Abstract
Free full text

Imatinib-treated chronic myeloid leukemia patients with discordant response between cytogenetic and molecular tests at 3 and 6 month time-points have a reduced probability of subsequent optimal response
The 2013 version of the European LeukemiaNet (ELN) recommendations for the management of chronic myeloid leukemia (CML) patients defines as optimal response the achievement of a partial cytogenetic response (PCyR) and/or BCR-ABL1 transcript ≤10%IS at 3 months, and of a complete cytogenetic response (CCyR) and/or BCR-ABL1 transcript ≤1%IS at 6 months.1 Obtaining less than PCyR (i.e. Ph+ 36–95%) and/or BCR-ABL1 >10%IS at 3 months, and less than CCyR and/or BCR-ABL1 >1%IS at 6 months are regarded as warning.1 Patients with discordant response between cytogenetic and molecular tests (e.g. PCyR and BCR-ABL1 >10%IS at 3 months) may be alternatively considered at the same timepoint as optimal or warning. There is no information currently available on the outcome of these patients. The objective of this analysis was to provide the first description of the outcome of CML patients with discordant results between cytogenetic and molecular tests.
We retrospectively analyzed our cohort of early chronic phase CML patients for which both cytogenetic and molecular responses were evaluable at 3 and/or 6 months after imatinib start. Individual charts were reviewed and clinical data were extracted. Informed consent was obtained before the start of therapy in accordance with the Declaration of Helsinki. All patients received front-line treatment with imatinib 400 mg daily, starting within 8 weeks from diagnosis (median 2.7 weeks) with the exception of two female patients, who having been diagnosed during pregnancy, were observed without any treatment until delivery (32 and 34 weeks, respectively). Cytogenetic analysis was performed in bone marrow cells with conventional G-banding technique; only samples with at least 20 metaphases were considered evaluable. PCyR and CCyR were defined as 1–35% and 0% Ph+ metaphases, respectively. Molecular response was assessed by quantitative polymerase chain reaction (Q-PCR); results were expressed as the BCR-ABL1/ABL1 transcript ratio (International Scale – IS).2 A major molecular response (MMR) was defined as BCR-ABL1/ABL1 ratio ≤ 0.1%. A deep molecular response (MR4.0) was defined as BCR-ABL1/ABL1 ratio ≤ 0.01% or undetectable transcript with at least 104 copies of ABL1. Failure-free survival (FFS) was measured from the start of imatinib to the date of any of the following events: progression to accelerated or blastic phase (ABP), death by any cause at any time, imatinib dose increase (≥ 600 mg/day) or a switch to nilotinib/dasatinib for primary or secondary hematologic or cytogenetic resistance, according to the definitions at the time of the occurrence of the event (principally 2009 ELN recommendations).3 Patients switching to other tyrosine-kinase inhibitor (TKI) for intolerance or other reasons (e.g. availability of a clinical trial) were censored at the time of imatinib cessation. Cumulative responses and survival probabilities were estimated by the Kaplan-Meier method and compared by log-rank test; differences among variables were evaluated by the Fisher’s exact test.
A total of 216 evaluable patients were retrieved from a cohort of 350 consecutive CML patients. Median age at diagnosis was 55 (range 20–84) years. The distribution according to the Sokal score4 was: 92 (42.6%), 87 (40.3%) and 36 (16.7%) patients for low, intermediate and high risk, respectively (for one patient Sokal risk was not evaluable). Stratification according to the EUTOS score5 identified 195 (90.3%) and 20 (9.7%) patients belonging to the low and high risk group, respectively.
At 3 months, 183 patients (84.7%) were evaluable for cytogenetic response, 181 patients (83.8%) for molecular response, 163 patients (75.5%) for both analyses, and 158 patients (73.1%) for “combined” response analysis, as 5 patients with primary cytogenetic failure (i.e. 100% Ph+) were excluded. At 6 months, 179 (82.9%) and 196 (90.7%) patients were evaluable for cytogenetic and molecular response, respectively, 176 (81.5%) for both tests and 144 (66.7%) for “combined” response analysis (32 patients were excluded for cytogenetic and/or molecular failure) (Table 1).
Table 1.
Response to imatinib at 3 and 6 month timepoints.
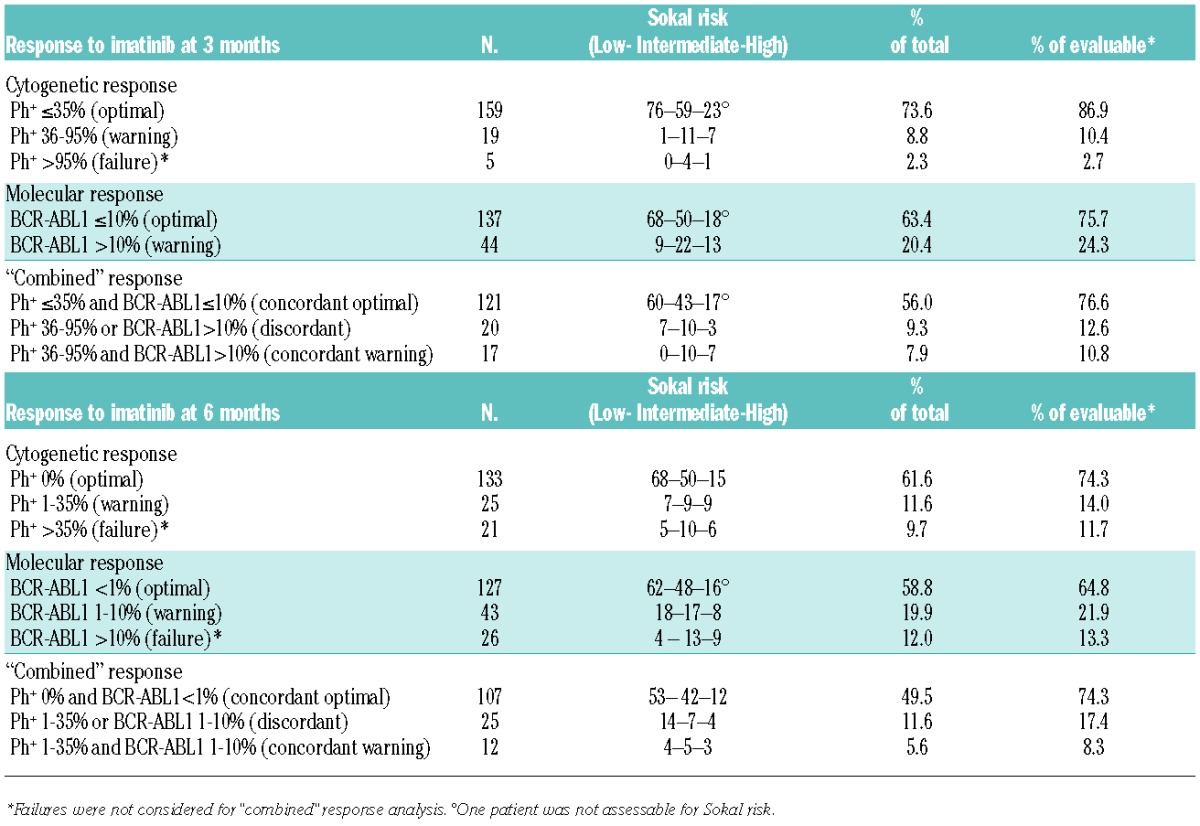
Patients with concordant optimal response at 3 months had a significantly superior chance of subsequent optimal response. CCyR rates at 6 months for concordant optimal, discordant or concordant warning patients were 85%, 37% and 17%, respectively (p<0.001 for concordant optimal vs. others). MMR rates at 12 months for the three cohorts were 62%, 12% and 0%, respectively (P<0.001 for concordant optimal vs. others). Median time to CCyR was shorter for patients with concordant optimal response than for patients with discordant or concordant warning response (3.5 vs.11.9 months vs. median not reached, respectively, P<0.001). In addition, median time to MMR was significantly different in the three groups (9.1 vs. 49.6 months vs. median not reached, respectively, P<0.001).
Patients with concordant optimal response at 6 months had a significantly higher probability of obtaining MMR at 12 months (82%) compared to patients with discordant (17%) or concordant warning (10%) response. Additionally, median time to MMR was significantly shorter in the first group compared to the others (8.1 vs. 30.5 vs. 25.7 months, respectively, P=0.006).
Patients evaluable for “combined” response analysis at both timepoints were 128: 71 out of 100 patients with concordant optimal response at 3 months maintained concordant optimal response at 6 months, while only 2/16 patients with discordant and no patients with concordant warning results at 3 months gained concordant optimal response at 6 months.
Scrutinizing deeper responses, 37 out of 62 (60%) evaluable patients with concordant optimal response at 3 months obtained a subsequent MR4.0 at a median time of 17 months. Notably, only 3 out of 11 (27%) evaluable patients with discordant and no patient with concordant warning response at the same timepoint obtained a subsequent MR4.0.
At a median follow-up of 48 months (range 5–124) FFS was significantly different between concordant optimal, discordant and concordant warning patients at 3 months (80.6% vs. 38.9% vs. 15.4%, respectively) (Figure 1A), while patients with concordant optimal results at 6 months had a significantly better FFS than the other two groups (87.6% vs. 65.2% and 70%, respectively) (Figure 1B). Overall, 12 patients progressed to ABP (median time from diagnosis: 7.5 months, range 4–53): response at 3 and 6 months did not significantly influence the probability of progression.
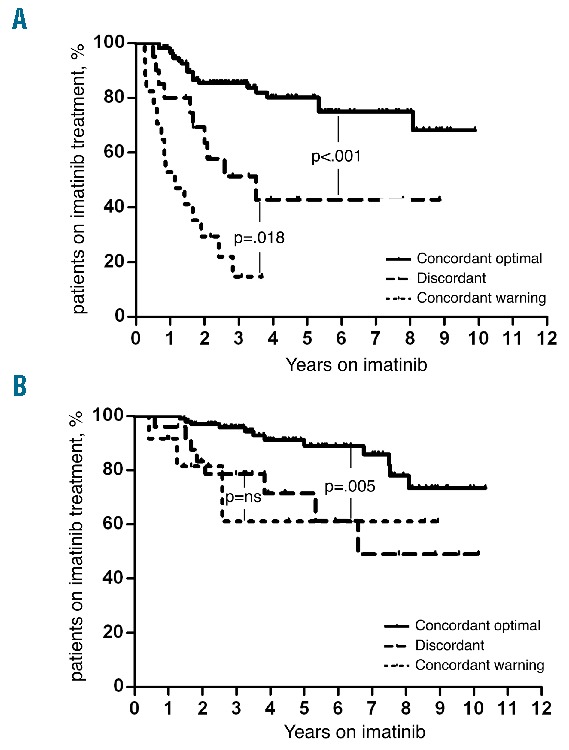
Failure-free survival according to 3-month (A) and 6-month (B) combined cytogenetic and molecular response.
There is growing evidence of the importance of early response to therapy. In particular, the role of a BCR/ABL1 transcript level lower than 10% as a positive predictive factor has been outlined in many studies, including the ELN recommendations, and reports from the Hammersmith group,6 the German CML-IV study7 and the NCCN guidelines.8 In the latter, as well as in data from the German7 and MD Anderson9 groups, an early cytogenetic response (i.e. PCyR at 3 months) was also recognized as a powerful predictor for better long-term outcome. However, little is known about those cases with discordant results at early timepoints between cytogenetics and molecular biology. In the MDACC report, among patients treated with imatinib (400 or 800 mg), 14% had less than PCyR at 3 months, while only 3% had BCR/ABL1 >10%.9 In the present series of 163 patients with both cytogenetic and molecular analysis evaluable at 3 months, 121 (74.2%) resulted concordantly optimal, 20 (12.3%) concordantly warning and 17 (10.4%) discordant, all but two being in optimal cytogenetic response (i.e. PCyR) with BCR/ABL1 transcript level >10%; the remaining 5 patients (3.1%) showed primary cytogenetic failure (i.e. 100% Ph+). After 6 months of treatment, 176 patients were evaluable for combined cytogenetic and molecular responses, with 107 (60.8%) concordant optimal, 12 (6.8%) concordant warning and 25 (14.2%) discordant, mainly due to optimal cytogenetic response (i.e. CCyR) with warning BCR/ABL1 transcript level. The remaining 32 patients (18.2%) had either concordant failure results, or discordant results (warning-failure) between cytogenetic and molecular tests, and only 3 of them subsequently obtained MMR with imatinib. Our results indicate that only those patients with concordant cytogenetic and molecular optimal response at the earliest timepoints have an excellent probability of obtaining subsequent MMR, deeper MR and favorable long-term FFS. In particular, patients with CCyR and BCR/ABL1 <1% (i.e. concordant optimal response) at 6 months of standard dose imatinib had 3-year FFS of 95.8% (95%CI: 91.7–99.8), which is comparable to that reported by Jain et al. (89%) in patients treated with different TKIs, including nilotinib and dasatinib. Conversely, patients with at least one warning test at 3 or 6 months have an inferior outcome, not statistically different to that observed in cases with both cytogenetic and molecular warning results. We therefore suggest that CML patients not attaining both cytogenetic and molecular optimal response, as defined by the 2013 ELN recommendations, after 3 and 6 months of imatinib, might be considered at higher risk of treatment failure.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
Articles from Haematologica are provided here courtesy of Ferrata Storti Foundation
Full text links
Read article at publisher's site: https://doi.org/10.3324/haematol.2015.124685
Read article for free, from open access legal sources, via Unpaywall:
https://haematologica.org/article/download/7473/46968
Citations & impact
Impact metrics
Citations of article over time
Article citations
How many chronic myeloid leukemia patients who started a frontline second-generation tyrosine kinase inhibitor have to switch to a second-line treatment? A retrospective analysis from the monitoring registries of the italian medicines agency (AIFA).
Cancer Med, 9(12):4160-4165, 22 Apr 2020
Cited by: 18 articles | PMID: 32319737 | PMCID: PMC7300412
Molecular monitoring in CML: how deep? How often? How should it influence therapy?
Hematology Am Soc Hematol Educ Program, 2018(1):168-176, 01 Nov 2018
Cited by: 13 articles | PMID: 30504306 | PMCID: PMC6246017
Review Free full text in Europe PMC
Molecular monitoring in CML: how deep? How often? How should it influence therapy?
Blood, 132(20):2125-2133, 01 Nov 2018
Cited by: 7 articles | PMID: 30429156
Review
Omitting cytogenetic assessment from routine treatment response monitoring in chronic myeloid leukemia is safe.
Eur J Haematol, 100(4):367-371, 07 Feb 2018
Cited by: 3 articles | PMID: 29288559
The significance of early warning in chronic myeloid leukemia.
Expert Rev Hematol, 11(4):265-266, 15 Jun 2017
Cited by: 1 article | PMID: 28598209
Go to all (7) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Concordant optimal molecular and cytogenetic responses at both 3 and 6 months predict a higher probability of MR4.5 achievement in patients with chronic myeloid leukemia treated with imatinib.
Leuk Lymphoma, 58(6):1384-1393, 12 Oct 2016
Cited by: 1 article | PMID: 27733081
Switching to second-generation tyrosine kinase inhibitor improves the response and outcome of frontline imatinib-treated patients with chronic myeloid leukemia with more than 10% of BCR-ABL/ABL ratio at 3 months.
Cancer Med, 4(7):995-1002, 10 Mar 2015
Cited by: 5 articles | PMID: 25756742 | PMCID: PMC4529338
The BCR-ABL1 transcript type influences response and outcome in Philadelphia chromosome-positive chronic myeloid leukemia patients treated frontline with imatinib.
Am J Hematol, 92(8):797-805, 30 May 2017
Cited by: 46 articles | PMID: 28466557
Chronic Myeloid Leukemia--Prognostic Value of Mutations.
Asian Pac J Cancer Prev, 16(17):7415-7423, 01 Jan 2015
Cited by: 21 articles | PMID: 26625737
Review




