Abstract
Introduction
Global epidemiological studies have revealed considerable geographical differences in prevalence of atopic dermatitis (AD).Aim
To present the epidemiology of AD, risk factors and co-occurrence of allergic diseases in the Polish population.Material and methods
The present paper is a part of the Epidemiology of Allergic Disorders in Poland study. We studied 22 703 participants by ECRHS/ISAAC questionnaire; 18 617 (53.8% female, 24.2% 6-7 y.o., 25.4% 13-14 y.o., 50.4% 20-44 y.o.) completed questionnaires were accepted. Four thousand seven hundred and eighty-three participants (25.7%) have undergone a medical examination.Results
Atopic dermatitis was diagnosed in 3.91% (6-7 y.o. 5.34%, 13-14 y.o. 4.3%, adults 3.02%), more often in females (OR = 1.52; 95% CI: 0.56-0.77), in the cities (OR = 2.23; 95% CI: 1.61-3.09), in mothers (OR = 2.07; 95% CI: 1.72-2.48) and fathers (OR = 2.00; 95% CI: 1.61-2.49) with atopy, higher education (OR = 1.61; 95% CI: 1.11-2.32) and economic status (OR = 1.35; 95% CI: 1.04-1.74). The highest prevalence was found in Katowice (4.89%) and lowest in rural areas (1.9%). Coexisting AD and allergic rhinitis (AR) was found in 26.17%, AR and asthma in 9.09% and AD, AR and asthma in 14.6%. Atopic dermatitis was diagnosed by allergologists in 6.5% (6-7 y.o. 8.7%, 13-14 y.o. 9.0%, adults 3.6%). Most diagnoses were made in Poznan (16.76%) and smallest in rural area (3.67%). 78.8% of subjects were diagnosed with AD for the first time although they had earlier experienced its symptoms.Conclusions
Atopic dermatitis prevalence in Poland is below the mean rate for Europe, but the risk factor profile is similar to other countries. Atopic dermatitis is more frequent in well-educated females with atopic parents and high socioeconomic status and who live in a city.Free full text

Atopic dermatitis is a serious health problem in Poland. Epidemiology studies based on the ECAP study
Abstract
Introduction
Global epidemiological studies have revealed considerable geographical differences in prevalence of atopic dermatitis (AD).
Aim
To present the epidemiology of AD, risk factors and co-occurrence of allergic diseases in the Polish population.
Material and methods
The present paper is a part of the Epidemiology of Allergic Disorders in Poland study. We studied 22 703 participants by ECRHS/ISAAC questionnaire; 18 617 (53.8% female, 24.2% 6–7 y.o., 25.4% 13–14 y.o., 50.4% 20–44 y.o.) completed questionnaires were accepted. Four thousand seven hundred and eighty-three participants (25.7%) have undergone a medical examination.
Results
Atopic dermatitis was diagnosed in 3.91% (6–7 y.o. 5.34%, 13–14 y.o. 4.3%, adults 3.02%), more often in females (OR = 1.52; 95% CI: 0.56–0.77), in the cities (OR = 2.23; 95% CI: 1.61–3.09), in mothers (OR = 2.07; 95% CI: 1.72–2.48) and fathers (OR = 2.00; 95% CI: 1.61–2.49) with atopy, higher education (OR = 1.61; 95% CI: 1.11–2.32) and economic status (OR = 1.35; 95% CI: 1.04–1.74). The highest prevalence was found in Katowice (4.89%) and lowest in rural areas (1.9%). Coexisting AD and allergic rhinitis (AR) was found in 26.17%, AR and asthma in 9.09% and AD, AR and asthma in 14.6%. Atopic dermatitis was diagnosed by allergologists in 6.5% (6–7 y.o. 8.7%, 13–14 y.o. 9.0%, adults 3.6%). Most diagnoses were made in Poznan (16.76%) and smallest in rural area (3.67%). 78.8% of subjects were diagnosed with AD for the first time although they had earlier experienced its symptoms.
Conclusions
Atopic dermatitis prevalence in Poland is below the mean rate for Europe, but the risk factor profile is similar to other countries. Atopic dermatitis is more frequent in well-educated females with atopic parents and high socioeconomic status and who live in a city.
Introduction
Epidemiological data are a prerequisite for obtaining a fuller picture and better understanding of allergic disorders, including atopic dermatitis (AD), as an important medical and socioeconomic problem [1]. The incidence of allergic disorders has increased in recent years as confirmed by global epidemiological studies (The International Study of Asthma and Allergies in Childhood (ISAAC), The European Community Respiratory Health Survey (ECRHS)), which also revealed considerable geographical differences in prevalence. The ISAAC Phase One found that the prevalence of AD in children and adolescents varied from less than 2% in China to approximately 20% in Australia, the United Kingdom and Scandinavia [2, 3]. Harrop et al. [4] conducted an epidemiological study according to ECRHS II protocol in 12 European countries which showed a very considerable variability in the prevalence of eczema in adults aged 20–44 years, the mean prevalence rate being 7.1% with the lowest rate of 2.2% reported in Switzerland and the highest rate of 17.6% reported in Estonia. Although increases in incidences are observed everywhere, rapidly developing countries and the regions with previously relatively low morbidity rates are the most affected.
In the last 30 years, Poland, and Central and East Europe in general have undergone a very deep transformation of the political and economic system with the adoption of Western lifestyle, improved hygiene level, and rapid industrial and economic development. As a result, the number of new cases of allergy-related disorders has increased and now the relevant epidemiological data are similar to those reported in the Western Europe [5]. As early as 2003, this was confirmed by Lis et al. in a study using the ISAAC methodology [6]. In Poland, the first questionnaire-based surveys to estimate the prevalence of allergic disorders were carried out in the 1990s and they confirmed that allergy was a serious epidemiological problem. However, most of the Polish data are derived from small-scale studies conducted in selected cities. In children, depending on the region and diagnostic criteria, the estimated prevalence of AD ranged from 1.6% [7] to 4.7% [8] to 5.8% [9] to 12.9% [10] to 26.6% [6]. In adults, the prevalence rate ranged from 0.4% to 1.5% [7, 8]. However, the studies assessing the epidemiology of AD did not include rural areas or all regions of Poland, employed different methodology and were based on different material [6, 9–12]. Many authors emphasize that numerous environmental and genetic factors play a role in the development of AD and their contribution depends on the region, intensity and interaction [5]. So far, there have been no comprehensive studies to assess the significance of various risk factors for the development of AD symptoms in the Polish population.
Aim
The aim of the present study was to evaluate the prevalence of AD in the population of Polish children, adolescents and young adults. In addition, we analyze the role of risk factors and co-morbidity of allergic diseases. The presented work is part of the Epidemiology of Allergic Disorders in Poland (ECAP study).
Material and methods
Study group
A questionnaire survey was carried out on a group of 22703 subjects with the response rate of 64.4% and eventually 18617 completed questionnaires were accepted. The study involved two age groups of children, 6–7 year olds and 13–14 year olds, and adults aged 20–44. There were 4510 (24.2%) 6–7 year olds, 4721 (25.4%) 13–14 year olds and 9386 (50.4%) adults. Of the respondents 10 011 (53.8%) were females and 8606 (46.2%) were males. In the medical evaluation part of the study 4783 patients (25.7% of the respondents) were assessed on an outpatient basis, including 1329 6–7 year olds, 1321 13–14 year olds and 2133 adults.
Methodology
The ECAP used the methodology of ISAAC and ECRHS II [2, 3, 13, 14] and the study areas were selected according to the ECRHS guidelines. The ECAP involved the populations of eight largest urban agglomerations in Poland (each with over 150 000 inhabitants) and additionally one rural region, also with a population of over 150 000 since the rural population accounts for 39% of the entire Polish population. The study areas were specifically chosen but the study subjects were selected by stratified random sampling based on the national identification number PESEL. The Computer Assisted Personal Interviewing (CAPI) technique was used in the survey. The data were transmitted by General Packet Radio Service (GPRS) and recorded online on the server of the Medical University of Warsaw. The system allowed a three-stage quality control.
Questionnaire-based survey
The questionnaire was based on the translated and validated ECRHS and ISAAC questionnaires. The following diagnostic criteria were accepted.
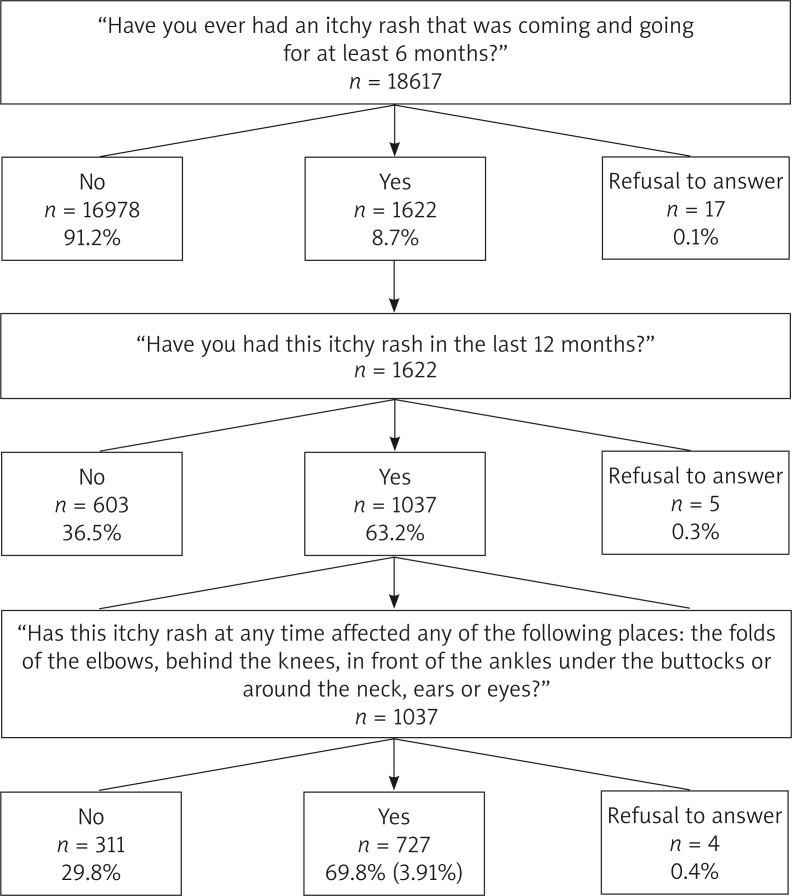
The diagnosis of AD was made in three stages. When the answer to the question “Have you ever had an itchy rash that was coming and going for at least 6 months?” was “Yes”, the subject was directed to the next question “Have you had this itchy rash in the last 12 months?”. When the answer again was “Yes”, the next question asked was “Has this itchy rash at any time affected any of the following places: the folds of the elbows, behind the knees, in front of the ankles, under the buttocks or around the neck, ears or eyes?”. The answer “Yes” meant the diagnosis of atopic dermatitis (Figure 1).
Self-reported AD (s-rAD) was recorded based on the answer to the question “Have you ever had eczema or any kind of skin allergy?” [4, 5, 14].
Allergic rhinitis (AR) was diagnosed when the answer to the question “Do you have any nasal allergies, including hay fever?” was “Yes”.
Self-reported asthma (DA) was recorded with the question “Have you ever had asthma?” answered in the affirmative, and symptomatic asthma (SA) when the respondents answered “Yes” when asked “Have you had wheezing or whistling in your chest at any time in the last months?” [2, 13].
The family's economic status was assessed as the joint monthly income from all sources for all members of the household in PLN [converted to € in this paper]. The respondents were classified into three groups by income: < PLN 1500, € 1500–3500 (below the average monthly income for Poland) and > € 3500 (above the average monthly income for Poland). The respondents’ education (or the mother's education) was recorded as: primary education (no school education, primary not completed, primary plus basic vocational), secondary education (secondary not completed, secondary comprehensive, secondary vocational, post-secondary vocational), higher education (higher not completed, bachelor's degree, master's degree).
Medical evaluation
Allergologists diagnosed AD (clinically diagnosed atopic dermatitis – CAD) using the Hanifin and Rajka criteria [15], asthma according to the GINA criteria [16] and allergic rhinitis (clinically diagnosed allergic rhinitis – CAR) using the ARIA criteria [17].
Statistical analysis
A χ2 test or Fisher's exact test (for small sample sizes) were used in the statistical analysis. The χ2 statistics and odds ratio (OR) were calculated to determine the strength of association and dependence between variables. Results were considered to be statistically significant at p < 0.05.
The study was approved by the institutional Bioethics Committee. It was carried out as part of the project “Implementation of the system for prevention and early diagnosis of allergic disorders in Poland” (No. 6 P05 2005 C/06572) funded by the Minister for Health and the Minister for Science.
Results
Questionnaire-based survey
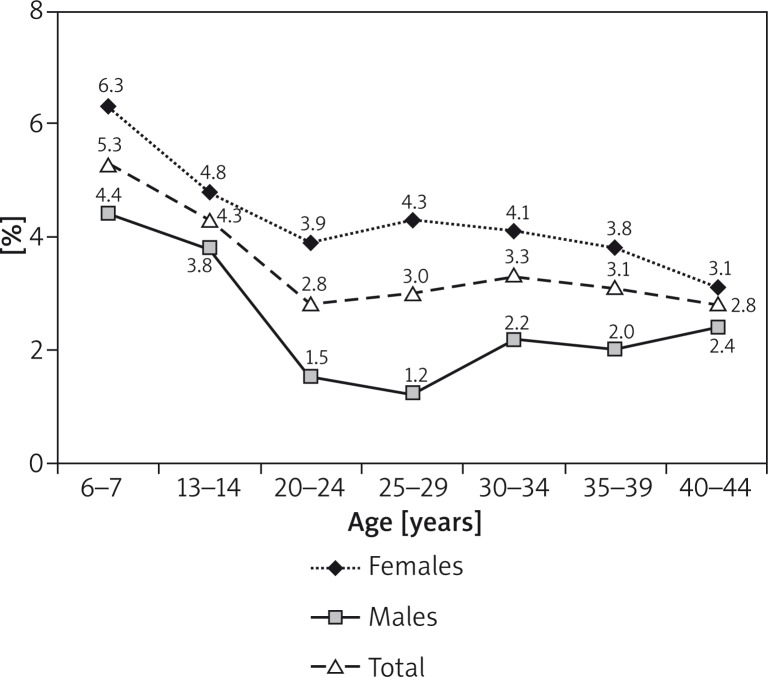
Atopic dermatitis was diagnosed in 3.91% (n = 727) of the subjects, self-reported asthma was found in 4.62% (n = 857), symptomatic asthma in 15.7% (n = 2914), allergic rhinitis in 21.11% (n = 3913) (Table 1). Atopic dermatitis was significantly more frequent in children (5.34% of 6–7 year olds and 4.30% of 13–14 year olds vs. 3.2% of adults; p < 0.001) (Figure 2). The prevalence of AD was varied depending on the region of Poland (Table 2). The lowest percentage was found in rural areas (Zamosc area) 1.9% (n = 39). Comparing the incidence of AD in urban and rural areas, we noticed more than twice as frequent manifestation of the disease in urban areas (4.1% vs. 1.9%, OR = 2.23, 95% CI: 1.61–3.09, p < 0.001). Atopic dermatitis was significantly more frequent in females of all age groups (mean prevalence 4.61% vs. 3.08%, OR = 1.52, 95% CI: 1.30–1.77, p < 0.0005) (Figure 2). In the entire population, the self-reported AD was in 37.98% (n = 7071) and depended on the place of residence (Table 2) and age (45.8%, n = 2066 6–7 year olds; 39.0%, n = 1842 13–14 year olds and 33.7%, n = 3163 adults).
Table 1
Allergic disorders diagnosed based on a questionnaire and physical examination
| Diagnosis | Total (n = 18617) | 6–7 year olds (n = 4510) | 13–14 year olds (n = 4721) | Adults (n = 9386) |
|---|---|---|---|---|
| Atopic dermatitis (AD) | 3.9 | 5.3 | 4.3 | 3.0 |
| Self-reported eczema (s-rAD) | 38.0 | 45.9 | 39.1 | 33.7 |
| Clinically diagnosed atopic dermatitis (CAD) (n = 4783) | 6.5 | 8.7 | 9.0 | 3.6 |
| Declared asthma (DA) | 4.6 | 4.4 | 6.2 | 4.0 |
| Symptomatic asthma (AS) | 15.7 | 19.2 | 15.9 | 13.8 |
| Allergic rhinitis (AR) | 22.6 | 23.7 | 24.6 | 21.0 |
Values in percent.
Table 2
Atopic dermatitis diagnosed based on a questionnaire and physical examination depending on the place of residence
| Parameter | Katowice | Warszawa | Wroclaw | Gdansk | Bialystok | Krakow | Lublin | Poznan | Zamosc |
|---|---|---|---|---|---|---|---|---|---|
| Atopic dermatitis based on questionnaire | |||||||||
| Total | 4.89 | 4.68 | 4.48 | 4.25 | 4.08 | 3.84 | 3.39 | 3.37 | 1.90 |
| 6–7 year olds | 6.86 | 5.90 | 7.33 | 4.61 | 5.80 | 4.22 | 4.31 | 6.09 | 3.25 |
| 13–14 year olds | 4.55 | 4.51 | 4.08 | 5.96 | 4.68 | 5.92 | 3.51 | 2.71 | 2.21 |
| Adults | 4.36 | 4.17 | 3.05 | 2.89 | 2.74 | 2.47 | 3.04 | 2.27 | 1.14 |
| Self-reported eczema | |||||||||
| Total | 34.40 | 46.63 | 45.25 | 48.94 | 41.48 | 33.68 | 33.99 | 29.64 | 25.41 |
| 6–7 year olds | 40.54 | 57.46 | 50.42 | 52.27 | 50.55 | 40.26 | 43.93 | 39.79 | 29.04 |
| 13–14 year olds | 33.32 | 46.13 | 44.61 | 50.84 | 44.26 | 35.13 | 35.94 | 27.45 | 25.08 |
| Adults | 32.71 | 41.67 | 42.40 | 45.63 | 34.77 | 29.12 | 30.01 | 25.43 | 24.03 |
| Atopic dermatitis based on doctor diagnosis | |||||||||
| Total | 7.18 | 6.31 | 6.38 | 5.95 | 4.42 | 2.02 | 6.98 | 16.76 | 3.67 |
| 6–7 year olds | 11.11 | 9.22 | 7.58 | 5.65 | 9.00 | 4.11 | 9.94 | 21.70 | 2.56 |
| 13–14 year olds | 7.08 | 10.50 | 9.09 | 9.22 | 5.43 | 2.04 | 9.47 | 22.61 | 4.65 |
| Adults | 5.08 | 2.15 | 3.14 | 3.66 | 0.95 | 0.00 | 4.77 | 8.72 | 3.69 |
Values in percent.
Atopic dermatitis was more frequently reported in adults or children of mothers having higher education and in subjects with a higher economic status. A statistically significant association was also established between the diagnosis of AD and atopy in parents and siblings. Atopic dermatitis was more frequently reported in subjects with dampness inside the home (Table 3). However, no correlation was established between AD and other environmental factors (having a twin sibling, p = 0.70; mother's age, p = 0.93; body mass index (BMI), p = 0.09; smoking, p = 0.17 and exposure to tobacco smoke, p = 0.33; a cat, p = 0.31, dog, p = 0.14 or birds, p = 0.07 kept inside the house; surface area of the flat (house), p = 0.24, type of heating, p = 0.32; how old the building was, p = 0.19).
Table 3
Association between environmental factors and diagnosis of AD. Significant values at the 5% level are in bold
| Parameter | AD based on questionnaire | AD by doctor diagnosis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | Value of p | OR | 95% CI | Value of p | |
| Gender: | ||||||
 Female (n = 10011) Female (n = 10011) | 1.00 | |||||
 Male (n = 8606) Male (n = 8606) | 0.66 | 0.56–0.77 | < 0.001 | 0.91 | 0.73–1.15 | 0.48 |
| Monthly salary (PLN): | ||||||
 < 1500 (n = 2883) < 1500 (n = 2883) | 1.00 | |||||
 1500–3500 (n = 5965) 1500–3500 (n = 5965) | 1.12 | 0.88–1.42 | 0.08 | 1.24 | 0.84–1.82 | 0.15 |
 > 3500 (n = 3177) > 3500 (n = 3177) | 1.35 | 1.04–1.74 | 0.05 | 1.45 | 0.96–2.19 | 0.19 |
| Education adults: | ||||||
 Primary (n = 1892) Primary (n = 1892) | 1.00 | |||||
 Secondary (n = 4096) Secondary (n = 4096) | 1.51 | 1.05–2.17 | 0.03 | 0.87 | 0.43–1.76 | 0.69 |
 Higher (n = 3330) Higher (n = 3330) | 1.61 | 1.11–2.32 | 0.03 | 1.01 | 0.50–2.03 | 0.83 |
| Education of the mother: | ||||||
 6–7 year olds: 6–7 year olds: | ||||||
  Primary (n = 1098) Primary (n = 1098) | 1.00 | |||||
  Secondary (n = 1874) Secondary (n = 1874) | 1.25 | 0.87–1.80 | 0.81 | 1.49 | 0.80–2.76 | 0.78 |
  Higher (n = 1511) Higher (n = 1511) | 1.62 | 1.13–2.33 | 0.03 | 2.88 | 1.60–5.17 | < 0.001 |
 13–14 year olds: 13–14 year olds: | ||||||
  Primary (n = 1330) Primary (n = 1330) | 1.00 | |||||
  Secondary (n = 2141) Secondary (n = 2141) | 0.99 | 0.69–1.42 | 0.09 | 2.44 | 1.37–4.35 | < 0.001 |
  Higher (n = 1182) Higher (n = 1182) | 1.58 | 1.09–2.30 | 0.03 | 2.46 | 1.32–4.58 | < 0.001 |
| Familial allergy: | ||||||
 Mother allergy Mother allergy | 2.07 | 1.72–2.48 | < 0.001 | 2.19 | 1.68–2.87 | < 0.001 |
 Father allergy Father allergy | 2.00 | 1.61–2.49 | < 0.001 | 2.36 | 1.74–3.19 | < 0.001 |
 Siblings Siblings | 1.84 | 1.55–2.18 | < 0.001 | 1.39 | 1.05–1.84 | 0.01 |
| Moisture in the house | 1.39 | 1.17–1.65 | < 0.001 | 1.21 | 1.09-1.54 | 0.02 |
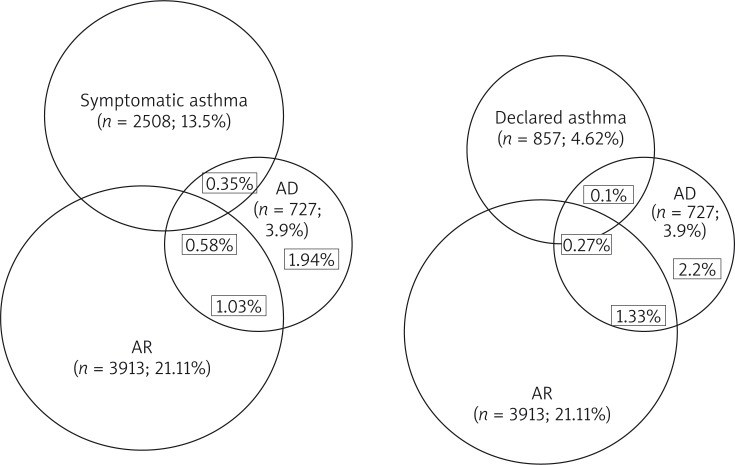
The concomitant occurrence of AD, declared asthma, asymptomatic asthma and AR in the entire study population are presented in Figure 3. In subjects with diagnosed AD, AD alone was reported in 49.72% (n = 361), coexisting AD and AR in 26.17% (n = 190), AR and AS in 9.09% (n = 66), and coexisting AD, AR and AS in 14.60% (n = 106).
Medical evaluation
Four thousand seven hundred eighty-three participants (1329 children aged 6–7 years, 1321 13–14 year olds and 2133 adults) were included in the medical evaluation. Atopic dermatitis was diagnosed in 311 (6.5%) study subjects (Table 1), including 6.75% (n = 176) of females and 6.21% (n = 135) of males (OR = 1.09, 95% CI: 0.88–1.38, p < 0.485). Most diagnoses of AD were made in Poznan (16.76%), Katowice (7.18%), Lublin (6.98%), and the smallest in the rural area (3.67%) (Figure 4). Table 2 shows the prevalence of AD depending on the place of residence. No association was established between either the economic status (p = 0.199) or education (p = 0.836) and the diagnosis of AD established by an allergologist (Table 3).
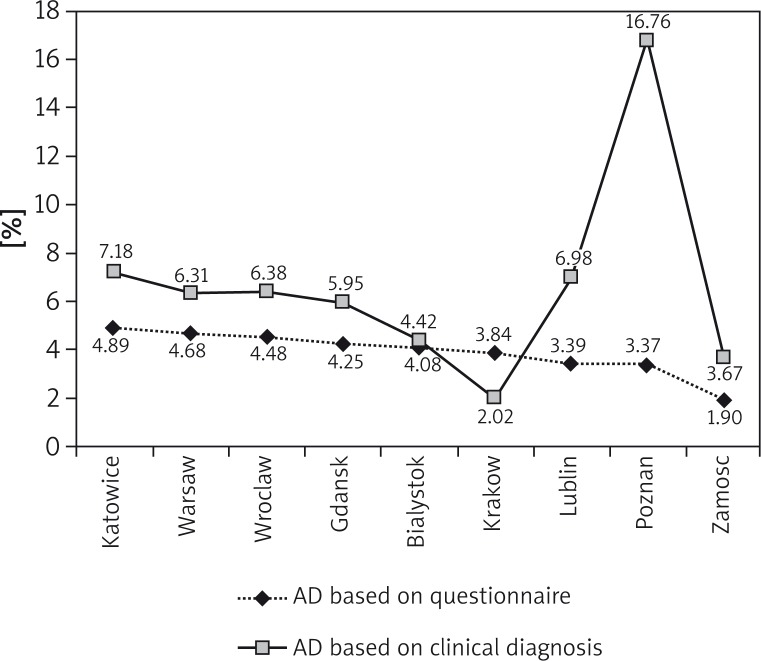
Diagnosis of atopic dermatitis based on the questionnaires (n = 18 617) vs. clinically diagnosed atopic dermatitis (n = 4783) depending on place of residence
Table 4 shows the occurrence of atopic dermatitis based on the questionnaires vs. clinically diagnosed atopic dermatitis. The consistency of the questionnaire-based diagnoses and those made by allergologists was estimated at 91.05%. A questionnaire-based diagnosis of AD was confirmed by a physician's evaluation (CAD) in 66 subjects (1.38%) while in 4288 subjects (89.67%) it was not found by both questionnaire and physician's evaluation. Of the respondents without AD reported from the questionnaire-based survey, a physician diagnosed AD in 245 subjects (5.1% of the entire study population; 95% CI: 4.5–5.7%) (Table 4). Of the respondents with AD reported from the questionnaire survey (n = 250), AD was confirmed by a study allergologist in 26.4% of the cases. Of the subjects with the clinically diagnosed AD (n = 311), the condition was self-reported in the questionnaires by 21.1% of the subjects. This finding shows that as many as 78.8% (n = 245) of the subjects undergoing evaluation by an allergologist within ECAP were then first diagnosed with AD although they had earlier experienced its symptoms.
Table 4
Diagnosis of atopic dermatitis based on the questionnaires vs. clinically diagnosed atopic dermatitis
| Atopic dermatitis based on questionnaire (AD) | |||
|---|---|---|---|
| Yes | No | ||
| Clinically diagnosed atopic dermatitis (CAD) | Yes | 66 (1.38%) | 245 (5.10%) |
| No | 184 (3.85%) | 4288 (89.67%) | |
Discussion
Epidemiological data are a prerequisite for obtaining a fuller picture and better understanding of allergic disorders, including atopic dermatitis, as an important medical and socioeconomic problem. They are also needed to assess the efficacy of prophylactic and therapeutic measures undertaken. It also allows the assessment of the significance of the disease in different age groups and regions, dependent variables of race, gender, living conditions [1]. These objectives guided the conception of aims and methodological principles of the ECAP project. This study is the largest epidemiological study of allergic diseases in Poland and in this part of Europe. Additionally, very important and greatly enhancing the value of this study is the ECAP conducted employing the same methodology as major global epidemiological studies (ISAAC and ECRHS). The sensitivity of those studies, using the same questions and diagnostic criteria, was estimated at 73.1% and the specificity was 87.3% in subjects with other skin disorders and 97.1% in subjects without skin disorders [18].
According the ISAAC Phase Three findings published in 2006, eczema in children was the most prevalent in Scandinavia and the United Kingdom and the least prevalent in Lithuania and Albania (Figure 5) [2]. The prevalence of AD in Poland as estimated by ECAP was in all age groups lower than the European average. ISAAC estimated the prevalence of AD in Polish children at 11.5% in 6–7 year olds and 8.5% in 13–14 year olds, which differs from the present results of our study. The difference might be due to a larger number of children in 8 largest cities as well as in rural areas included in our study. Our findings seem to offer a fuller and more precise picture of the AD epidemiology in Poland. In ECRHS, the mean prevalence of AD in adults was estimated at 7.1% [4]. Similarly to younger age groups, also in adults the highest prevalence of AD was found in Scandinavia and the United Kingdom. In our findings, the prevalence of AD in adults in Poland is below the mean rate for Europe and similar to the prevalence rates in Spain and Switzerland (Figure 5). The comparison of data from European countries suggests a geographical location is not the main factor affecting the occurrence of AD. The questionnaire-based part of our study showed a statistically significant correlation between the AD symptoms and higher than average income and lower than minimum income. This might explain the position of Poland, a country in an early stage of dynamic development among European countries with lower prevalence rates of AD. The changing economic status of Polish families has a direct impact on their lifestyle and as a result may lead to an increase in the prevalence of AD, similar to that observed in more affluent European countries. Simultaneously, the level of education in Poland increases systematically and rapidly, which may also account for the increasing prevalence of AD observed in our study. A higher level of education has been claimed to be of significance for the increased incidence of AD [19–22], although some authors do not agree with the hypothesis [23].
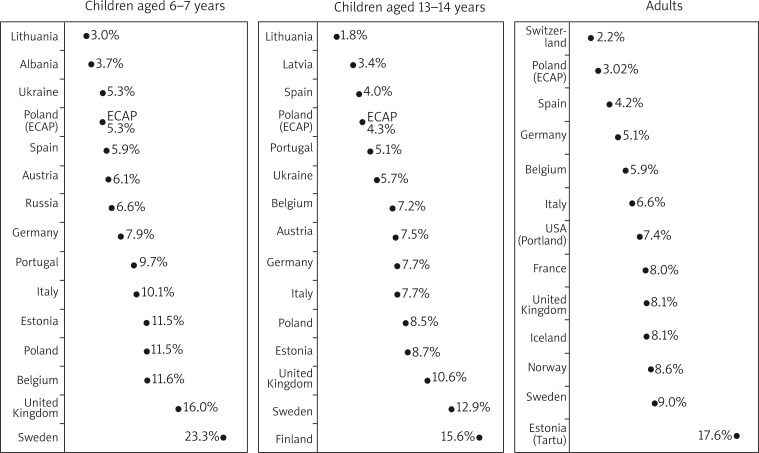
Epidemiology of atopic dermatitis according to ISAAC, ECRHS and ECAP studies [2, 4 modified by authors]
Our findings confirm earlier reports [21, 22], of a significantly more frequent occurrence of AD in children compared to adults. We did not find any significant differences when the adult population was stratified by age (Figure 2). Thus, a decrease in AD prevalence with age is seen in children and adolescents only, while in adults it remains at a similar level irrespective of age.
The prevalence of AD by gender is another important question. In adolescents, Italian [24], Spanish [25], and German [19] studies or the PARSIFAL study [23] showed a more frequent occurrence of AD in girls. We found a similar predominance of females, especially in younger children. However, the opposite tendency or no correlation with gender was seen in several studies carried out outside Europe [21, 26–28]. Only one European study reported a higher prevalence in boys, but the difference was not statistically significant [29]. A higher prevalence of AD may be due to a westernized lifestyle among girls, quality of water or housing conditions in Europe. Similarly, in adults our findings confirmed earlier reports of significantly higher proportions of women with AD compared to men [4, 30]. As suggested by some authors [31], this might reflect very frequent allergic skin disorders of the hands in women, possibly due to more extensive exposure to water and detergents associated with washing, cleaning and cooking, although different morphological and physiological features of female skin have also been implicated [22].
Our data indicate a lower prevalence of AD in rural areas compared to cities. A similar tendency was observed in a number of earlier studies from other countries [21, 32–34] and Poland [11] demonstrating the adverse impact of living in big cities on health. The environmental factors may offer the explanation. One factor is less environmental pollution in rural areas [2]. Another, presented by von Hertzen and Haahtela, is the hygiene hypothesis of allergy development whereby increased exposure to microorganisms and plants has a protective function [35]. Additionally, higher hygiene levels (frequent washing and drying of the hands or bath-taking, using large amounts of soap and shower gels) in city dwellers may damage or destroy the natural barrier function of the skin with the resulting development of skin disorders, including AD [5, 36]. At the same time, the structure of families living in rural areas (a larger number of children, housing conditions) seems to have a role in the prevention of allergic diseases.
It must be borne in mind that the development or progress of AD is not determined by a single environmental or genetic factor, but by their combinations [5]. Despite numerous studies, we still do not know which of the environmental and genetic factors play a major role. Apart from those discussed earlier, the list of risk factors for AD also includes a family history of atopy, housing conditions and environment or smoking. A positive family history of allergy seems to be the most significant risk factor for AD development [19, 28]. Our results fully support this hypothesis and are similar to the data reported by other authors [22, 23, 37]. Especially atopy found in one or both parents is a strong risk factor. Thus, a genetic predisposition to atopic disorders seems to be an unquestionable and most important factor in the development of AD and other allergic disorders. There is also a general agreement on the adverse effect of wet or damp spots on surfaces inside the home [19, 22, 38]. Our findings confirm this correlation, but it is of a lesser importance than atopy in family members.
Several studies published in recent years suggest a beneficial effect of close contact with furry pets. In 2007, based on a systematic review Langan suggested that a dog and cat ownership may protect against AD, OR of 0.79 (95% CI: 0.62–0.92) and of 0.68 (95% CI: 0.53–0.87), respectively [39]. Newer studies do not report this significant effect of close contact with the cat and dog [19, 23, 24, 28]. In the present study, no correlation between allergy and a cat or a dog in the home was established [40]. This discrepancy points to the need for further studies to confirm or refute the earlier hypothesis of a beneficial effect of furry pets.
Our study did not confirm either the significance of such factors as smoking cigarettes, exposure to tobacco smoke, flat (house) size, maternal age, BMI or mode of delivery. No correlation between the above factors and AD has been confirmed by other recent studies [21–23].
The results of a questionnaire-based survey should be verified by laboratory investigations and assessment by physicians who make a more precise diagnosis based on physical examination. A study by Flohr et al. showed flexural eczema in 3.9% of patients examined by a physician vs. 9.4% identified from questionnaires (ISAAC Phase Two) [41]. It showed that, based on the questionnaire, AD is overdiagnosed. This aspect was pointed out by a group of Polish investigators [42, 43]. In our study, on the other hand, significantly more cases of AD were identified by physicians (6.5%) than were self-reported in the questionnaires (3.9%). This proves that a properly conducted survey on adequately prepared questionnaires is methodologically a reliable source of information on the epidemiology of AD. Proper translation and validation of the original questionnaire is the most crucial, which is particularly emphasized by the authors of the original English-speaking questions [44]. A larger number of diagnoses based on doctor examination may indicate that medical examination is likely to be preferred and come more frequently for people with health problems than healthy ones, which can in some way falsify the results. Perhaps this situation occurred in our study in the Poznan center (diagnoses based on medical examination are almost five times higher than diagnoses based on the questionnaire) and Lublin. This could have an impact on the overall frequent detection of AD in clinical examination.
When the agreement of the two kinds of diagnosis, i.e. made by a study physician and self-reported was analyzed, AD was found to be seriously underdiagnosed in Poland. Out of the study subjects undergoing evaluation by an allergologist as many as 78.8% were first diagnosed with AD although they had developed the symptoms earlier. This finding may suggest underdiagnosis of AD in Poland, but on the other hand it may result from the imprecise definition of AD in the questionnaire and the methodology of recognizing AD used in the study. A respondent is required to answer questions about skin changes (an itchy rash) at an unspecified time in the past (“Have you ever had…”) and in the last 12 months, which may falsify the data. This pitfall has already been identified by Zutavern et al. [19]. Thus, we cannot ignore the number of “Yes” answers to one question about having ever had skin changes suggestive of allergy. The question is very general and non-specific so the “Yes” answer cannot be treated as the diagnosis of AD. Overall, as many as 38% of the respondents answered “Yes”. The question though non-specific, is characterized by a very high sensitivity and the answer identifies the number of people with any skin problem. When comparing the ECAP findings with the ISAAC data published in 2008, the proportion of “Yes” answers in the Polish pediatric population is similar to the countries with the highest rates (Sweden 38.6% and the UK 36.1%) [5]. In addition, in adults, the percentage of self-declared eczematous changes at any time reported in our study was similar to the ECRHS findings, i.e. 38.4% on average [4].
Studies in large groups of children and adolescents have demonstrated that AR coexists with AD in 8% [45] to over 21% of cases [19]. Asthma accompanies AD in fewer cases, although in some studies concomitant AD and asthma were found in 23.2% [46] and 19.6% of subjects [19]. Our study confirms the tendency of more frequent coexistence of AR and AD than of asthma and AD. Over 40% of AD patients had the coexisting symptoms of AR and in 23.7% of the subjects skin manifestations were associated with wheezes (AS). This finding shows that allergic disorders affect many organ systems and AD patients should be evaluated for AR and asthma with preventive measures introduced when necessary.
Conclusions
The findings of the reported study clearly demonstrate that AD is a serious health problem in Poland, although its prevalence is below the mean rate for Europe. Based on our data, we can assume that from 4% to 6.5% of the Polish population suffers from AD. It gives approximately 1.5 to 2.5 million people. It should also be noted that due to the methodology of project, we included children aged 6–7 and 13–14 years and 20–44 year-old adults, which is not the age range in which AD is the most common. It gives you assume that younger children have atopic dermatitis is more common, and is therefore a very serious medical and socioeconomic problem. When different risk factors were assessed it was found that AD should be more frequently expected in girls whose parents suffer from atopic disease themselves, are well educated, with a high socioeconomic status, and live in a city.
Acknowledgments
The study was performed in collaboration with Professor Anna Bodzenta-Łukaszyk (Bialystok), Professor Anna Bręborowicz (Poznan), Professor Andrzej Emeryk (Lublin), Professor Andrzej M. Fal (Wroclaw), Professor Radosław Gawlik (Zabrze), Professor Wiesław Gliński (Warsaw), Teresa Hofman Ph.D. (Poznan), Professor Mirosław Jarosz (Lublin), Professor Ewa Jassem (Gdansk), Professor Piotr Kuna (Lodz), Professor Jerzy Kruszewski (Warsaw), Professor Teresa Kulik (Lublin), Professor Marek Kulus (Warsaw), Professor Grzegorz Lis (Krakow), Professor Sławomir Majewski (Warsaw), Professor Michał Musielak (Poznan), Professor Barbara Rogala (Katowice), Professor Wojciech Silny (Poznan), Professor Andrzej Szpak (Bialystok), and Professor Jan Zejda (Katowice).
Conflict of interest
The authors declare no conflict of interest.
References
Articles from Advances in Dermatology and Allergology/Postȩpy Dermatologii i Alergologii are provided here courtesy of Termedia Publishing
Full text links
Read article at publisher's site: https://doi.org/10.5114/pdia.2014.40935
Read article for free, from open access legal sources, via Unpaywall:
https://www.termedia.pl/Journal/-7/pdf-22341-10?filename=Atopic dermatitis is a serious.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
The Prevalence of Atopic Dermatitis and Food Allergy in Children Living in an Urban Agglomeration-Is There a Current Relationship?
J Clin Med, 12(18):5982, 15 Sep 2023
Cited by: 1 article | PMID: 37762923 | PMCID: PMC10531722
The importance of specific IgE antibodies in the epidemiology of allergic rhinitis and asthma (ECAP survey): part four. The relationship between the concentration of specific IgE antibodies in serum and types of asthma.
Postepy Dermatol Alergol, 40(5):611-616, 02 Aug 2023
Cited by: 1 article | PMID: 38028409 | PMCID: PMC10646703
The importance of specific IgE antibodies in the epidemiology of allergic rhinitis and asthma (ECAP survey): part five. The relationship between the concentration of specific IgE antibodies in serum and types of rhinitis.
Postepy Dermatol Alergol, 40(5):617-624, 04 Aug 2023
Cited by: 0 articles | PMID: 38028415 | PMCID: PMC10646722
12-month prevalence of atopic dermatitis in resource-rich countries: a systematic review and meta-analysis.
Sci Rep, 12(1):15125, 06 Sep 2022
Cited by: 3 articles | PMID: 36068263 | PMCID: PMC9448775
Review Free full text in Europe PMC
The potential action of SSRIs in the treatment of skin diseases including atopic dermatitis and slow-healing wounds.
Pharmacol Rep, 74(5):947-955, 07 Oct 2022
Cited by: 2 articles | PMID: 36203121 | PMCID: PMC9584846
Review Free full text in Europe PMC
Go to all (15) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Epidemiology of atopic dermatitis in Poland according to the Epidemiology of Allergic Disorders in Poland (ECAP) study.
J Dermatol, 42(2):140-147, 06 Dec 2014
Cited by: 20 articles | PMID: 25483345
Filaggrin gene defects are independent risk factors for atopic asthma in a Polish population: a study in ECAP cohort.
PLoS One, 6(2):e16933, 18 Feb 2011
Cited by: 38 articles | PMID: 21365004 | PMCID: PMC3041817
Prevalence of rhinitis in Polish population according to the ECAP (Epidemiology of Allergic Disorders in Poland) study.
Otolaryngol Pol, 63(4):324-330, 01 Jul 2009
Cited by: 40 articles | PMID: 19999749
Epidemiology of atopic dermatitis: a review.
Allergy Asthma Proc, 33(3):227-234, 01 May 2012
Cited by: 116 articles | PMID: 22584191
Review

 1,2
1,2