Abstract
Unlabelled
Dengue virus (DENV) is a major public health threat worldwide. Infection with one of the four serotypes of DENV results in a transient period of protection against reinfection with all serotypes (cross-protection), followed by lifelong immunity to the infecting serotype. While a protective role for neutralizing antibody responses is well established, the contribution of T cells to reinfection is less clear, especially during heterotypic reinfection. This study investigates the role of T cells during homotypic and heterotypic DENV reinfection. Mice were sequentially infected with homotypic or heterotypic DENV serotypes, and T cell subsets were depleted before the second infection to assess the role of DENV-primed T cells during reinfection. Mice primed nonlethally with DENV were protected against reinfection with either a homotypic or heterotypic serotype 2 weeks later. Homotypic priming induced a robust neutralizing antibody response, whereas heterotypic priming elicited binding, but nonneutralizing antibodies. CD8(+) T cells were required for protection against heterotypic, but not homotypic, reinfection. These results suggest that T cells can contribute crucially to protection against heterotypic reinfection in situations where humoral responses alone may not be protective. Our findings have important implications for vaccine design, as they suggest that inducing both humoral and cellular responses during vaccination may maximize protective efficacy across all DENV serotypes.Importance
Dengue virus is present in more than 120 countries in tropical and subtropical regions. Infection with dengue virus can be asymptomatic, but it can also progress into the potentially lethal severe dengue disease. There are four closely related dengue virus serotypes. Infection with one serotype results in a transient period of resistance against all serotypes (cross-protection), followed by lifelong resistance to the infecting serotype, but not the other ones. The duration and mechanisms of the transient cross-protection period remain elusive. This study investigates the contribution of cellular immunity to cross-protection using mouse models of DENV infection. Our results demonstrate that cellular immunity is crucial to mediate cross-protection against reinfection with a different serotype, but not for protection against reinfection with the same serotype. A better understanding of the mediators responsible for the cross-protection period is important for vaccine design, as an ideal vaccine against dengue virus should efficiently protect against all serotypes.Free full text

CD8+ T Cells Can Mediate Short-Term Protection against Heterotypic Dengue Virus Reinfection in Mice
ABSTRACT
Dengue virus (DENV) is a major public health threat worldwide. Infection with one of the four serotypes of DENV results in a transient period of protection against reinfection with all serotypes (cross-protection), followed by lifelong immunity to the infecting serotype. While a protective role for neutralizing antibody responses is well established, the contribution of T cells to reinfection is less clear, especially during heterotypic reinfection. This study investigates the role of T cells during homotypic and heterotypic DENV reinfection. Mice were sequentially infected with homotypic or heterotypic DENV serotypes, and T cell subsets were depleted before the second infection to assess the role of DENV-primed T cells during reinfection. Mice primed nonlethally with DENV were protected against reinfection with either a homotypic or heterotypic serotype 2 weeks later. Homotypic priming induced a robust neutralizing antibody response, whereas heterotypic priming elicited binding, but nonneutralizing antibodies. CD8+ T cells were required for protection against heterotypic, but not homotypic, reinfection. These results suggest that T cells can contribute crucially to protection against heterotypic reinfection in situations where humoral responses alone may not be protective. Our findings have important implications for vaccine design, as they suggest that inducing both humoral and cellular responses during vaccination may maximize protective efficacy across all DENV serotypes.
IMPORTANCE Dengue virus is present in more than 120 countries in tropical and subtropical regions. Infection with dengue virus can be asymptomatic, but it can also progress into the potentially lethal severe dengue disease. There are four closely related dengue virus serotypes. Infection with one serotype results in a transient period of resistance against all serotypes (cross-protection), followed by lifelong resistance to the infecting serotype, but not the other ones. The duration and mechanisms of the transient cross-protection period remain elusive. This study investigates the contribution of cellular immunity to cross-protection using mouse models of DENV infection. Our results demonstrate that cellular immunity is crucial to mediate cross-protection against reinfection with a different serotype, but not for protection against reinfection with the same serotype. A better understanding of the mediators responsible for the cross-protection period is important for vaccine design, as an ideal vaccine against dengue virus should efficiently protect against all serotypes.
INTRODUCTION
The four serotypes of dengue virus (DENV) are the etiologic agent of dengue, a rapidly spreading arboviral disease that is present in more than 120 countries (1,–5). Recent estimates suggest that more than 3.5 billion people living in tropical and subtropical regions are at risk of infection, with 390 million infections per year, of which 96 million are symptomatic (1,–3).
Infection with DENV is often asymptomatic (6, 7), but if disease is apparent, it ranges from dengue fever to severe dengue (formerly known as dengue hemorrhagic fever [DHF] or dengue shock syndrome [DSS]) (5, 8). Dengue fever is a self-limited illness characterized by headache, retro-orbital pain, nausea, muscle and joint pain, and leukopenia. Warning signs of severe dengue disease include abdominal pain, persistent vomiting, fluid accumulation, mucosal bleeding, lethargy, liver enlargement, increased hematocrit, and low platelet count (5, 8, 9). Signs of severe dengue are severe plasma leakage, severe bleeding, respiratory distress, and severe organ involvement (liver, kidney, heart, central nervous system) (5, 8, 9).
Infection with one serotype of DENV results in lifelong immunity to that serotype due to induction of a robust serotype-specific neutralizing antibody response (10,–12). Additionally, after infection, there is a period of protection against heterotypic infection with other serotypes (cross-protection) (13,–17). The duration of the cross-protection period remains a matter of debate (13,–15, 17). A recent reanalysis of historical data suggests a duration of 8 weeks (17). Based on serology and on epidemic modeling, other estimates vary from 1 to 2 weeks to 1 year or more (13,–15). In addition to the duration, the mechanism of cross-protection remains elusive too (17). The transient cross-protection is often assumed to rely on high titers of cross-reactive antibodies reactive for all DENV serotypes (18,–20), but experimental evidence is scarce. Therefore, the precise features of the transient cross-protection remain unclear.
While the importance of a robust neutralizing antibody response for protection against DENV is undisputed (10,–12, 21), less is known about the importance of T cells during reinfection, in particular T cells previously activated by another DENV serotype (heterotypic T cells). It has been hypothesized that altered responses from heterotypic T cells can result in a “cytokine storm” and exacerbation of disease (22). However, increasing evidence suggests a protective role for T cells during DENV infection (23,–30), including for heterotypic T cells (23, 25).
A better understanding of the contribution of T cells (both homotypic and heterotypic) to protection against reinfection is crucial to support DENV vaccine development. Efforts to develop a vaccine that efficiently protects against all DENV serotypes are still ongoing (31,–33). Inducing a balanced neutralizing antibody response against all four DENV serotypes has been the overarching goal of most vaccine developers, but it is increasingly recognized that the presence of neutralizing antibodies (as measured by 50% plaque reduction neutralization test [PRNT50]) may not be an optimal correlate of protection (34,–36). A vaccine that efficiently induces both humoral and cellular responses may be preferable.
In this study, we investigated in mice the contribution of cellular immunity to protection during homotypic or heterotypic reinfection 2 weeks after a primary infection. 129/Sv mice lacking type I and II interferon (IFN) receptors (AG129) or type I IFN receptor only (IFNAR−/−) were nonlethally primed with DENV serotype 2 (DENV2) or DENV serotype 4 (DENV4) prior to challenge with DENV2 2 weeks later. In order to maximize the chance of capturing the potential contribution of T cells, we chose to challenge with virus shortly after the peak of the T cell response (expected around days 7 to 10), but before T cell responses return to the prepriming level (28). This approach also allowed us to be within the 2-week period that has been postulated as being the shortest period of cross-protection according to the most conservative estimates (14, 17).
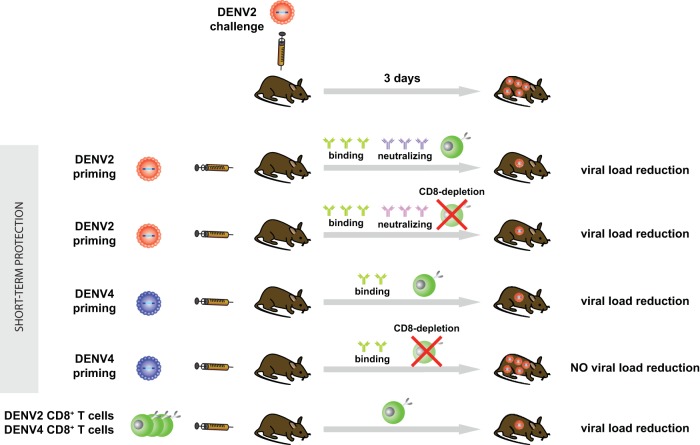
Both homotypic (DENV2) and heterotypic (DENV4) priming reduced viral load, morbidity, and/or mortality upon challenge with DENV2. Depletion of CD8+ T cells prior to challenge abrogated protection in the case of heterotypic, but not homotypic, priming. CD8+ T cells were likely not required for protection after homotypic priming due to the induction of a robust serotype-specific neutralizing antibody response. However, transfer of DENV2 (homotypic)- or DENV4 (heterotypic)-primed CD8+ T cells both reduced viral load upon reinfection with DENV2. Therefore, while both homotypic and heterotypic CD8+ T cells efficiently reduced viral load upon reinfection, CD8+ T cells were necessary for viral load reduction only after heterotypic priming.
Taken together, our results demonstrate that 2 weeks after priming, CD8+ T cells were necessary for protection against heterotypic reinfection but not homotypic reinfection. This study suggests a protective role for CD8+ T cells during heterotypic reinfection, which implies that inducing both humoral and cellular responses during vaccination may maximize protective efficacy across all DENV serotypes.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (37), the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals (38), and the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All experimental procedures were approved and performed according to the guidelines set by the La Jolla Institute for Allergy and Immunology Animal Care and Use Committee (protocol number AP-28SS1-0809).
Mice.
129/Sv mice deficient in type I and II interferon receptors (AG129) and 129/Sv mice deficient in type I interferon receptor (IFNAR−/−) were housed under specific-pathogen-free (SPF) conditions at the La Jolla Institute for Allergy and Immunology (LJI). Sex-matched 5- to 6-week-old mice were used. For survival studies, mice were sacrificed when moribund or at the first signs of paralysis.
Virus production.
DENV serotype 2 (DENV2) strain PL046 (39), DENV2 strain S221 (28), DENV serotype 3 (DENV3) strain UNC3001 (40) (obtained from Aravinda deSilva, University of North Carolina [UNC] Chapel Hill), and DENV serotype 4 (DENV4) strain H241 (purchased from ATCC) were amplified on C6/36 cells as described in reference 41. DENV2 PL046, DENV2 S221, DENV3 UNC3001, and DENV4 H241 were quantified by standard plaque assay on baby hamster kidney (BHK) cells as previously described (42). DENV2 S221 organ titers were quantified by real-time quantitative reverse transcription-PCR (qRT-PCR) as previously described (41). There are approximately 5 × 104 genomic equivalents (GE) per PFU for DENV2 strain S221.
Infections.
All infections were performed intravenously; virus was diluted to a total volume of 200 μl phosphate-buffered saline (PBS) with 10% fetal calf serum (FCS). When needed, the viral dose used for priming was adjusted between experiments to account for differences in virulence between different strains and batches of virus.
T cell depletions.
T cell-depleting antibodies 2.43 (IgG2b anti-mouse CD8), GK1.5 (IgG2b anti-mouse CD4), and the isotype control LTF2 (IgG2b) were purchased from BioXCell. CD8+ or CD4+ T cells were depleted by administering 250 μg of 2.43 or GK1.5 antibody intraperitoneally in a total volume of 200 μl in PBS 3 days and 1 day before challenge with virus as previously described (30). CD8+ depletion efficiency was more than 98% as verified by flow cytometry.
Viral RNA quantification in organs.
Viral RNA was quantified in organs by real-time qRT-PCR as described previously (41).
DENV-specific IgG detection by ELISA.
DENV-reactive IgG was detected by enzyme-linked immunosorbent assay (ELISA) on DENV S221-coated plates as previously described (30). To compare the relative amount of DENV-reactive IgG in different sera, the optical density (OD) values of three samples were averaged and plotted against the log of the dilution factor. Subsequently, a linear regression was done with the points in the linear range of the curve. Based on the equation of the linear regression, a dilution factor can be calculated for any desired OD in the linear range and compared to the dilution factor of another group at the same OD. When sera were compared, this was done for ODs of 0.3 to 0.7 with 0.1 increments, and the factors obtained from those five OD measures were averaged.
Neutralizing activity of serum.
Anti-DENV neutralizing antibodies (Abs) were quantified by 50% plaque reduction neutralization test (PRNT50) as previously described (30). Serum was serially diluted 1:2 with the starting dilution being 1:10. The highest dilution reducing more than 50% of the plaques is reported as “50% neutralizing titer.”
Generation of DENV-immune serum.
IFNAR−/− mice were infected intravenously (i.v.) with 2 × 106 PFU DENV2 strain S221, and serum was collected on day 15 and 32; fractions collected on day 15 and 32 were pooled.
Direct in vivo intracellular cytokine staining (ICS).
As previously described (43,–45), this method allows for detection and enumeration of T cells that are actively producing cytokines in vivo after contact with DENV antigen. One hour after challenge with DENV2, 200 μg brefeldin A (Sigma) diluted in PBS containing 20% ethanol (EtOH) (vol/vol) was injected i.v. into mice in a total of 200 μl to retain cytokines in the cells. Mice were sacrificed 6 h later, and spleens were passed through 70-μm cell strainers to obtain single-cell suspensions. Without any other stimulation, cells were stained for CD3, CD8, CD44, and CD62L (extracellularly) as well as gamma interferon (IFN-γ) and CD107a (intracellularly). Samples were acquired on an LSR II flow cytometer (BD Bioscience) and analyzed with FlowJo software (Tree Star). The following antibodies were used: anti-CD3-efluor 450 (anti-CD3 antibody conjugated to efluor 450) (clone 17A2; eBioscience), anti-CD8a-PerCP-Cy5.5 (anti-CD8a antibody conjugated to peridinin chlorophyll protein [PerCP] and Cy5.5) (clone 53-6.7; eBioscience), anti-CD62L-Alexa Fluor 700 (clone MEL-14; eBioscience), anti-CD44-PE-Cy7 (anti-CD44 conjugated to phycoerythrin [PE] and Cy7) (BD Pharmingen, clone IM7), anti-IFN-γ-FITC (anti-IFN-γ conjugated to fluorescein isothiocyanate [FITC]) (clone XMG1.2; Tonbo Biosciences), and anti-CD107a-PE (clone 1D4B; eBioscience).
In vivo cytotoxicity assay.
IFNAR−/− recipient mice were infected with 1 × 105 PFU DENV2 (PL046) or DENV4 (H241). Two weeks later, donor splenocytes were obtained from naive IFNAR−/− mice. Red blood cells (RBC) were lysed (RBC lysis buffer; eBioscience), and cells were resuspended at 1 × 107 cells/ml in RPMI 1640 containing 2% FCS, penicillin, and streptomycin (Gibco). The cells were pulsed with 0.5 μg/ml of DENV peptide NS4B99-107 (NS4B with amino acids 99 to 107) (28) or no peptide (1 h, 37°C). The cells were washed three times and labeled with carboxyfluoroscein succinimidyl ester (CFSE) (CellTrace; Invitrogen/Molecular Probe) in PBS with 0.1% bovine serum albumin (BSA) for 10 min at 37°C. DENV peptide-pulsed cells (target cells) were labeled with 1 μM CFSE (CFSEhi), and the non-peptide-pulsed cells (marker cells) were labeled with 100 nM CFSE (CFSElo). After the cells were washed, the two cell populations were mixed at a 1:1 ratio and 3.5 × 106 cells from each population were injected i.v. into naive or infected recipient mice. After 15 h, the ratio of target (CFSEhi) to marker (CFSElo) cells was determined in the spleens of recipient mice by flow cytometry. Cytolytic activity in the recipient is expected to reduce the peptide-labeled target population, but not the unlabeled marker population.
CD8+ T cell isolation and transfer.
CD8+ T cells were isolated by magnetically activated cell sorting (MACS) positive selection with a kit from Miltenyi Biotech (CD8a Ly-2), and total T cells were isolated by MACS negative selection with the Pan T cell isolation kit II from Miltenyi Biotech according to the manufacturer's instructions. After the cells were isolated, they were injected intravenously in a total volume of 200 μl PBS.
Statistical analysis.
For viral RNA titers, P values were calculated with Prism (GraphPad Software) using the unpaired t test with Welch's correction. For statistical analysis, the value of the limit of detection was attributed to the samples that were under the detection limit.
For survival curves, P values were calculated with Prism (GraphPad Software) using the log rank (Mantel-Cox) test.
RESULTS
Nonlethal DENV priming protects from heterotypic lethal DENV2 challenge in a CD8-dependent manner.
To evaluate the effect of nonlethal DENV priming on a subsequent lethal challenge with a different DENV serotype (heterotypic challenge) shortly after priming, we first used a model of DENV infection in 129/Sv mice lacking type I and II IFN receptors (AG129 mice). We chose this model because some DENV strains replicate in AG129 mice and cause a disease that recapitulates many features of DENV infection in humans and can be lethal (39, 41, 46, 47).
AG129 mice were nonlethally primed with 1 × 104 PFU DENV4 (strain H241) 2 weeks prior to challenge with 1 × 105 PFU DENV2 (strain S221), and survival was monitored. A control group was challenged with 1 × 105 PFU DENV2 without priming. All the nonprimed animals died by day 20, whereas 62.5% of the DENV4-primed animals survived without signs of disease until day 25, when the experiment was terminated (Fig. 1A). These results show that nonlethal DENV4 priming reduced mortality upon DENV2 challenge.
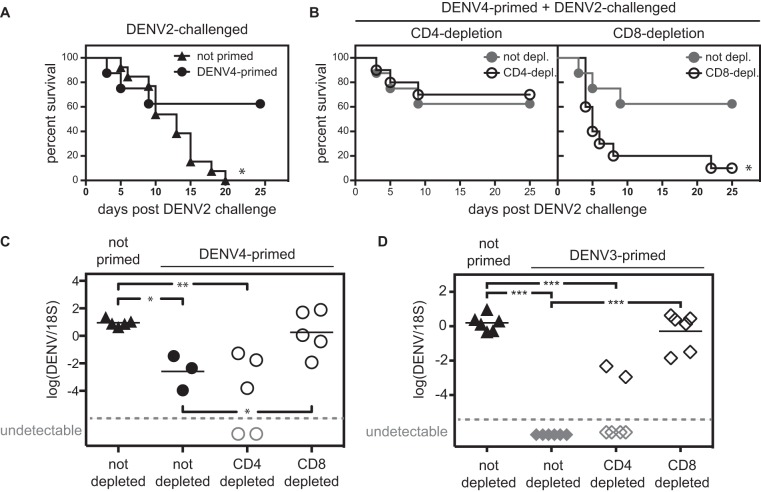
DENV priming protects from heterotypic lethal DENV2 challenge in a CD8-dependent manner. (A) AG129 mice were either left untreated or primed with 1 × 104 PFU DENV4 (strain H241) 2 weeks prior to challenge with 1 × 105 PFU DENV2 (strain S221) (number of mice [n] = 8 to 13). The survival of nonprimed mice was significantly different (P ≤ 0.05) from the survival of primed mice by the log rank (Mantel-Cox) test. This is indicated by an asterisk. (B) Mice were primed and challenged as described above for panel A, and their CD4+ or CD8+ T cell populations were depleted before challenge. The DENV4-primed nondepleted group shown in panel A is also depicted in panel B for clarity. Survival was monitored (n = 8 to 10). The survival of mice depleted of their CD8+ T cells was significantly different (P ≤ 0.05) from the survival of nondepleted mice by the log rank (Mantel-Cox) test. This is indicated by an asterisk. (C) AG129 mice were primed with 1 × 105 PFU DENV4 (strain H241) 2 weeks prior to challenge with 1 × 104 PFU DENV2 (strain S221). Before challenge, mice were depleted of their CD4+ or CD8+ T cell populations or not depleted. Viral RNA titers were measured in the livers 3 days after challenge. (D) AG129 mice were primed with 5 × 104 PFU DENV3 (strain UNC3001) 2 weeks prior to challenge with 1 × 104 PFU DENV2 (strain S221). Before challenge, mice were depleted of their CD4+ or CD8+ T cell populations or not depleted. Viral RNA titers were measured in the liver 3 days after challenge. Gray symbols represent samples that were under the detection limit (broken line) and therefore have no numerical value. In panels C and D, each symbol represents the value for an individual mouse, and the bar represents the mean value for the group of mice. The mean is not shown when some of the samples were under the detection limit. Values that were significantly different in panels C and D by unpaired t test with Welch's correction are indicated by bars and asterisks as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
The 2-week period between priming and challenge was chosen to give enough time for the T cell response to develop (28). In order to maximize our chance of observing any potential effect of T cells, we chose to challenge with virus soon after the peak, before the T cell levels were back to prepriming levels. Also, challenging 2 weeks after priming ensured that we were challenging within the shortest duration that has been postulated for the cross-protection period observed after DENV infection (14, 17).
Previous studies from our laboratory have demonstrated that both CD4+ and CD8+ T cells can contribute to protection against DENV infection (24, 27,–30). Therefore, we assessed the role of T cells in the heterotypic protection observed 2 weeks after nonlethal DENV4 priming. AG129 mice were primed (DENV4) and challenged (DENV2) as described above, and CD4+ or CD8+ T cells were depleted just before challenge with DENV2. Depletion of CD4+ T cells before challenge had no significant effect on survival after DENV2 challenge (Fig. 1B), whereas depletion of CD8+ T cells abolished the protection mediated by DENV4 priming (Fig. 1B).
To confirm the survival phenotype, we next investigated how DENV4 priming would influence the viral load after DENV2 challenge. AG129 mice were primed with 1 × 105 PFU DENV4 and challenged 2 weeks later with 1 × 104 PFU DENV2. DENV2 RNA titers were measured in the liver 3 days after DENV2 challenge. The liver was chosen as we have previously demonstrated that high liver viral RNA titers on day 3 are predictive of severe disease and short survival time (30, 48). Compared to the nonprimed control group, DENV4-primed mice had about 1,000-fold-lower viral RNA titers in the liver 3 days after DENV2 challenge (Fig. 1C). As in the previous experiment, CD4+ or CD8+ T cells were depleted in some animals prior to DENV2 challenge. Consistent with the survival data shown in Fig. 1B, depletion of CD8+ T cells, but not CD4+ T cells, abrogated the viral load reduction mediated by DENV4 priming (Fig. 1C).
To confirm that a similar phenomenon would be observed after priming with another DENV serotype, AG129 mice were primed with 5 × 104 PFU DENV3 (strain UNC3001) 2 weeks prior to challenge with 1 × 104 PFU DENV2 (strain S221). As in the previous experiment, CD4+ or CD8+ T cells were depleted before challenge in some of the animals. One control group without priming (challenge only) was included. Three days after challenge, viral RNA was present in the livers of the nonprimed mice but not detected in the livers of DENV3-primed mice (Fig. 1D). As found in the DENV4 priming experiments, CD8 depletion, but not CD4 depletion, abolished the viral load reduction mediated by DENV3 priming (Fig. 1D). Taken together, these results show that 2 weeks after DENV priming, CD8+ T cells contribute to protection against heterotypic reinfection.
CD8+ T cells are required to reduce viral load upon heterotypic, but not homotypic, reinfection.
After demonstrating the importance of CD8+ T cells in protection after heterotypic priming, we evaluated whether CD8+ T cells were also required for protection after homotypic DENV priming. AG129 mice were nonlethally primed with 1 × 105 PFU DENV2 (strain PL046) or DENV4 (strain H241) 2 weeks prior to challenge with 1 × 104 PFU DENV2 (strain S221). The DENV2 strain PL046 was chosen for nonlethal priming, as it is less virulent than the strain used for challenge, DENV2 strain S221 (39). A nonprimed group (challenge only) was included. Prior to challenge with DENV2, half of the animals were depleted of their CD8+ T cell population. Liver viral RNA titers were measured on day 3 (Fig. 2A). In the nonprimed group, CD8+ T cell depletion had no effect on the liver RNA titer on day 3, likely because 3 days are insufficient for CD8+ T cells to become activated and functional after primary infection (24). In DENV2-primed mice (homotypic priming), the liver RNA titer was reduced by 100-fold or more compared to the titer in the nonprimed mice, and CD8 depletion had no effect on the viral load reduction. In comparison, in DENV4-primed mice (heterotypic priming), viral load reduction was observed in the CD8-competent, but not in the CD8-depleted animals. Similar results were observed when viral RNA titers were measured in the spleen (Fig. 2B).
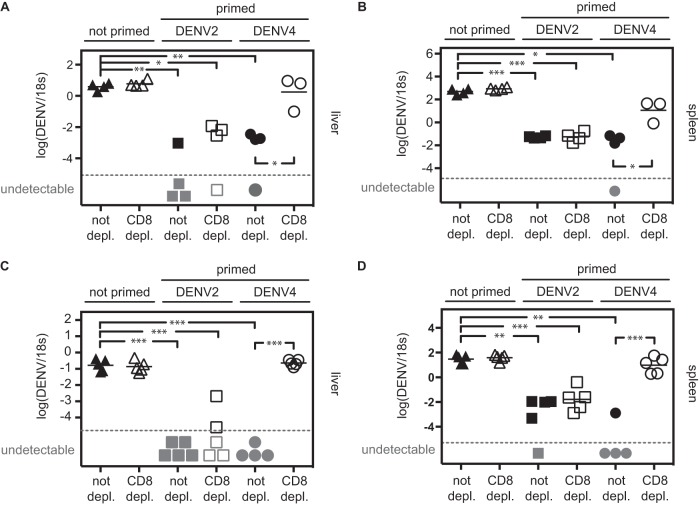
CD8+ T cells are necessary to reduce viral load upon heterotypic, but not homotypic, reinfection. (A and B) AG129 mice were primed with 1 × 105 PFU DENV2 (strain PL046) or DENV4 (strain H241) 2 weeks prior to challenge with 1 × 104 PFU DENV2 (strain S221). One control group was not primed. CD8+ T cells were depleted in half of the mice before challenge. Viral RNA titers (DENV2) were monitored in the liver (A) and the spleen (B) 3 days after challenge. (C and D) IFNAR−/− mice were primed with 1 × 106 PFU DENV2 (strain PL046) or DENV4 (strain H241) 2 weeks prior to challenge with 2 × 105 PFU DENV2 (strain S221). One control group was not primed. Before challenge, CD8+ T cells were depleted in half of the mice. Viral RNA titers (DENV2) were monitored in the liver (C) and the spleen (D) 3 days after challenge. Gray symbols represent samples that were under the detection limit (broken line) and therefore have no numerical value. Each symbol represents the value for an individual mouse, and the bar represents the mean value for the group of mice. The mean is not shown when some of the samples were under the detection limit. Values that were significantly different by unpaired t test with Welch's correction are indicated by bars and asterisks as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Because IFN-γ production is a major function of CD8+ T cells, we wanted to confirm our results in mice with intact IFN-γ receptor signaling. Therefore, we next performed similar experiments with 129/Sv mice deficient in type I IFN receptor only (IFNAR−/− mice). As these mice are less immunocompromised than AG129 mice, they are more resistant to DENV infection and manifest fewer signs of disease than AG129 mice. However, some DENV strains replicate in IFNAR−/− mice to detectable levels (47), which allows for assessment of the effect of priming on subsequent DENV challenge. Those experiments would be impossible in wild-type (WT) mice in which DENV does not replicate to measurable levels (47). Consistent with results obtained from experiments using AG129 mice, homotypic priming with 1 × 106 PFU DENV2 (strain PL046) 2 weeks prior to DENV2 challenge (2 × 105 PFU, strain S221) reduced viral RNA titers in the livers of IFNAR−/− mice upon challenge, even when CD8+ T cells were depleted before challenge (Fig. 2C). After heterotypic priming (DENV4 strain H241, 1 × 106 PFU), a reduction in viral RNA titer was observed upon DENV2 challenge in CD8-sufficient, but not CD8-depleted, mice (Fig. 2C). Similar results were obtained in the spleen (Fig. 2D). Collectively, these results demonstrate that while both homotypic and heterotypic DENV priming reduce viral RNA titer upon reinfection, CD8+ T cells are necessary to mediate viral load reduction after heterotypic, but not homotypic, priming. If not stated otherwise, all subsequent experiments are performed in IFNAR−/− mice.
CD8+ T cells are necessary to reduce morbidity upon heterotypic, but not homotypic reinfection.
Next, we examined whether the decrease in viral load observed after DENV2 challenge in DENV2- or DENV4-primed animals correlated with a reduction in morbidity. IFNAR−/− mice were primed with 1 × 106 PFU DENV2 (strain PL046) or DENV4 (strain H241) 2 weeks prior to challenge with 2 × 106 PFU DENV2 (strain S221) in the presence of 10 μl of DENV2-immune serum (see Materials and Methods). This elevated viral challenge dose was chosen in order to increase the chance of observing signs of disease. DENV-immune serum was given to the mice based on our unpublished observation that this serum amount causes antibody-dependent enhancement of infection and increases the severity of disease when administered with this viral challenge strain and dose. To confirm the importance of CD8+ T cells in heterotypic protection, CD8+ T cells were depleted in half of the mice between priming and challenge, and the health of the mice was monitored. In the nonprimed control group, all animals but one were sick (hunchback posture, ruffled fur, or reduced mobility) or dead by day 4, and more than 50% were dead by day 6 regardless of the presence of CD8+ T cells (Fig. 3A, top). In the DENV2-primed animals (homotypic priming), no sign of disease was observed on day 4 or 6, regardless of the presence of CD8+ T cells (Fig. 3A, middle). In DENV4-primed animals (heterotypic priming), all animals were sick on day 4 in the absence of CD8+ T cells, whereas the CD8-competent animals were all healthy on day 4 (Fig. 3A, bottom).
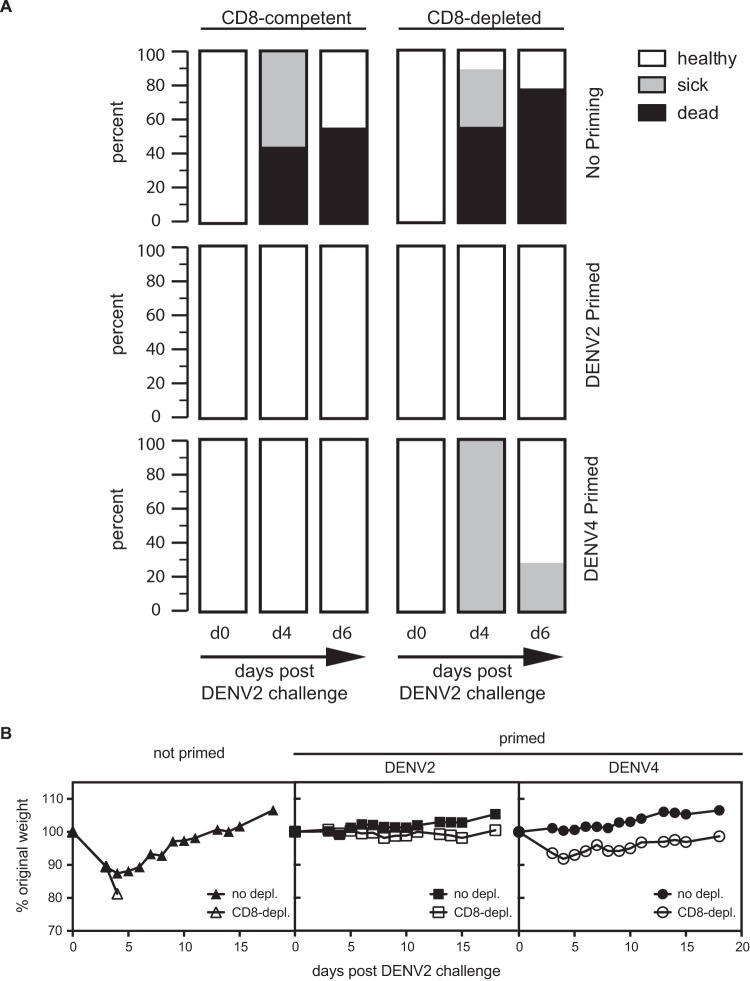
CD8+ T cells are necessary to reduce morbidity upon heterotypic, but not homotypic, reinfection. IFNAR−/− mice were primed with 1 × 106 PFU DENV2 (strain PL046) or DENV4 (strain H241) 2 weeks prior to challenge with 2 × 106 PFU DENV2 (strain S221) in the presence of 10 μl of immune serum, an amount that increases the severity of disease. One control group was not primed. Before challenge, CD8+ T cells were depleted in half of the mice. The health (A) and weight (B) of the animals were assessed daily. The data in panel A are pooled from two experiments. The number of mice (n) was 7 to 9 for panel A and 3 to 5 for panel B.
In addition to monitoring health, weight loss was recorded as a sign of morbidity. IFNAR−/− mice were primed with DENV2 or DENV4 or not primed prior to challenge with DENV2 as described above. Half of the animals were depleted of their CD8+ T cell population before challenge. As shown in Fig. 3B, weight loss was observed in all nonprimed mice, regardless of the presence of CD8+ T cells. All nonprimed CD8-depleted mice and 60% of the nonprimed, CD8-sufficient mice had lost weight (20% weight loss) by day 5 and were sacrificed. Weight loss was not observed in DENV2-primed (CD8-depleted or not) animals, whereas in DENV4-primed animals, weight loss was observed in CD8-depleted, but not in CD8-sufficient, animals. These results show that CD8+ T cells are necessary to reduce morbidity and weight loss upon DENV2 challenge in IFNAR−/− mice primed with DENV4 (heterotypic priming). However, after homotypic priming (DENV2), no sign of disease or weight loss occurred, irrespective of the presence of CD8+ T cells.
Priming with DENV4 induces DENV2-reactive antibodies, but not DENV2 neutralizing antibodies.
We analyzed the humoral immune response after DENV2 or DENV4 priming. IFNAR−/− mice were primed with 1 × 106 PFU DENV2 (strain PL046) or DENV4 (strain H241) 2 weeks prior to challenge with 2 × 105 PFU DENV2 (strain S221). One group of mice was not primed prior to DENV2 challenge. Three days after challenge, the levels of DENV2-reactive IgG were measured in serum by ELISA (Fig. 4A). DENV2-reactive IgG antibodies were present in both DENV2- and DENV4-primed animals, but the levels were 5-fold higher in DENV2-primed mice than in DENV4-primed mice. No DENV2-reactive IgG antibodies were detected in nonprimed (DENV2-challenged) mice on day 3, indicating that the viral challenge without priming did not result in IgG production by day 3. The neutralizing activity of the serum was analyzed by 50% plaque reduction neutralization test (PRNT50) on BHK21 cells. As shown in Fig. 4B, DENV2 neutralizing antibodies were detected in DENV2-primed, but not in DENV4-primed, animals. Therefore, both DENV2 and DENV4 priming induced production of DENV2-reactive IgG, but only DENV2 priming induced a DENV2 neutralizing antibody response. This result implies a minimal role for the humoral response in mediating the short-term heterotypic protection in DENV4-primed mice upon DENV2 challenge.
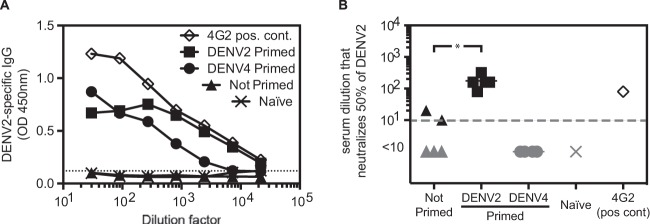
Priming with DENV4 induces DENV2-reactive antibodies, but not DENV2 neutralizing antibodies. IFNAR−/− mice were primed with 1 × 106 PFU DENV2 (strain PL046) or DENV4 (strain H241) 2 weeks prior to challenge with 2 × 105 PFU DENV2 (strain S221). One control group was challenged but not primed. On day 3 after challenge, serum was harvested, and DENV2-reactive IgG antibodies were measured by ELISA (n = 3) (A), and DENV2-neutralizing antibodies were quantified by PRNT50 (B). Naive serum and 5 μg of the DENV-specific neutralizing antibody 4G2 were included as negative and positive controls, respectively.
T cells are responding to challenge with DENV2 in both DENV2- and DENV4-primed mice.
To further support our hypothesis that CD8+ T cells can mediate heterotypic protection during DENV2 challenge in DENV4-primed mice, we analyzed the T cell response during DENV2 challenge in nonprimed or DENV2- or DENV4-primed mice. IFNAR−/− mice were primed with DENV2 (strain PL046) or DENV4 (strain H241) 2 weeks prior to challenge with DENV2 (strain S221). One control group was not primed. The percentage of T cells producing cytokines was monitored by “direct” in vivo intracellular cytokine staining (ICS) (43,–45) 7 h after challenge with DENV2. In vivo administration of brefeldin A (which causes retention of cytokines in the Golgi complex) 1 h after DENV2 challenge allows for detection and enumeration of T cells that are actively producing cytokines in vivo after exposure to DENV. As expected, the percentage of activated CD8+ T cells (characterized by CD44hi, IFN-γ-positive [IFN-γ+], or CD107a+) was significantly higher in both DENV2- or DENV4-primed mice than in nonprimed animals (Fig. 5A). However, the percentage of IFN-γ+ CD8+ T cells was lower in DENV4-primed mice than in DENV2-primed mice. These results demonstrate that both DENV2 and DENV4 priming leads to CD8+ T cell activation, but DENV4-primed CD8+ T cells are less efficient than DENV2-primed CD8+ cells in producing IFN-γ after DENV2 challenge.
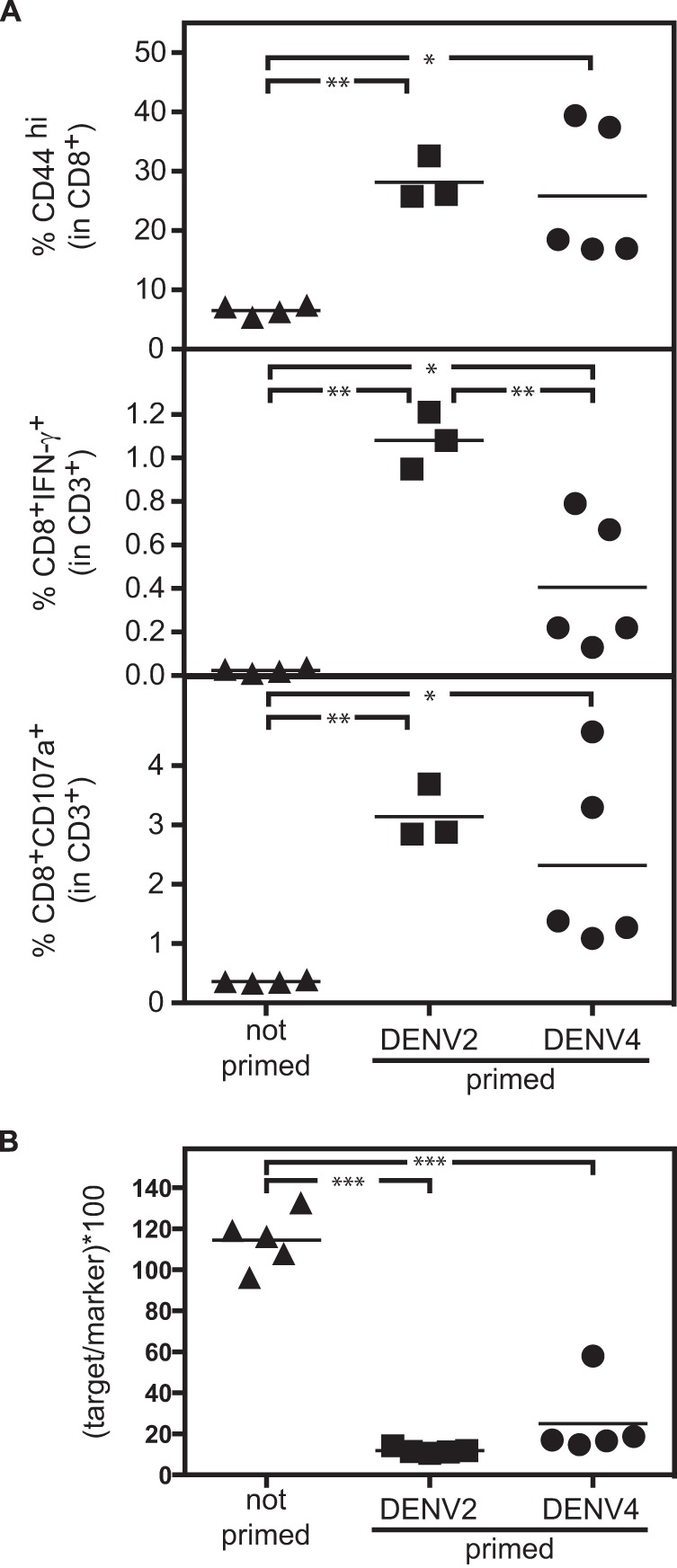
T cells are activated and functional during challenge with DENV2 in mice primed with homotypic or heterotypic DENV. (A) IFNAR−/− mice were primed with 1 × 105 PFU DENV2 (strain PL046) or DENV4 (strain H241) 2 weeks prior to challenge with 2 × 105 PFU DENV2 (strain S221). One control group was not primed. T cell activity was monitored by in vivo ICS 7 h after challenge with DENV2. (Top) Percent CD44hi cells of CD8+ T cells; (middle) percent IFN-γ-producing CD8+ T cells; (bottom) percent CD8+ T cells expressing degranulation marker CD107a+. (B) The cytolytic activity of T cells was assessed by in vivo cytotoxicity assay in IFNAR−/− mice 2 weeks after priming with 1 × 105 PFU DENV2 (strain PL046) or DENV4 (strain H241). Equal numbers of naive splenocytes labeled with the DENV peptide NS4B99-107 (CFSEhi target cells) and unlabeled splenocytes (CFSElo marker cells) were transferred into naive or DENV2- or DENV4-infected recipient mice. The target/marker ratio was determined by flow cytometry in the spleens of recipient mice 15 h after transfer.
To confirm the functionality of the CD8+ T cells activated by DENV2 or DENV4 priming, the cytolytic activity of the CD8+ T cells was assessed by in vivo cytotoxicity assay in IFNAR−/− mice primed with DENV2 (strain PL046) or DENV4 (strain H241). Equal numbers of naive splenocytes labeled with the DENV peptide NS4B99-107 (CFSEhi target cells) and splenocytes without peptide (CFSElo marker cells) were transferred into naive mice or DENV2- or DENV4-infected recipient mice 2 weeks after priming. The target/marker ratio was determined by flow cytometry of spleen cells of recipient mice 15 h after transfer. Similar cytolytic activity was detected in both DENV2- and DENV4-infected mice, and as expected, no cytotoxic activity was detected in naive mice (Fig. 5B). Taken together, these results suggest that priming with either DENV2 or DENV4 induced a CD8+ T cell response that was both measurable and functional upon DENV2 challenge.
CD8+ T cells from DENV2- or DENV4-primed mice are sufficient to reduce viral load when transferred into naive recipients prior to challenge with DENV2.
Finally, we investigated whether CD8+ T cells activated by DENV2 or DENV4 priming would be sufficient to reduce viral load upon DENV2 challenge. IFNAR−/− mice were primed with DENV2 (strain PL046) or DENV4 (strain H241), and 2 weeks later, their CD8+ T cells were isolated (positive selection of CD8+ T cells). CD8+ T cells (2 × 107) from either DENV2- or DENV4-primed mice were transferred into naive IFNAR−/− mice 1 day prior to challenge with DENV2 (strain S221). Viral RNA titers were monitored in the liver and spleen 3 days after challenge with DENV2 (Fig. 6A and andB).B). Recipients that received DENV2- or DENV4-primed CD8+ T cells had a lower viral RNA titer in the liver (Fig. 6A) and spleen (Fig. 6B) 3 days after DENV2 challenge compared to the mice that did not receive CD8+ T cells. The viral RNA titer was lower in mice that received homotypic (DENV2-primed) CD8+ T cells than in mice that received heterotypic (DENV4-primed) CD8+ T cells, which correlated with the higher frequency of IFN-γ+ CD8+ T cells observed in DENV2-primed mice compared to DENV4-primed mice upon challenge with DENV2 (Fig. 5). To assess the duration of protection mediated by the DENV2- or DENV4-primed T cells against DENV2 reinfection, IFNAR−/− mice were primed with DENV2 or DENV4, and 6 weeks later, total T cells were isolated (MACS negative selection of total T cells), and various numbers of T cells were transferred into naive AG129 recipients. One day later, mice were challenged with DENV2, and viral titers were measured in the liver by qRT-PCR 3 days after challenge (Fig. 6C). Both DENV2- and DENV4-primed T cells efficiently reduced viral load upon challenge with DENV2, demonstrating that both homotypic and heterotypic T cells can be protective for at least 6 weeks after priming.
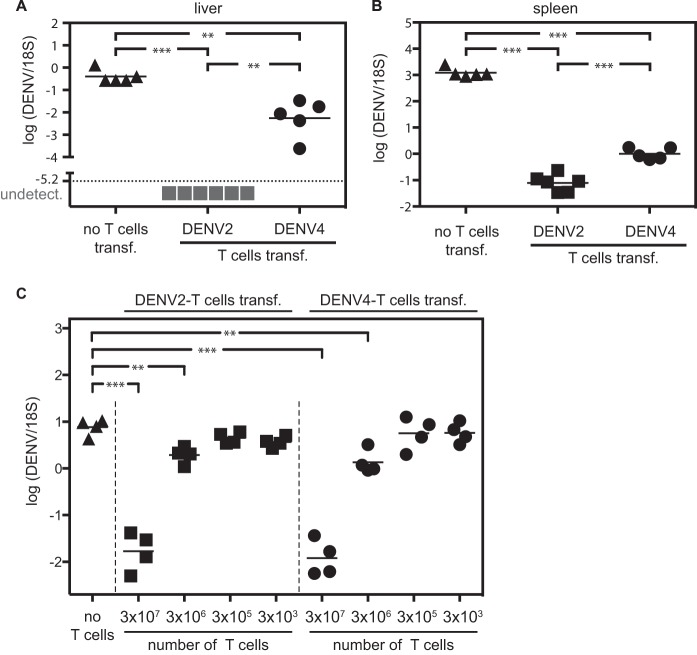
CD8+ T cells from DENV2- and DENV4-primed mice reduce viral load when transferred into naive recipients prior to challenge with DENV2. (A and B) IFNAR−/− mice were primed with 1 × 105 PFU DENV2 (strain PL046) or DENV4 (strain H241). Two weeks later, CD8+ T cells were isolated, and 2 × 107 CD8+ cells were transferred into naive IFNAR−/− mice prior to challenge with 2 × 105 PFU DENV2 (strain S221) 1 day later. Viral RNA titers were monitored in the liver (A) and spleen (B) 3 days after challenge with DENV2. (C) IFNAR−/− mice were primed with 2 × 105 PFU DENV2 (strain S221) or 1 × 106 PFU DENV4 (strain H241). Six weeks later, total T cells were isolated, and various numbers of T cells were transferred into naive AG129 mice prior to challenge with 1 × 104 PFU DENV2 (strain S221) 1 day later. Viral RNA titers were monitored in the liver 3 days after challenge with DENV2.
Collectively, these results demonstrated that both homotypic (DENV2-primed) and heterotypic (DENV4-primed) CD8+ T cells were functional and sufficient to reduce viral load upon DENV2 challenge. However, when equal numbers of CD8+ T cells were transferred, CD8+ T cells activated by DENV2 (homotypic priming) caused a larger viral RNA reduction than CD8+ T cells activated by DENV4 (heterotypic priming).
DISCUSSION
In this study, we used a murine model to investigate the contribution of T cells to protection shortly after DENV reinfection. Our results showed that CD8+ T cells were necessary for protection against heterotypic reinfection in mice that were nonlethally primed with one DENV serotype 2 weeks before challenge with another DENV serotype. However, CD8+ T cells were not required for protection against a homotypic challenge (priming and challenge with the same serotype).
Homotypic (DENV2) priming induced a robust neutralizing antibody response against the serotype used for challenge (DENV2), whereas heterotypic (DENV4) priming induced antibodies that bound to, but did not neutralize, DENV2. Perhaps CD8+ T cells were not necessary for protection after homotypic priming due to the presence of serotype-specific neutralizing antibodies but were required after heterotypic priming because no cross-neutralizing antibodies were present. We demonstrated by adoptive transfer experiments that both homotypic and heterotypic CD8+ T cells were able to reduce viral load upon reinfection. Therefore, we hypothesize that cross-protective CD8+ T cell responses can efficiently compensate for the absence of cross-protective antibodies upon heterotypic reinfection; in contrast, after homotypic priming, humoral responses are sufficient to protect from reinfection. Figure 7 shows a graphical summary of our findings.
In our experiments, we did not detect DENV2 neutralizing antibodies after DENV4 priming. This could be due to a lower level of replication of the DENV4 strain used for priming compared to the DENV2 strain. In the absence of cross-neutralizing antibodies, cross-protection relied on cellular immunity. However, it is possible that under other experimental conditions (other viral strains or higher viral doses), cross-neutralizing antibodies can be induced and can contribute to protection.
While the protective efficacy of a robust neutralizing antibody response is undisputed (10,–12, 21), we suggest that cellular immunity can contribute to protection, especially when the antibody response is not fully protective by itself, as may be the case during heterotypic challenge early after DENV priming. The absence of cross-neutralizing antibodies early after infection with DENV in humans has been reported (49, 50). One study analyzed the humoral immune response 1 to 2 weeks after the onset of fever and reported that cross-reactive antibodies were readily detected against all serotypes, whereas neutralizing antibodies were found only for the infecting serotype (50). In another study, antibody found in convalescent-phase serum was weakly neutralizing for other serotypes (49).
In all our experiments, CD8+ T cell depletion always abolished the viral load reduction observed on day 3 after heterotypic challenge. However, upon DENV2 challenge of IFNAR−/− mice, signs of disease and mortality on day 6 were lower in CD8+ T cell-depleted DENV4-primed mice than in CD8+ T cell-depleted nonprimed mice. This suggests that other protective mechanisms (as for example innate immunity or nonneutralizing antibodies) also play a role besides CD8+ T cells upon heterotypic priming.
Overall, we do not exclude a protective role for cross-neutralizing antibodies, nor do we imply that heterotypic CD8+ T cells are the exclusive mediators of cross-protection. Simply, we suggest (i) that heterotypic CD8+ T cells can play a beneficial role if humoral responses fail to be fully protective and (ii) that cross-protection may not rely exclusively on humoral responses.
Cellular immunity efficiently protected from heterologous reinfection in our study. However, when equal numbers of CD8+ T cells from DENV2- or DENV4-primed mice were transferred into naive recipients before challenge with DENV2, CD8+ T cells from DENV2-primed mice (homotypic) reduced the viral load to a greater degree than CD8+ T cells from DENV4-primed mice. This could be explained by at least two nonmutually exclusive hypotheses. First, CD8+ T cells in DENV4-primed mice may have a lower state of activation than CD8+ T cells in DENV2-primed mice due to lower replication of the DENV4 strain used compared to the DENV2 strain. Alternatively, due to antigenic differences between the DENV2 and DENV4 strains used for priming, only some of the CD8+ T cells primed by DENV4 are reactive to peptides also present in DENV2, resulting in lower CD8+ T cell responses upon challenge with DENV2 in mice primed by DENV4 than in animals primed by DENV2. Our finding that upon challenge with DENV2, fewer cells produced IFN-γ or expressed CD107a in DENV4-primed mice than in DENV2-primed mice is consistent with both hypotheses.
Use of mice deficient in both type I and II IFN receptor (AG129) or in type I IFN receptor only (IFNAR−/−) is not ideal, as both strains lack an important component of the immune system. Therefore, caution must be used when extrapolating our results to dengue disease in humans. However, the advantage of using these mice is that some DENV strains replicate to measurable levels and cause a dengue-like disease (39, 46), thereby allowing for investigation of the effect of priming on subsequent DENV infection. The experiments described here would be impossible in WT mice, in which DENV does not replicate. The lack of replication in WT mice may be explained by the fact that DENV interferes with IFN signaling to establish infection in humans but is unable to do so in mice (51,–54). Therefore, mice deficient in IFN signaling have been increasingly used as a model to study DENV-induced pathology and immunity (26, 30, 47, 48, 55,–61).
Both the mechanism and duration of cross-protection after DENV infection remain controversial (17). Estimates for the length of the cross-protection period vary between 1 and 2 weeks to 1 year or longer, potentially depending on the person or viral serotype (13,–17). Both viral and host factors may thus influence the nature of cross-protection. Our study was designed to investigate experimentally the role of CD8+ T cells during homotypic and heterotypic DENV reinfection. The 2-week period between priming and challenge was chosen because we wished to maximize our chance of observing any potential effect of the cellular responses. Therefore, we chose to wait until after the peak cellular responses (expected around days 7 to 10 in mice [27, 28]) but before the contraction of the T cell compartment. Our study, focusing mainly on short-term protection, can be viewed as a proof-of-principle demonstration of the importance of T cells for protection against heterotypic reinfection. Although a comprehensive study of the long-term protective capacity of CD8+ T cells is required in the future, our data suggest that both homotypic and heterotypic cellular responses can be protective against DENV challenge for at least 6 weeks.
A better understanding of the mechanisms that mediate short- and long-term protection against both homotypic and heterotypic reinfection with DENV is crucial, as the ultimate goal of a DENV vaccine is to provide long-term protection against all serotypes and genotypes within a serotype. Despite years of effort, this goal has not yet been attained (31,–34, 36). The role of cellular immunity in mediating protection against DENV reinfection or after DENV vaccination has not received much attention, as most protection studies focused mainly on neutralizing antibodies. Our study does not exclude a role for cross-reactive and/or cross-neutralizing antibodies at other time points after heterotypic challenge or under different priming conditions. However, our results do suggest that early after infection, CD8+ T cells are crucial to mediate protection against heterotypic reinfection if cross-neutralizing antibodies are absent.
Our study adds to the increasing experimental evidence supporting an important role for cellular immunity during DENV infection in humans and mouse models (23, 25,–30). Our data also support the hypothesis that a DENV vaccine that efficiently induces both humoral and cellular immunity may offer better protection than a vaccine that elicits humoral immune responses alone (25,–28, 30). The phase IIb and III results of the most advanced DENV vaccine candidate, a live-attenuated tetravalent formulation developed by Sanofi Pasteur, is consistent with this hypothesis (31,–33). The overall efficacy of the Sanofi vaccine, composed of the structural proteins of DENV and nonstructural proteins of yellow fever 17D, ranged from 30 to 65%. In addition, the efficacy was much lower in DENV-naive individuals (35% efficacy) than in individuals who were DENV seropositive at baseline (74% efficacy) (31, 62). As the DENV structural proteins are the major targets of anti-DENV antibody but not T cell responses, the limited efficacy of the Sanofi vaccine against certain DENV serotypes or in naive people may be due to the absence of robust T cell responses.
In summary, our results suggest that protection against heterotypic reinfection may not rely exclusively on antibody and show that CD8+ T cells can contribute crucially to protection during heterotypic reinfection and/or if humoral responses alone are not fully protective. Our findings have important implications for vaccine design, as they suggest that inducing both humoral and cellular responses during vaccination may maximize protective efficacy across all DENV serotypes.
ACKNOWLEDGMENTS
This project was supported by NIH grants U54AI057517 from the Southeastern Regional Center of Excellence for Emerging Infectious and Biodefense (S.S.), R56 A1085063; (S.S.), U01 AI082185; (S.S.), and NIH contract HHSN272200900042C to Alessandro Sette and an LJI Center for Infectious Disease Research Fellowship to R.M.Z.
REFERENCES
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.00036-15
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/89/12/6494.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
The Flavivirus Non-Structural Protein 5 (NS5): Structure, Functions, and Targeting for Development of Vaccines and Therapeutics.
Vaccines (Basel), 12(8):865, 01 Aug 2024
Cited by: 0 articles | PMID: 39203991 | PMCID: PMC11360482
Review Free full text in Europe PMC
Epitope(s) involving amino acids of the fusion loop of Japanese encephalitis virus envelope protein is(are) important to elicit protective immunity.
J Virol, 98(4):e0177323, 26 Mar 2024
Cited by: 0 articles | PMID: 38530012 | PMCID: PMC11019926
Unveiling a Shield of Hope: A Novel Multiepitope-Based Immunogen for Cross-Serotype Cellular Defense against Dengue Virus.
Vaccines (Basel), 12(3):316, 16 Mar 2024
Cited by: 0 articles | PMID: 38543950 | PMCID: PMC10975250
Human coronavirus OC43-elicited CD4<sup>+</sup> T cells protect against SARS-CoV-2 in HLA transgenic mice.
Nat Commun, 15(1):787, 26 Jan 2024
Cited by: 6 articles | PMID: 38278784 | PMCID: PMC10817949
Safety and immunogenicity of a synthetic nanoparticle-based, T cell priming peptide vaccine against dengue in healthy adults in Switzerland: a double-blind, randomized, vehicle-controlled, phase 1 study.
EBioMedicine, 99:104922, 20 Dec 2023
Cited by: 2 articles | PMID: 38128414 | PMCID: PMC10776924
Go to all (64) article citations
Data
Protocols & materials
Related Immune Epitope Information - Immune Epitope Database and Analysis Resource
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Protective Role of Cross-Reactive CD8 T Cells Against Dengue Virus Infection.
EBioMedicine, 13:284-293, 07 Oct 2016
Cited by: 69 articles | PMID: 27746192 | PMCID: PMC5264312
Complexity of Neutralizing Antibodies against Multiple Dengue Virus Serotypes after Heterotypic Immunization and Secondary Infection Revealed by In-Depth Analysis of Cross-Reactive Antibodies.
J Virol, 89(14):7348-7362, 01 Jul 2015
Cited by: 46 articles | PMID: 25972550 | PMCID: PMC4473561
Immune response to dengue virus and prospects for a vaccine.
Annu Rev Immunol, 29:587-619, 01 Jan 2011
Cited by: 262 articles | PMID: 21219187
Review
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.
Front Immunol, 10:1316, 11 Jun 2019
Cited by: 36 articles | PMID: 31244855 | PMCID: PMC6579874
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (3)
Grant ID: U01 AI082185
Grant ID: HHSN272200900042C
Grant ID: U54AI057517
PHS HHS (2)
Grant ID: HHSN272200900042C
Grant ID: R56 A1085063