Abstract
Background
Increasing antimicrobial resistance among pathogens causing complicated intra-abdominal infections (cIAIs) supports the development of new antimicrobials. Ceftolozane/tazobactam, a novel antimicrobial therapy, is active against multidrug-resistant Pseudomonas aeruginosa and most extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae.Methods
ASPECT-cIAI (Assessment of the Safety Profile and Efficacy of Ceftolozane/Tazobactam in Complicated Intra-abdominal Infections) was a prospective, randomized, double-blind trial. Hospitalized patients with cIAI received either ceftolozane/tazobactam (1.5 g) plus metronidazole (500 mg) every 8 hours or meropenem (1 g) every 8 hours intravenously for 4-14 days. The prospectively defined objectives were to demonstrate statistical noninferiority in clinical cure rates at the test-of-cure visit (24-32 days from start of therapy) in the microbiological intent-to-treat (primary) and microbiologically evaluable (secondary) populations using a noninferiority margin of 10%. Microbiological outcomes and safety were also evaluated.Results
Ceftolozane/tazobactam plus metronidazole was noninferior to meropenem in the primary (83.0% [323/389] vs 87.3% [364/417]; weighted difference, -4.2%; 95% confidence interval [CI], -8.91 to .54) and secondary (94.2% [259/275] vs 94.7% [304/321]; weighted difference, -1.0%; 95% CI, -4.52 to 2.59) endpoints, meeting the prespecified noninferiority margin. In patients with ESBL-producing Enterobacteriaceae, clinical cure rates were 95.8% (23/24) and 88.5% (23/26) in the ceftolozane/tazobactam plus metronidazole and meropenem groups, respectively, and 100% (13/13) and 72.7% (8/11) in patients with CTX-M-14/15 ESBLs. The frequency of adverse events (AEs) was similar in both treatment groups (44.0% vs 42.7%); the most common AEs in either group were nausea and diarrhea.Conclusions
Treatment with ceftolozane/tazobactam plus metronidazole was noninferior to meropenem in adult patients with cIAI, including infections caused by multidrug-resistant pathogens.Clinical trials registration
NCT01445665 and NCT01445678.Free full text

Ceftolozane/Tazobactam Plus Metronidazole for Complicated Intra-abdominal Infections in an Era of Multidrug Resistance: Results From a Randomized, Double-Blind, Phase 3 Trial (ASPECT-cIAI)
Abstract
Background. Increasing antimicrobial resistance among pathogens causing complicated intra-abdominal infections (cIAIs) supports the development of new antimicrobials. Ceftolozane/tazobactam, a novel antimicrobial therapy, is active against multidrug-resistant Pseudomonas aeruginosa and most extended-spectrum β-lactamase (ESBL)–producing Enterobacteriaceae.
Increasing antimicrobial resistance among pathogens causing complicated intra-abdominal infections (cIAIs) supports the development of new antimicrobials. Ceftolozane/tazobactam, a novel antimicrobial therapy, is active against multidrug-resistant Pseudomonas aeruginosa and most extended-spectrum β-lactamase (ESBL)–producing Enterobacteriaceae.
Methods. ASPECT-cIAI (Assessment of the Safety Profile and Efficacy of Ceftolozane/Tazobactam in Complicated Intra-abdominal Infections) was a prospective, randomized, double-blind trial. Hospitalized patients with cIAI received either ceftolozane/tazobactam (1.5 g) plus metronidazole (500 mg) every 8 hours or meropenem (1 g) every 8 hours intravenously for 4–14 days. The prospectively defined objectives were to demonstrate statistical noninferiority in clinical cure rates at the test-of-cure visit (24–32 days from start of therapy) in the microbiological intent-to-treat (primary) and microbiologically evaluable (secondary) populations using a noninferiority margin of 10%. Microbiological outcomes and safety were also evaluated.
ASPECT-cIAI (Assessment of the Safety Profile and Efficacy of Ceftolozane/Tazobactam in Complicated Intra-abdominal Infections) was a prospective, randomized, double-blind trial. Hospitalized patients with cIAI received either ceftolozane/tazobactam (1.5 g) plus metronidazole (500 mg) every 8 hours or meropenem (1 g) every 8 hours intravenously for 4–14 days. The prospectively defined objectives were to demonstrate statistical noninferiority in clinical cure rates at the test-of-cure visit (24–32 days from start of therapy) in the microbiological intent-to-treat (primary) and microbiologically evaluable (secondary) populations using a noninferiority margin of 10%. Microbiological outcomes and safety were also evaluated.
Results. Ceftolozane/tazobactam plus metronidazole was noninferior to meropenem in the primary (83.0% [323/389] vs 87.3% [364/417]; weighted difference, −4.2%; 95% confidence interval [CI], −8.91 to .54) and secondary (94.2% [259/275] vs 94.7% [304/321]; weighted difference, −1.0%; 95% CI, −4.52 to 2.59) endpoints, meeting the prespecified noninferiority margin. In patients with ESBL-producing Enterobacteriaceae, clinical cure rates were 95.8% (23/24) and 88.5% (23/26) in the ceftolozane/tazobactam plus metronidazole and meropenem groups, respectively, and 100% (13/13) and 72.7% (8/11) in patients with CTX-M-14/15 ESBLs. The frequency of adverse events (AEs) was similar in both treatment groups (44.0% vs 42.7%); the most common AEs in either group were nausea and diarrhea.
Ceftolozane/tazobactam plus metronidazole was noninferior to meropenem in the primary (83.0% [323/389] vs 87.3% [364/417]; weighted difference, −4.2%; 95% confidence interval [CI], −8.91 to .54) and secondary (94.2% [259/275] vs 94.7% [304/321]; weighted difference, −1.0%; 95% CI, −4.52 to 2.59) endpoints, meeting the prespecified noninferiority margin. In patients with ESBL-producing Enterobacteriaceae, clinical cure rates were 95.8% (23/24) and 88.5% (23/26) in the ceftolozane/tazobactam plus metronidazole and meropenem groups, respectively, and 100% (13/13) and 72.7% (8/11) in patients with CTX-M-14/15 ESBLs. The frequency of adverse events (AEs) was similar in both treatment groups (44.0% vs 42.7%); the most common AEs in either group were nausea and diarrhea.
Conclusions. Treatment with ceftolozane/tazobactam plus metronidazole was noninferior to meropenem in adult patients with cIAI, including infections caused by multidrug-resistant pathogens.
Treatment with ceftolozane/tazobactam plus metronidazole was noninferior to meropenem in adult patients with cIAI, including infections caused by multidrug-resistant pathogens.
Clinical Trials Registration. NCT01445665 and NCT01445678.
NCT01445665 and NCT01445678.
Complicated intra-abdominal infections (cIAIs) are tissue-invasive infections leading to abscess formation or generalized peritonitis. The management of cIAIs involves operative or percutaneous intervention to obtain surgical control of the source. Nonetheless, patients with cIAIs are at risk of sepsis and mortality [1–4].
Empiric antimicrobial therapy with appropriate agents is an important component of treatment [5, 6]. Initial empiric therapy that is not effective against infecting pathogens increases costs, treatment failure, and death [7–10]. Because of this, cIAIs are an important infection category for evaluation of the efficacy of investigational agents.
The well-recognized appearance of antimicrobial resistance among gram-negative bacteria has stimulated the development of novel agents [11], particularly those targeting Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBLs) [12], which confer resistance to most β-lactam antimicrobial agents [1].
Ceftolozane/tazobactam consists of a novel cephalosporin and an established β-lactamase inhibitor that is being developed to address antimicrobial resistance in serious infections caused by gram-negative pathogens, including cIAI, complicated urinary tract infection/pyelonephritis (cUTI), and ventilated nosocomial pneumonia. In vitro activity of ceftolozane/tazobactam has been confirmed against ESBL-producing Enterobacteriaceae, drug-resistant Pseudomonas aeruginosa [13–16], and some Streptococcus species [17]. The results from a phase 2 study with ceftolozane/tazobactam in combination with metronidazole in cIAI supported further development for this indication [18].
We now report the results from ASPECT-cIAI (Assessment of the Safety Profile and Efficacy of Ceftolozane/Tazobactam in Complicated Intra-abdominal Infections), a large global phase 3 clinical program that evaluated intravenous ceftolozane/tazobactam plus metronidazole vs meropenem for the treatment of hospitalized adult patients with cIAI.
PATIENTS AND METHODS
Study Design
Two identical multicenter, prospective, randomized, double-blind, placebo-controlled trials were initiated in December 2011 at 196 study centers worldwide (ClinicalTrials.gov identifiers NCT01445665 and NCT01445678).
The trials were designed in compliance with current clinical guidelines and the regulatory requirements of the US Food and Drug Administration (FDA) and the European Medicines Agency, approved by local institutional review boards, and conducted in accordance with the International Conference on Harmonisation/Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. All patients were required to provide written informed consent prior to participation. In 2012, the FDA released a draft guidance providing for a single study pathway for approval of antibiotics in cUTI and cIAI [19]. The sponsor sought and received prospective permission from the relevant regulatory agencies to pool the data from the 2 protocols upon completion to form a single dataset for analysis. Enrollment in the 2 protocols was completed in September 2013, and data were pooled after database lock in each study.
Inclusion Criteria
Patients were to be ≥18 years of age, with clinical evidence of cIAI. Operative or percutaneous drainage of an infectious focus was either planned or had been performed recently (within 24 hours), confirming the presence of cIAI.
Exclusion Criteria
The following exclusions applied: cIAI managed by staged abdominal repair in which the fascia was not closed; low likelihood of adequate source control at surgery; creatinine clearance <30 mL/minute; or use of systemic antimicrobial therapy for IAI for >24 hours prior to the first dose of study drug (unless this treatment failed, defined by the need for additional intervention and persistent signs of ongoing infection with a positive culture of intra-abdominal abscess or peritonitis fluid, despite >48 hours of prior antimicrobial therapy).
Randomization and Treatment
Randomization numbers were computer-generated. Patients were assigned (1:1) by the study site's pharmacist to intravenous ceftolozane/tazobactam 1.5 g (containing 1 g ceftolozane and 500 mg tazobactam) plus metronidazole (500 mg every 8 hours) or intravenous meropenem (1 g every 8 hours) plus placebo for 4–10 days. Treatment could be continued for up to 14 days in patients who had 1 of the following: multiple abscesses; non-appendix-related diffuse peritonitis, failure of prior antimicrobial therapy, or hospital-acquired infection. The dose of ceftolozane/tazobactam was based on data from previous clinical studies [18, 20]. The comparator, meropenem, is recommended by the Surgical Infection Society and the Infectious Diseases Society of America as appropriate first-line empiric therapy for high-risk and severe cIAI, and is prescribed commonly for this indication [5, 6]. In patients with moderate renal impairment (creatinine clearance, 30–50 mL/minute), the ceftolozane/tazobactam dose was reduced to 750 mg every 8 hours and the meropenem dose to 1 g every 12 hours. Placebo saline infusions were used to maintain blinding. Drug assignment was concealed from the patient and all clinical and study staff.
Assessments
At baseline, intra-abdominal specimens were collected from aspirates (collected with a needle or a syringe) for culture of aerobes and anaerobes. Samples were inoculated into aerobic and anaerobic culture bottles, incubated at 35°C–37°C, and transferred to the microbiology laboratory. Signs and symptoms of the index infection were recorded daily. Blood samples for culture were drawn in patients with hospital-acquired infections, those who had failed prior antimicrobial therapy, and those with signs of severe sepsis [21]. Other baseline assessments included measurement of disease severity, as determined by the Acute Physiology and Chronic Health Evaluation (APACHE) II score; measurements of hematology, chemistry, and coagulation; urinalysis; and estimation of creatinine clearance.
Outcome Assessment
Clinical outcomes were assessed at the end of therapy (within 24 hours of last dose of treatment), the test-of-cure (TOC) visit (24–32 days after start of therapy), and the late follow-up visit (38–45 days after start of therapy). Clinical cure was defined as complete resolution or significant improvement in signs and symptoms of the index infection, such that no additional antimicrobials or interventions were required. Events defining clinical failure included death due to cIAI prior to the TOC visit, persisting or recurrent infection requiring additional intervention, treatment with additional antimicrobials for ongoing symptoms of IAI, and/or surgical site infection [22]. An indeterminate response was recorded when trial data were not available for evaluation of efficacy for any reason, including death unrelated to the index infection, or in extenuating circumstances that precluded classification as cure or failure. Analysis populations are defined in Supplementary Table 1. Patients with missing clinical outcome data or indeterminate responses were considered to have failed treatment in the intent-to-treat (ITT) and microbiological ITT (MITT) analyses, but were excluded from the per-protocol (clinically evaluable [CE] and microbiologically evaluable [ME]) analyses.
Pathogen susceptibility to ceftolozane/tazobactam was defined as a minimum inhibitory concentration (MIC) ≤8 mg/L, intermediate as an MIC of 16 mg/L, and resistant as an MIC ≥32 mg/L. MIC cutoffs for determination of susceptibility to meropenem were based on Clinical Laboratory Standards Institute (CLSI) definitions. Enterobacteriaceae susceptibility was defined as MIC ≤1 mg/L, intermediate as MIC 2 mg/L, and resistant as MIC ≥4 mg/L. For P. aeruginosa, the MIC cutoffs were ≤2 mg/L, 4 mg/L, and ≥8 mg/L, respectively [23]. Multidrug resistance in P. aeruginosa was based on the CLSI breakpoints and was defined as resistance or nonsusceptibility to ≥3 drug classes that are known to be active against P. aeruginosa. Enterobacteriaceae organisms with an ESBL phenotype (predefined criteria: MIC ≥2 mg/L for any cephalosporin, ≥3 dilution change in MIC when an antibiotic was combined with a β-lactamase inhibitor) identified prior to database lock were characterized by JMI Laboratories (North Liberty, Iowa) using a commercial MicroArray System Check-MDR CT101 kit (Check-points, Wageningen, the Netherlands). Safety was assessed by review of adverse events (AEs), vital signs, physical examination findings, and clinical laboratory results.
Source Control Review
All patients with a baseline pathogen and an investigator-assigned outcome of failure, and those with an outcome of clinical cure who underwent a second procedure, were reviewed by an independent blinded surgical review panel comprising 3 surgeons and 2 radiologists. Source control was considered adequate when the physical and mechanical measures were consistent with current local standards of practice to eliminate the source of infection, control ongoing contamination, and restore gastrointestinal function [24]. Patients who were considered to have had inadequate source control were excluded from the per-protocol analyses.
Statistical Analysis
Prior to completion of the studies, statistical analyses were planned based on pooled data from the 2 trials. The planned pooled sample size ensured a minimum of 90% power to demonstrate the noninferiority of ceftolozane/tazobactam plus metronidazole to meropenem at a 10% noninferiority margin at a 1-sided significance level of 0.025. These calculations also assumed that 80% of randomized patients would meet the criteria to be included in the MITT population and that the clinical cure rate in both arms would be 75%.
The noninferiority hypothesis was tested through a 2-sided 95% confidence interval (CI) approach. The weighted difference in cure rates and the 95% CI around the difference in cure rates between study treatments were calculated using a stratified Newcombe CI with minimum risk weights [25, 26]. If the lower bound of the 95% CI for the difference (ceftolozane/tazobactam plus metronidazole minus meropenem) was above −10 percentage points, noninferiority was claimed.
Other secondary endpoints of clinical cure in the ITT and CE populations, various ME population subgroups, and per-baseline pathogen in the ME population were analyzed using a 95% CI calculated by the Wilson score methodology. Safety and tolerability of ceftolozane/tazobactam plus metronidazole were also evaluated. Statistical analyses were performed using SAS version 9.1.3 or higher (SAS Institute, Cary, North Carolina).
RESULTS
Patient Disposition and Baseline Characteristics
In total, 993 patients were randomized to ceftolozane/tazobactam plus metronidazole (n = 487) or meropenem (n = 506), and 806 (81.2%) qualified for the MITT population (Figure (Figure1).1). Approximately 50% of patients in each treatment group received therapy for up to 7 days, and an additional 36.5% received treatment for up to 10 days. The mean duration of treatment for patients who were eligible to continue therapy beyond 10 days is shown in Table Table11.
Table 1.
Duration of Therapy for Patients Who Were Eligible for Extension of Treatment Beyond 10 Days (Microbiological Intent-to-Treat Population)
| Diagnosis | Mean Duration of Therapy, d | Min–Max, d |
|---|---|---|
| Multiple abscess (n = 64) | 9.6 | 2–15 |
| Diffuse peritonitis, nonappendix (n = 149) | 8.6 | 2–15 |
| Failed prior therapy, nonappendix (n = 42) | 9.3 | 2–15 |
| Localized complicated appendicitis (n = 254)a | 7.0 | 2–15 |
a Patients were not eligible for extension of treatment beyond 10 days.
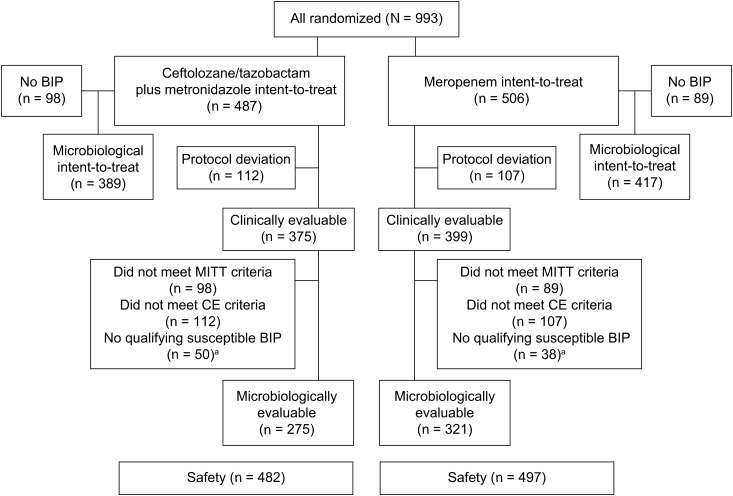
Patient disposition in ASPECT-cIAI (Assessment of the Safety Profile and Efficacy of Ceftolozane/Tazobactam in Complicated Intra-abdominal Infections). aPatients could be excluded for more than one reason. Abbreviations: BIP, baseline infecting pathogen; CE, clinically evaluable; MITT, microbiological ITT.
The majority of participants were from Europe (76.9%), followed by South America (10.5%), North America (7.6%), and other geographic regions (5.0%). A list of participating investigators and their sites is provided in the Supplementary Data. Baseline demographic characteristics were similar between treatment groups (Table (Table2).2). The most common origin of infection was the appendix, and the most common diagnosis was appendiceal perforation or abscess (Table (Table33).
Table 2.
Baseline Demographics (Microbiological Intent-to-Treat Population)
| Characteristic | Ceftolozane/Tazobactam Plus Metronidazole (n = 389) | Meropenem (n = 417) |
|---|---|---|
| Sex, male, No. (%) | 218 (56.0) | 248 (59.5) |
| Race, white, No. (%) | 367 (94.3) | 388 (93.0) |
| Age, y | ||
 Mean (SD) Mean (SD) | 50.8 (18.3) | 50.4 (16.9) |
 18–64, No. (%) 18–64, No. (%) | 289 (74.3) | 332 (79.6) |
 65–74, No. (%) 65–74, No. (%) | 54 (13.9) | 48 (11.5) |
 ≥75, No. (%) ≥75, No. (%) | 46 (11.8) | 37 (8.9) |
| Body mass index, kg/m2, mean (SD) | 26.76 (5.5) | 27.07 (5.3) |
| Baseline APACHE II score, No. (%)a | ||
 Mean (SD) Mean (SD) | 6.2 (4.2) | 6.0 (4.1) |
 0–5 0–5 | 191 (49.2) | 213 (51.1) |
 6–10 6–10 | 143 (36.9) | 153 (36.7) |
 11–15 11–15 | 42 (10.8) | 38 (9.1) |
 >15 >15 | 12 (3.1) | 13 (3.1) |
| Presence of bacteremia | 8 (2.1) | 12 (2.9) |
| Creatinine clearance, No. (%) | ||
 Mild renal impairment (50 to 79 mL/min) Mild renal impairment (50 to 79 mL/min) | 98 (25.2) | 109 (26.1) |
 Moderate renal impairment (30–49 mL/min) Moderate renal impairment (30–49 mL/min) | 23 (5.9) | 13 (3.1) |
 Severe renal impairment (<30 mL/min) Severe renal impairment (<30 mL/min) | 0 | 0 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; SD, standard deviation.
a Data missing in 1 patient.
Table 3.
Site of Infections, Diagnoses, Disease Characteristics, and Source Control Procedures (Microbiological Intent-to-Treat Population)
| Characteristic | Ceftolozane/Tazobactam Plus Metronidazole (n = 389) | Meropenem (n = 417) |
|---|---|---|
| Origin of current infection, No. (%)a | ||
 Appendix Appendix | 179 (46.0) | 205 (49.2) |
 Biliary–cholecystitis Biliary–cholecystitis | 73 (18.8) | 69 (16.5) |
 Colon Colon | 56 (14.4) | 62 (14.9) |
 Stomach/duodenum Stomach/duodenum | 40 (10.3) | 39 (9.4) |
 Small bowel Small bowel | 23 (5.9) | 19 (4.6) |
 Parenchymal (liver) Parenchymal (liver) | 16 (4.1) | 17 (4.1) |
 Parenchymal (spleen) Parenchymal (spleen) | 2 (0.5) | 2 (0.5) |
 Biliary–cholangitis Biliary–cholangitis | 1 (0.3) | 0 |
| Diagnosis, No. (%)b | ||
 Appendiceal perforation or abscess Appendiceal perforation or abscess | 175 (45.0) | 203 (48.7) |
 Cholecystitis with rupture, perforation, or progression of infection Cholecystitis with rupture, perforation, or progression of infection | 72 (18.5) | 69 (16.5) |
 Peritonitis due to other perforated viscus or following a prior operative procedure Peritonitis due to other perforated viscus or following a prior operative procedure | 41 (10.5) | 33 (7.9) |
 Acute gastric or duodenal perforation Acute gastric or duodenal perforation | 38 (9.8) | 33 (7.9) |
 Diverticular disease with perforation or abscess Diverticular disease with perforation or abscess | 29 (7.5) | 36 (8.6) |
 Other IAI abscess (including liver and spleen) Other IAI abscess (including liver and spleen) | 29 (7.5) | 36 (8.6) |
 Traumatic intestinal perforation Traumatic intestinal perforation | 5 (1.3) | 7 (1.7) |
| Abscess present, No. (%) | 219 (56.3) | 240 (57.6) |
 Multiple abscessesc Multiple abscessesc | 33 (15.1) | 32 (13.3) |
| Peritonitis present, No. (%) | 337 (86.6) | 340 (81.5) |
 Locald Locald | 198 (58.8) | 203 (59.7) |
 Diffused Diffused | 139 (41.2) | 137 (40.3) |
| Localized complicated appendicitis, No. (%) | 115 (29.6) | 142 (34.1) |
| Etiological mechanism, No. (%) | ||
 Postoperative infection Postoperative infection | 27 (6.9) | 28 (6.7) |
 Trauma Trauma | 4 (1.0) | 8 (1.9) |
 Spontaneous rupture Spontaneous rupture | 277 (71.2) | 302 (72.4) |
 Malignancy Malignancy | 11 (2.8) | 13 (3.1) |
 Other Other | 70 (18.0) | 66 (15.8) |
| Procedure type, No. (%)e | ||
 Laparotomy Laparotomy | 274 (70.4) | 272 (65.2) |
 Laparoscopy Laparoscopy | 86 (22.1) | 105 (25.2) |
 Percutaneous aspiration Percutaneous aspiration | 24 (6.2) | 37 (8.9) |
 Otherf Otherf | 5 (1.3) | 4 (1.0) |
Percentages are calculated as 100 × (no./No.), except where indicated by a footnote.
Abbreviation: IAI, intra-abdominal infection.
a Investigator could choose >1 site; totals are not mutually exclusive.
b Investigator could chose only 1 diagnosis.
c Percentages are calculated 100 × (no./No. of patients with abscess present).
d Percentages are calculated 100 × (no./No. of patients with peritonitis present).
e Patients could have multiple procedure types.
f Other procedures included appendectomy and esophagogastroduodenoscopy.
Pathogens at Baseline
The incidence and distribution of baseline pathogens were similar between the treatment groups. The most common gram-negative aerobes isolated at baseline from intra-abdominal specimens in the MITT population were Escherichia coli (525/806 [65.1%]), Klebsiella pneumoniae (76/806 [9.4%]), and P. aeruginosa (72/806 [8.9%]). The majority of infections were polymicrobial (257/389 [66.1%] and 288/417 [69.1%] patients in the ceftolozane/tazobactam plus metronidazole and meropenem treatment groups, respectively). There were 29 ESBL-producing Enterobacteriaceae isolated in each treatment group, an overall rate of 7.2% (58/806). Of 52 individual P. aeruginosa isolates for which MIC data were available, 3 (5.8%) isolates were resistant to ≥3 drug classes known to be active against P. aeruginosa, and 6 (11.5%) were nonsusceptible to ≥3 antipseudomonal drug classes.
The MIC required to inhibit the growth of 90% (MIC90) of Enterobacteriaceae was 1 mg/L for ceftolozane/tazobactam and 0.063 mg/L for meropenem. For P. aeruginosa, the MIC90 values for each study drug were 1 mg/L and 2 mg/L, respectively. Susceptibility rates to ceftolozane/tazobactam and meropenem were 97.4% and 99.9% for Enterobacteriaceae and 98.6% and 89.9% for P. aeruginosa, respectively.
Source Control Review Panel Findings
The surgical review panel reviewed 73 patients. Twenty-four patients (12 in each treatment group) were considered to have had inadequate source control and were excluded from the CE and ME populations. Of the 9 patients who were considered to have obtained clinical cure by the investigator but required a second procedure, the surgical review panel changed 2 patients' outcomes (1 per treatment arm) from cure to failure because of evidence of ongoing infection at the time of the second intervention.
Efficacy Analysis
For the primary endpoint, clinical cure rates were 83.0% (323/389) with ceftolozane/tazobactam plus metronidazole and 87.3% (364/417) with meropenem in the MITT population at the TOC visit, which occurred at a median of 27 days (interquartile range, 26–28 days) after the TOC visit. The weighted difference in clinical cure rates (ceftolozane/tazobactam plus metronidazole minus meropenem) was −4.2% with a 2-sided 95% CI of −8.91% to .54%, thus meeting the statistical criteria for noninferiority. Statistical noninferiority was also demonstrated for the ME population, where clinical cure rates were 94.2% and 94.7% (weighted difference, −1.0; 95% CI, −4.52 to 2.59) at the TOC visit (Figure (Figure2).2). Clinical cure rates in the ITT population at TOC were 83.6% for ceftolozane/tazobactam plus metronidazole and 86.2% for meropenem (difference, −2.6; 95% CI, −7.08 to 1.87), similar to those observed in the MITT population. In the CE population, cure rates were 94.1% and 94.0%, respectively (difference, 0.1; 95% CI, −3.30 to 3.55). At the end of therapy, clinical cure rates in the MITT population were higher in both treatment groups: 89.2% for ceftolozane/tazobactam plus metronidazole and 92.3% for meropenem (difference, −3.1; 95% CI, −7.23 to .89).

Primary and secondary analysis endpoints at the test-of-cure visit. In the microbiological intent-to-treat (MITT) population, a treatment failure approach was used, where indeterminate clinical responses were imputed as failures. In the microbiologically evaluable (ME) population, a data-as-observed approach was used, where indeterminate clinical responses were excluded from the analysis. Abbreviations: CI, confidence interval; NI, noninferiority margin.
In both treatment groups, 8.2% of patients in the MITT populations failed treatment at the TOC visit (Figure (Figure2).2). The most common reasons for failure were persisting or recurrent abdominal infection requiring an additional intervention (2.8% of failures in the ceftolozane/tazobactam plus metronidazole group and 3.6% in the meropenem group) and the requirement for additional antibiotics for ongoing cIAI (3.3% and 2.6%, respectively, for each treatment group). Other reasons for failure were postsurgical wound infection and death due to cIAI.
Clinical outcomes in the subgroup analyses were generally consistent with the primary and secondary analyses, with no meaningful differences recorded between treatments. Clinical cure rates with both treatments were generally lower in high-risk patients (elderly patients and those with higher APACHE II scores, moderate renal impairment, or small bowel and colon infections) vs the overall ME population (Table (Table44).
Table 4.
Subgroup Analysis of Clinical Cure at the Test-of-Cure Visit (Microbiologically Evaluable Population)
| Clinical Cure no./No. (%) | Ceftolozane/Tazobactam Plus Metronidazole (n = 275) | Meropenem (n = 321) | Percentage Difference (95% CI) |
|---|---|---|---|
| Sex | |||
 Male Male | 147/157 (93.6) | 182/189 (96.3) | −2.7 (−7.97 to 2.05) |
 Female Female | 112/118 (94.9) | 122/132 (92.4) | 2.5 (−4.04 to 8.91) |
| Age, y | |||
 18–64 18–64 | 206/214 (96.3) | 249/262 (95.0) | 1.2 (−2.80 to 5.03) |
 65–74 65–74 | 30/35 (85.7) | 36/38 (94.7) | −9.0 (−24.59 to 5.43) |
 ≥75 ≥75 | 23/26 (88.5) | 19/21 (90.5) | −2.0 (−20.76 to 18.79) |
| Region | |||
 Eastern Europe Eastern Europe | 213/221 (96.4) | 239/247 (96.8) | −0.4 (−4.10 to 3.12) |
 Western Europe Western Europe | 2/5 (40.0) | 7/11 (63.6) | −23.6 (−58.95 to 22.86) |
 North America North America | 8/11 (72.7) | 10/11 (90.9) | −18.2 (−48.41 to 15.40) |
 South America South America | 28/28 (100) | 40/42 (95.2) | 4.8 (−7.78 to 15.79) |
 Rest of world Rest of world | 8/10 (80.0) | 8/10 (80.0) | 0 (−34.14 to 34.14) |
| APACHE II score | |||
 <10 <10 | 213/222 (95.9) | 262/274 (95.6) | 0.3 (−3.61 to 3.98) |
 ≥10 ≥10 | 45/52 (86.5) | 42/47 (89.4) | −2.8 (−16.08 to 10.92) |
| Baseline CrCl, mL/min | |||
 <50 <50 | 8/11 (72.7) | 5/7 (71.4) | 1.3 (−34.37 to 40.92) |
 ≥50 ≥50 | 251/264 (95.1) | 299/314 (95.2) | −0.1 (−3.95 to 3.43) |
| Prior antibiotic use | |||
 Yes Yes | 133/145 (91.7) | 163/177 (92.1) | −0.4 (−6.81 to 5.69) |
 No No | 126/130 (96.9) | 141/144 (97.9) | −1.0 (−5.76 to 3.30) |
| Primary site of infection | |||
 Bowel (small or large) Bowel (small or large) | 35/43 (81.4) | 51/57 (89.5) | −8.1 (−23.17 to 5.74) |
 Other site of IAI Other site of IAI | 224/232 (96.6) | 253/264 (95.8) | 0.7 (−2.97 to 4.28) |
| Anatomic site of infection | |||
 Appendix Appendix | 136/141 (96.5) | 172/179 (96.1) | 0.4 (−4.55 to 4.79) |
 Nonappendix Nonappendix | 123/134 (91.8) | 132/142 (93.0) | −1.2 (−7.86 to 5.33) |
 Biliary–cholangitis Biliary–cholangitis | 1/1 (100) | 0/0 | NC |
 Biliary–cholecystitis Biliary–cholecystitis | 49/50 (98.0) | 43/46 (93.5) | 4.5 (−4.99 to 15.63) |
 Colon Colon | 25/31 (80.6) | 39/42 (92.9) | −12.2 (−29.77 to 3.41) |
 Parenchymal (liver) Parenchymal (liver) | 9/10 (90.0) | 8/8 (100) | −10.0 (−40.42 to 23.46) |
 Parenchymal (spleen) Parenchymal (spleen) | 2/2 (100) | 1/1 (100) | 0 (−65.76 to 79.35) |
 Small bowel Small bowel | 11/13 (84.6) | 12/15 (80.0) | 4.6 (−25.20 to 32.12) |
 Stomach/duodenum Stomach/duodenum | 28/30 (93.3) | 26/26 (100) | −6.7 (−21.32 to 7.08) |
 Other Other | 5/5 (100) | 5/6 (83.3) | 16.7 (−28.88 to 56.35) |
| Peritonitis type | |||
 Localized Localized | 126/132 (95.5) | 152/161 (94.4) | 1.0 (−4.62 to 6.34) |
 Diffuse Diffuse | 98/104 (94.2) | 108/114 (94.7) | −0.5 (−7.37 to 6.02) |
| No. of abscesses | |||
 Single Single | 116/124 (93.5) | 146/152 (96.1) | −2.5 (−8.65 to 2.90) |
 Multiple Multiple | 22/25 (88.0) | 23/25 (92.0) | −4.0 (−22.86 to 14.69) |
| Procedure type | |||
 Percutaneous aspiration Percutaneous aspiration | 14/14 (100) | 23/23 (100) | 0 (−21.53 to 14.31) |
 Laparoscopy Laparoscopy | 63/66 (95.5) | 73/81 (90.1) | 5.3 (−3.98 to 14.27) |
 Laparotomy Laparotomy | 181/193 (93.8) | 207/217 (95.4) | −1.6 (−6.42 to 2.90) |
Regions are defined as follows: Eastern Europe (Bulgaria, Croatia, Estonia, Georgia, Hungary, Latvia, Lithuania, Poland, Republic of Moldova, Romania, Russia, Serbia, Slovakia, Ukraine); Western Europe (Belgium, Germany, Spain); North America (Mexico, United States); South America (Argentina, Brazil, Chile, Colombia, Peru); and rest of world (Australia, Israel, South Africa, South Korea).
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CI, confidence interval; CrCl, creatinine clearance; IAI, intra-abdominal infection; NC, not calculated.
Per-pathogen clinical cure rates were similar between groups (Supplementary Table 2). In all patients with ESBL-producing Enterobacteriaceae, the clinical cure rate was 95.8% (23/24) in the ceftolozane/tazobactam plus metronidazole group and 88.5% (23/26) in the meropenem group (Supplementary Figure). In patients with CTX-M-14/15 ESBL-producing Enterobacteriaceae, clinical cure was observed in 13 of 13 (100%) and 8 of 11 (72.7%) patients, respectively.
Safety
The incidence of AEs that developed during treatment was similar between the ceftolozane/tazobactam plus metronidazole group and the meropenem group (44.0% and 42.7%, respectively), and most events were mild to moderate in severity. The AEs occurring in ≥2% of patients in either treatment group are shown in Table Table5.5. The most common laboratory AEs were increased alanine aminotransferase and aspartate aminotransferase, which occurred in 2.5% and 1.6% of all patients, respectively. Drug-related AEs leading to discontinuation were few, occurring in 3 patients (0.6%) in the ceftolozane/tazobactam plus metronidazole group and 4 patients (0.8%) in the meropenem group.
Table 5.
Adverse Events Occurring in ≥2% of Patients in Either Treatment Group (Safety Population)
| Adverse Event No. (%) | Ceftolozane/Tazobactam Plus Metronidazole (n = 482) | Meropenem (n = 497) |
|---|---|---|
| Any adverse event | 212 (44.0) | 212 (42.7) |
| Nausea | 38 (7.9) | 29 (5.8) |
| Diarrhea | 30 (6.2) | 25 (5.0) |
| Vomiting | 16 (3.3) | 20 (4.0) |
| Pyrexia | 25 (5.2) | 20 (4.0) |
| Hypokalemia | 14 (2.9) | 8 (1.6) |
| Insomnia | 17 (3.5) | 11 (2.2) |
| Headache | 12 (2.5) | 9 (1.8) |
| Anemia, postoperative | 10 (2.1) | 8 (1.6) |
| Hypertension | 9 (1.9) | 10 (2.0) |
Serious AEs occurred in 39 of 482 (8.1%) and 36 of 497 (7.2%) patients in the ceftolozane/tazobactam plus metronidazole and meropenem groups, respectively. Drug-related serious AEs occurred in 1 patient in each treatment group (both Clostridium difficile infection).
There were 11 deaths (2.3%) in the ceftolozane/tazobactam plus metronidazole group and 8 deaths (1.6%) in the meropenem group; no death was considered by the investigators to be related to study treatment.
DISCUSSION
The increasing prevalence of multidrug-resistant, gram-negative organisms in serious infections is an important concern, and has led to the development of new therapeutic agents [19, 27, 28]. In this trial, the efficacy and safety of the novel antimicrobial agent ceftolozane/tazobactam were evaluated in patients with mostly community-acquired cIAIs. One-fifth of patients were aged ≥65 years, one-third had renal impairment, >80% had peritonitis, and the most frequent site of infection was the appendix. These factors attest to the high degree of illness severity in the patients who were enrolled in this trial. Although the microbiology of infecting pathogens was similar to that observed in other phase 3 studies in this indication [29–32], the overall rate of infection with ESBL-positive isolates (7.2%) was higher than in previous observations [29, 30]. The key finding from this study was that intravenous ceftolozane/tazobactam (1.5 g) plus metronidazole (500 mg) every 8 hours was noninferior to the comparator treatment, intravenous meropenem (1 g) every 8 hours, when administered for 4–14 days for the treatment of cIAIs.
Ceftolozane/tazobactam plus metronidazole demonstrated high clinical cure rates in patients infected with the common cIAI pathogens including E. coli, K. pneumoniae, P. aeruginosa, Enterobacter cloacae, and Klebsiella oxytoca, as well as Streptococcus anginosus, Streptococcus constellatus, and Streptococcus salivarius.
As with other cephalosporins [31, 33–35], the AEs reported most frequently were gastrointestinal symptoms of nausea, vomiting, and diarrhea and are expected events in a postoperative population with cIAI.
Activity Against Extended-Spectrum β-Lactamases
In the past 10 years, ESBL-producing Enterobacteriaceae have become an important challenge for antibiotic treatment of infections, and ESBL carriage rates are increasing in nearly all geographic areas [36]. In the United States, up to 19% of healthcare-associated infections are due to ESBL-producing Enterobacteriaceae, constituting a serious threat of resistance [37]. For severe ESBL infections, carbapenems have become the drugs of choice [38].
Ceftolozane/tazobactam showed substantial clinical and microbiological activity against ESBL-producing E. coli and Klebsiella strains. The ESBL-positive rate is consistent with observations from the Study for Monitoring Antimicrobial Resistance Trends, a global surveillance program of gram-negative bacilli from IAIs. This European study characterized >3000 patient isolates in 2008, revealing ESBL rates of 11.6% in E. coli and 17.9% in K. pneumoniae [39].
CTX-M–type ESBLs are by far the most common ESBLs worldwide, and are often associated with multidrug resistance in Enterobacteriaceae [11, 36]. In our study, more than one-half of the ESBL-producing Enterobacteriaceae isolated at baseline were positive for CTX-M-14 or CTX-M-15–type enzymes, but no K. pneumoniae carbapenemase enzymes were identified. Ceftolozane/tazobactam plus metronidazole maintained clinical efficacy against these highly resistant strains (100%) compared with 72.7% with meropenem.
Activity Against Pseudomonas aeruginosa
Multidrug-resistant P. aeruginosa often requires complex antimicrobial regimens and, when treated inadequately, infections caused by this pathogen are associated with particularly poor outcomes including postoperative complications, longer hospital stays, and increased mortality [5, 6, 40–42]. In vitro studies have shown that ceftolozane/tazobactam is the most potent antipseudomonal agent, maintaining activity against many multidrug-resistant strains [13]. Ceftolozane/tazobactam demonstrated efficacy against P. aeruginosa, even though experience with multidrug-resistant P. aeruginosa was limited.
In conclusion, these results suggest that ceftolozane/tazobactam plus metronidazole is a potential alternative to the currently recommended antimicrobials for the treatment of cIAIs, especially when resistant Enterobacteriaceae or P. aeruginosa are suspected, such as in healthcare-associated infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the trial participants, the investigators who made this study possible, and the surgical review panel members. Additional investigators are listed in the Supplementary Appendix. Editorial support for this manuscript was provided by Kate Bradford of PAREXEL.
We thank the trial participants, the investigators who made this study possible, and the surgical review panel members. Additional investigators are listed in the Supplementary Appendix. Editorial support for this manuscript was provided by Kate Bradford of PAREXEL.
Financial support. This work was supported by Cubist Pharmaceuticals.
This work was supported by Cubist Pharmaceuticals.
Potential conflicts of interest. E. H., B. M., M. P., J. Steenbergen, M. Y., and S. C. are employees of Cubist Pharmaceuticals. I. F. and G. Y. are previous employees of Cubist Pharmaceuticals. C. E. has received research grant support from Wyeth (now Pfizer) and consultancy or speaker fees from AstraZeneca, Bayer, Merck Sharp & Dohme, Novartis, Pfizer, Wyeth, and Cubist. P. S. B. has served on speaker's bureaus for Pfizer, Merck, and Forest Laboratories and has acted as a consultant for Cubist, Forest, Astellas, and Durata. J. Solomkin is a consultant to Cubist, Bayer, AstraZeneca, Merck, Tetraphase, Rempex, and Pfizer, and has provided lectures supported by GlaxoSmithKline, Bayer, Merck, and Pfizer.
E. H., B. M., M. P., J. Steenbergen, M. Y., and S. C. are employees of Cubist Pharmaceuticals. I. F. and G. Y. are previous employees of Cubist Pharmaceuticals. C. E. has received research grant support from Wyeth (now Pfizer) and consultancy or speaker fees from AstraZeneca, Bayer, Merck Sharp & Dohme, Novartis, Pfizer, Wyeth, and Cubist. P. S. B. has served on speaker's bureaus for Pfizer, Merck, and Forest Laboratories and has acted as a consultant for Cubist, Forest, Astellas, and Durata. J. Solomkin is a consultant to Cubist, Bayer, AstraZeneca, Merck, Tetraphase, Rempex, and Pfizer, and has provided lectures supported by GlaxoSmithKline, Bayer, Merck, and Pfizer.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Articles from Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/cid/civ097
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/cid/article-pdf/60/10/1462/16787398/civ097.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Intra-abdominal infections survival guide: a position statement by the Global Alliance For Infections In Surgery.
World J Emerg Surg, 19(1):22, 08 Jun 2024
Cited by: 0 articles | PMID: 38851700
Review
The use of new antibacterial drugs against infections caused by multidrug-resistant Gram-negative bacteria: an Italian real-world evidence study in a Lombardy hospital.
Naunyn Schmiedebergs Arch Pharmacol, 397(10):8069-8075, 24 May 2024
Cited by: 1 article | PMID: 38789634
Cost-effectiveness analysis of CTZ/TAZ for the treatment of ventilated hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia in Japan.
BMC Health Serv Res, 24(1):389, 28 Mar 2024
Cited by: 0 articles | PMID: 38549158 | PMCID: PMC10976789
Ceftolozane/Tazobactam for the Treatment of Complicated Infections in Hospital Settings-A French Real-world Study.
Open Forum Infect Dis, 11(2):ofae037, 22 Feb 2024
Cited by: 0 articles | PMID: 38390458 | PMCID: PMC10883286
New Antibiotics Against Multidrug-Resistant Gram-Negative Bacteria in Liver Transplantation: Clinical Perspectives, Toxicity, and PK/PD Properties.
Transpl Int, 37:11692, 01 Feb 2024
Cited by: 0 articles | PMID: 38362283 | PMCID: PMC10867129
Review Free full text in Europe PMC
Go to all (199) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (2 citations) ClinicalTrials.gov - NCT01445678
- (2 citations) ClinicalTrials.gov - NCT01445665
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Efficacy of ceftolozane/tazobactam against urinary tract and intra-abdominal infections caused by ESBL-producing Escherichia coli and Klebsiella pneumoniae: a pooled analysis of Phase 3 clinical trials.
J Antimicrob Chemother, 72(1):268-272, 05 Oct 2016
Cited by: 51 articles | PMID: 27707990
Analysis of patients with diabetes and complicated intra-abdominal infection or complicated urinary tract infection in phase 3 trials of ceftolozane/tazobactam.
BMC Infect Dis, 17(1):316, 02 May 2017
Cited by: 9 articles | PMID: 28464828 | PMCID: PMC5414364
Multicenter, double-blind, randomized, phase II trial to assess the safety and efficacy of ceftolozane-tazobactam plus metronidazole compared with meropenem in adult patients with complicated intra-abdominal infections.
Antimicrob Agents Chemother, 58(9):5350-5357, 30 Jun 2014
Cited by: 60 articles | PMID: 24982069 | PMCID: PMC4135839
Ceftolozane/Tazobactam: A Review in Complicated Intra-Abdominal and Urinary Tract Infections.
Drugs, 76(2):231-242, 01 Feb 2016
Cited by: 20 articles | PMID: 26746849
Review





