancer assuming HLA-A*0201 and found 10 and 7 novel epitopes per cancer, respectively. The authors used allele-specific predictors BIMAS, SYFPEITHI, RANKPEP and NetMHC.
These MHC Class I predictors remain the most commonly used. SYFPEITHI, one of the first algorithms [26], has been used frequently in the cancer field for epitope prediction [27–29] at times in conjunction with other predictors [30]. BIMAS [31] has been used both in infectious disease studies [32,33], and to evaluate cancer-associated antigens [34,35], as has RANKPEP [36–38], although less frequently than SYFPEITHI or NetMHC.
The NetMHC programs are the best validated for peptide prediction in infectious diseases and oncology. NetMHC is allele-specific, and generates an IC50 score for the more common HLA types [39]. NetMHCpan is pan-specific and as such has broader applicability [24]. Hereafter, these programs are considered together as they are often cited in general terms by authors.
NetMHC has been used to predict epitopes in viruses associated with malignancy [40,41], neoantigens generated by drug-resistance mutations in Bcr-abl in CML [42] and to identify neoantigens thought to be responsible for spontaneous CD8+ T cell responses in patients with various malignancies [43●●]. The latter example also highlights the limits of predictive programs: although in most cases the cognate wild type peptide was predicted to bind MHC as strongly as the corresponding mutated peptide, in vitro only the mutated peptide stimulated cytolytic activity. This effect was hypothesized to result from the peptide in question affecting TCR interaction rather than MHC binding (24 of 26 cases), retention of one anchor amino acid (2 of 26 cases), or successful tolerance to the native peptide.
Finally, the IEDB Recommended tool provides an amalgam of some of the above predictive strategies (http://tools.immuneepitope.org/mhci/reference/). This program aims to choose the ‘best’ predictor for a given input peptide. While this would seem the ideal method, it has not yet been compared to the other programs listed above.
MHC Class II prediction
MHC Class II is highly polymorphic, and is comprised of, both the core and flanking residues, both of which influence binding to the MHC [44●●,45]. These features make MHC Class II binding prediction very difficult (reviewed in [15]), with MHC Class II predictors consistently underperforming MHC Class I [46]. Ideally, between 100 and 200 experimental peptide binding measurements are needed per MHC molecule for an adequate prediction to be calculated [47], but IEDB, the largest database of MHC binding peptides, has far fewer data points than this on which to train predictive programs.
Multiple tools for predicting MHC Class II binding have been created (Table 1), the most commonly used being TEPITOPEpan [48]. This program attempts to address the problem of insufficient in vitro data by assessing peptide sequence and peptide-MHC binding residues rather than actual binding data. TEPITOPE has been used in infectious disease [49–51] although users emphasize its limitations. TEPITOPE has also been used to detect Class II epitopes in malignancy, including for the melanoma antigen gp100 [52] and prostate-specific membrane antigen [53], among many other similar examples. OWA-PSSM is a recently developed tool from the authors of TEPITOPE still undergoing refinement and is not publically available (Wong HS, personal communication) [54].
The other Class II predictors are less commonly used. NetMHCIIpan uses artificial neural network learning to train the model on actual MHC Class II binding data [55]. In one analysis written by the authors of TEPITOPE that compared the pan-specific MHC II predictors, NetMH-CII was found to be superior to both MultiRTA and TEPITOPE [18●]. NetMHCII has been used in rheumatology [56], infectious diseases [57] and malignancy [58]. SYFPEITHI and RANKPEP have also been used in malignancy [59–63]. MULTIPRED2, software that combines the NetMHC tools as well as a peptide cleavage tool, is similar to ‘IEDB recommended’ in that it incorporates those programs with the highest validation rate, but it has not yet validated in vitro.
T cell interaction prediction
The MHC-T cell interaction is the most complex, and therefore the hardest to model bioinformatically (Table 2). The most frequently used program is NetCTL, which does not try to directly predict T cell binding. Rather, it uses a combination of MHC binding (NetMHC), C-terminal cleavage affinity (NetCHOP [64]) and transporter associated with antigen processing (TAP) transport [65] to generate its score. This program has been used commonly in infectious disease [66–69], and infrequently in malignancy [70,71], where again the authors comment on the limitations of this strategy, including limited selection of HLA alleles, and the presence of epitopes found experimentally that were not predicted bioinformatically. The IEDB T cell epitope prediction tool is a recently developed tool [72] that predicts immunogenicity based on peptide position and amino acid characteristics, but it has not yet been validated in vitro.
Table 2
T cell prediction tools
| Name | Website | Validated in vitro | Pros | Cons |
|---|---|---|---|---|
| EpicCapo [78] | http://pirun.ku.ac.th/~fsciiok/EpicCapoREF.zip | No | 9 amino acid input, with plan to expand to 8–11 | Predictions for selected HLA alleles |
| POPISK [74] | http://iclab.life.nctu.edu.tw/POPISK | No | Takes into account HLA type | Has not yet been validated |
| EpiMatrix [79●] | http://www.epivax.com/epimatrix/ | Yes [100,101] | Validated in vitro | Proprietary tool |
| NetCTLpan [102] | http://www.cbs.dtu.dk/services/NetCTL/ | Yes [66,67] | Validated in vitro; is really a combination of Class I, peptide cleavage and TAP transport models | Focuses on MHC presentation rather than TCR itself |
| iMatrix [75] (formerly PAComplex) | http://pacomplex.life.nctu.edu.tw/index_iM.php | No | User-friendly | Has not yet been validated |
| IEDB tool [72] | http://tools.immuneepitope.org/main/html/tcell_tools.html | Yes [103] | User-friendly; good in silico validation | Paucity of validation |
| CTLPred [76] | http://www.imtech.res.in/raghava/ctlpred/ | No | User-friendly | Has not yet been validated |
There are several other T cell epitope predictors, but none has been validated. POPI was the first T-cell reactivity predictor [73]; POPISK [74] takes into account MHC allele restriction has been used in infectious disease [57] but not malignancy. iMatrix models the TCR-peptide and peptide-MHC interfaces [75]. CTLPred [76] has been used retrospectively to predict epitopes from Ebola virus but these were not validated in vitro, and this program underperformed relative to SYFPEITHI and IEDB for predicting HIV-specific epitopes [77]. EpicCapo + REF has been used to search for promiscuous epitopes (epitopes presented by HLA supertypes) of influenza [78], but has not been used in cancer.
EpiMatrix [79●] has been used predominantly by the group involved in its creation to look at infectious organisms [80–83]. In its first use in malignancy, an abstract describes the use of EpiMatrix to identify CD4+ and CD8+ T cell epitopes of the protein survivin, which was used to induce tumor regression in a mouse model of malignant mesothelioma [84].
Summary: which tool, individually or concurrently?
At present, MHC prediction tools are typically used in malignancy to retrospectively predict which of a given tumor’s mutation(s) led to neoantigen formation. Two important questions remain: which tool should be used, and should multiple tools be used concurrently?
There have been several comparative assessments of the prediction tools. A 2007 study of 16 MHC Class I prediction tools evaluated each program separately and in conjunction. This work included the commonly used programs BIMAS, SYFPEITHI, RANKPEP and NetMHC, and reported a modest improvement in sensitivity when the programs were combined [85●]. An international competition for machine learning in 2009 found that NetMHC outperformed both PickPocket and the allele-specific programs BIMAS and SYFPEITHI (described in [16]). A 2012 comparison of the MHC Class I and Class II pan-specific methods found the NetMHC tools performed best for both classes [18●].
In light of these studies, and given existing data from both translational and in vitro studies, the NetMHC series programs have been best validated, with the strongest evidence for MHC Class I prediction, weaker for MHC Class II and the least for T cell recognition. The question of whether NetMHC should be combined with other predictive tools remains to be elucidated.
Preclinical data for neoantigen prediction in malignancy
There are several examples of preclinical models showing the importance of neoantigens in malignancy. As early as 1995, Mandelboim et al. described anti-tumor cytotoxic T lymphocyte responses against neoantigens from a murine lung carcinoma [86●●]. He showed that vaccination with these peptides both protected mice from new metastases and reduced metastatic load in mice with micrometastases.
Three landmark studies published in 2012 demonstrated the importance of tumor neoantigens to tumor control versus escape in mouse models, two of which used NetMHC for neoantigen prediction. In a mouse model of chemically induced sarcoma, Matsushita et al. identified the neoantigen responsible for spontaneous tumor rejection [87●●]. Cell lines harboring this mutation were rejected in most wild type mice, whereas 20% of these tumors underwent immunoediting of the neoantigen, and were able to grow. In a subsequent study, the same group defined the neoantigens responsible for the antitumor effects of anti-CTLA-4 and anti-PD-1 immunotherapy [88●●].
The Sahin lab similarly used next generation sequencing and NetMHC prediction to design tumor-specific vaccines using neoantigens derived from the melanoma cell line B16F10 [89●●]. They selected 50 mutations out of the 962 nonsynonymous exonic mutations for validation; of these, 16 elicited immune responses in immunized mice when delivered with polyI:C, and two vaccines delayed tumor growth.
The Jacks lab treated mice with soft tissue sarcoma with and without lentivirus that expressed known T-cell antigens [90●]. Immunocompetent mice had a delayed development of tumors that was even more dramatic in the presence of T-cell antigens, and that was abrogated upon treatment with anti-CD4+ and anti-CD8+ antibodies. These neoantigens were exogenously expressed, rather than representing the mutant form of an existing wild type peptide.
Moving into the clinic: tumor-associated antigens and neoantigens
Seminal papers in both solid and liquid tumors have demonstrated an important role for tumor neoantigens in immunotherapy. Lennerz et al. used a cDNA library from a patient-derived melanoma cell line to screen for tumor-reactive autologous T cells, and identified four neoantigens that induced T cell response as measured by IFN-gamma ELISPOT [7●]. The same year, Rosenberg and Robbins also discovered two neoantigens recognized by adoptively transferred tumor infiltrating lymphocytes in a single patient with metastatic melanoma treated with adoptive T cell immunotherapy [91●]. Intriguingly, their data suggests that the relevant T cell clones existed before their ex vivo expansion: the autologous T cell therapy was amplifying an existing antineoantigen tumor response.
More recently the same investigators used bioinformatic prediction to narrow down candidate neoantigens in three melanoma patients treated with TIL therapy [92●]. After predicting neoantigens from an HLA-A*0201 patient-derived cell line, the group tested the ability for autologous TILs to recognize 55 neoantigen peptides when presented by the cell line T2. Four peptides predicted by NetMHCPan2.4 led to IFN-gamma release by TIL. Little to no response was seen for the corresponding wild type peptides. For a two additional, non-A*0201 patients (in whom the T2 system could not be used), after incubation with HLA-matched target cells 3 of 53 and 4 of 46 tested peptides led to TIL activation. Importantly, responses to some of the neoantigens were measurable in the peripheral blood, albeit greater than two logs less than in the TILs.
The Rosenberg group has since expanded its therapies to include other tumor types. A patient with metastatic cholangiocarcinoma treated with adoptive transfer of tumor infiltrating lymphocytes containing approximately 25% of neoantigen-specific CD4+ T helper cells transiently responded to initial therapy [4●●]. When her cancer subsequently progressed, the patient was retreated with a near-pure population of neoantigen-specific T cells and responded. This case illustrates several important points: in addition to providing proof of principle that these therapies can work in epithelial malignancies, it also shows the necessity for expanding our neoantigen prediction strategies beyond MHC Class I/CD8+ T cell interaction, as the important population in this case was comprised CD4+ T cells.
In the first published study of neoantigens in the response to checkpoint blockade therapy, the Schumacher group sequenced the tumor of a patient with metastatic melanoma who had a partial response to treatment with CTLA-4 blockade by ipilimumab [93●●]. They then used NetMHC3.2, NetCHOP and RNA expression to predict 448 peptide neoantigens. After testing all these peptides using multimer technology, they found one strong and one weak neoantigen peptide. A T cell response to the strong neoantigen was seen both in TILs and in PBMC, and was not seen against the corresponding wild type peptide. Our group applied similar concepts to a study of 64 metastatic melanoma patients treated with the CTLA4 blockade agents ipilimumab or tremelimumab. Mutational load appeared to correlate with response but alone did not perfectly predict response, as there were some tumors with a high mutational load that did not respond to treatment, and conversely relatively lower burden tumors that did respond. Relying primarily on the NetMHC Class I prediction tool for neoantigen prediction, we found that specific neoantigens predicted to be presented by patient-specific HLA correlated with benefit from therapy [94●●]. These neoantigens appeared to have substrings in common, suggesting T cell degeneracy as has been described [95●]. This phenomenon, which is reminiscent of molecular mimicry, is being addressed by ongoing studies by our group. Several predicted neoantigens were validated in vitro with intracellular cytokine staining using autologous peripheral blood from the treated patients stimulated with synthetic peptides.
The Wu group has used neoantigen prediction extensively. In one example, the investigators used NetMHCpan v2.4 to predict peptide neoantigens occurring in chronic lymphocytic leukemia (CLL) that are presented by patient-specific MHC Class I [96●●]. They tested 9 and 10 amino acid peptides predicted to be presented by HLA-A or HLA-B in vitro in a competitive binding assay and validated 76.5% of peptides predicted to bind with an IC50 of less than 150, and 36% of weak binders (≤500 nM), for a total of a 55% correspondence between NetMHC prediction and the competitive binding assay. When they translated this into patient material, 2 out of 30 peptides tested for one patient, and 1 out of 37 for a second patient led to T cell responses. The authors emphasize that this underestimation of neoantigens on the part of NetMHC pre-dominantly results from the relative insensitivity of in vitro methods to validate T cell reactivity. Thus, the actual number of neoantigens is likely much higher. In an exciting next step for the field of neoantigen prediction and application, a trial using the prediction method to create a patient-specific vaccine (termed NeoVax) is currently being run out of the Harvard system cancer centers (Brigham and Women’s and Dana Farber Cancer Institute ()).
Summary and conclusions
With the increasing use of immunotherapies to treat malignancy, it will become critically important to accurately triage patients to treatments that are most likely to be effective. Novel peptide neoantigens resulting from somatic exonic mutations have been shown conclusively to elicit T cell responses in patients. Prediction tools have proliferated for MHC Class I, Class II and T cell interactions, as well as peptide cleavage. Of the existing tools, based on comparative studies and validation data to date, we recommend using the NetMHC programs for MHC Class I prediction. The apparent low positive predictive value of this tool may actually reflect the relatively insensitive methods used to validate the peptides in vitro, rather than a deficiency of the tool itself. The predictive tools for MHC Class II and T cell interaction are not yet sufficiently validated for routine use. Furthermore, a wild type peptide that is predicted to be presented does not necessarily imply that a peptide-specific response will be seen when tested using patient material. Ultimately, predictive tools in their current form may help narrow candidate peptides for in vitro testing, but these algorithms will need ongoing refinement before they are ready for routine clinical use.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
● of special interest
●● of outstanding interest
This study illustrated that expanded tumor infiltrating lymphocytes that recognized a neoantigen predicted by NetMHC led to the regression of a cholangiocarcinoma. Importantly, these were CD4+ TH1 cells.
This review discusses advantages and challenges of vaccination with neoantigens rather than the vaccine strategies used previously, which predominantly relied on overexpressed self antigens.
One of the pioneering studies in the neoantigen field, this study showed that anti-tumor cytotoxic lymphocytes recognized the mutant form for the connexin peptide.
This study, often cited in the neoantigen literature, identified both tumor-associated and mutated peptides using cDNA expression screening.
This review describes then evaluates pan-specific methods of MHC binding prediction.
This review discusses advances in MHC Class I and Class II prediction as well as the ways in which the latter is not yet as accurate as the former.
Perhaps the most cited paper in the neoantigen literature, this paper addressed the relationship between mutations and neoantigens in a series of breast and colorectal cancer patients using a composite of the methods listed in Table 1.
This restrospective study applies MHC Class I prediction to documented cases of spontaneous tumor regression, showing the utility of this approach to epitope selection.
This study describes the importance of the peptide flanking residues, in addition to the core binding residues, to MHC Class II binding, illustrative of the complexities of MHC Class II prediction.
This paper describes the development and mechanism of the predictor EpiMatrix in order to discover new HIV-1 epitopes. It also illustrates that certain peptides are conserved across strains and likely to be presented by more than one HLA molecule.
This study argues that combining prediction tools increases the predictive value over each individual tool; this paper does not include the most recent versions of each program, due to its publication in 2007.
This was among the first studies that showed the utility of a neoantigen-based tumor vaccine in protecting mice from metastases.
This study used the Immune Epitope Database tool to predict the neoantigen that led to tumor rejection in a murine model of sarcoma. It laid the foundation for a similar analysis in human melanoma tumors (Snyder A, Makarov V, Merghoub T, Yuan J et al, below).
This study used a multi-tool prediction method to identify two rejection neoantigens following anti-PD-1 or anti-CTLA-4 treatment of murine sarcomas.
This work showed that the non-immunogenic murine cell line B16F10 could be made immunogenic by vaccinating with mutant epitopes predicted from whole exome sequencing plus adjuvant.
This study identified rejection antigens, but using a genetically engineered authochthonous mouse model of sarcomagenesis rather than a carcinogen-induced model such as in the Schreiber work.
This study used a cDNA library screen to identify the neoantigens against which anti-tumor TILs were directed in a melanoma patient.
This study used exome sequencing followed by neoantigen prediction by NetMHCpan and peptide stimulation of autologous T cells to identify tumor neoantigens in melanoma patients treated with TIL therapy.
This was the first study of tumor neoantigens in a patient treated with immune blockade therapy, in this case ipilimumab.
This study elucidated the neoantigen patterns underlying clinical response to anti-CTLA4 therapy. This was the first multi-patient study of the mutational features influencing response to immune checkpoint blockade.
This study illustrated the ability of the T cell clones under study to recognize and proliferate in response to a diversity of peptides, including those with limited homology of the centrally-located amino acids.
This study demonstrates that the tumor neoantigen association with response also applies to a liquid tumor, CLL. It also discusses predicted neoantigens across in TCGA data.
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.gde.2014.12.003
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6657809?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.gde.2014.12.003
Article citations
Tumor-Derived Antigenic Peptides as Potential Cancer Vaccines.
Int J Mol Sci, 25(9):4934, 30 Apr 2024
Cited by: 0 articles | PMID: 38732150 | PMCID: PMC11084719
Review Free full text in Europe PMC
The role of the master cancer regulator Pin1 in the development and treatment of cancer.
Front Cell Dev Biol, 12:1343938, 30 Apr 2024
Cited by: 0 articles | PMID: 38745861 | PMCID: PMC11091292
Review Free full text in Europe PMC
Neo-intline: integrated pipeline enables neoantigen design through the in-silico presentation of T-cell epitope.
Signal Transduct Target Ther, 8(1):397, 18 Oct 2023
Cited by: 3 articles | PMID: 37848417 | PMCID: PMC10582007
The Efficacy of Tumor Mutation Burden as a Biomarker of Response to Immune Checkpoint Inhibitors.
Int J Mol Sci, 24(7):6710, 04 Apr 2023
Cited by: 13 articles | PMID: 37047684 | PMCID: PMC10095310
Review Free full text in Europe PMC
A Winning New Combination? Toward Clinical Application in Oncology.
Cancer Control, 30:10732748231175240, 01 Jan 2023
Cited by: 0 articles | PMID: 37166227 | PMCID: PMC10184224
Review Free full text in Europe PMC
Go to all (48) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Best practices for bioinformatic characterization of neoantigens for clinical utility.
Genome Med, 11(1):56, 28 Aug 2019
Cited by: 106 articles | PMID: 31462330 | PMCID: PMC6714459
Review Free full text in Europe PMC
MHC class II restricted neoantigen: A promising target in tumor immunotherapy.
Cancer Lett, 392:17-25, 16 Jan 2017
Cited by: 41 articles | PMID: 28104443
Review
HLA class I loss in metachronous metastases prevents continuous T cell recognition of mutated neoantigens in a human melanoma model.
Oncotarget, 8(17):28312-28327, 01 Apr 2017
Cited by: 16 articles | PMID: 28423700 | PMCID: PMC5438652
Characterizing neoantigens for personalized cancer immunotherapy.
Curr Opin Immunol, 46:58-65, 04 May 2017
Cited by: 31 articles | PMID: 28478383
Review