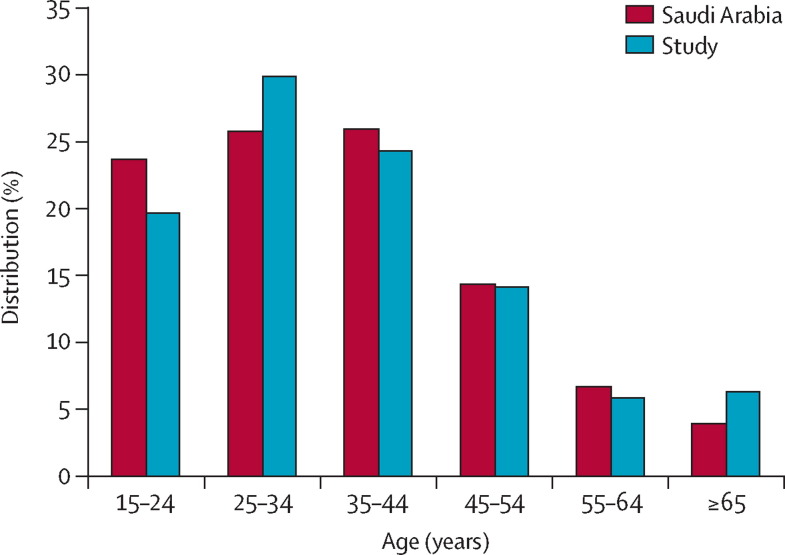Abstract
Background
Scientific evidence suggests that dromedary camels are the intermediary host for the Middle East respiratory syndrome coronavirus (MERS-CoV). However, the actual number of infections in people who have had contact with camels is unknown and most index patients cannot recall any such contact. We aimed to do a nationwide serosurvey in Saudi Arabia to establish the prevalence of MERS-CoV antibodies, both in the general population and in populations of individuals who have maximum exposure to camels.Methods
In the cross-sectional serosurvey, we tested human serum samples obtained from healthy individuals older than 15 years who attended primary health-care centres or participated in a national burden-of-disease study in all 13 provinces of Saudi Arabia. Additionally, we tested serum samples from shepherds and abattoir workers with occupational exposure to camels. Samples were screened by recombinant ELISA and MERS-CoV seropositivity was confirmed by recombinant immunofluorescence and plaque reduction neutralisation tests. We used two-tailed Mann Whitney U exact tests, χ(2), and Fisher's exact tests to analyse the data.Findings
Between Dec 1, 2012, and Dec 1, 2013, we obtained individual serum samples from 10,009 individuals. Anti-MERS-CoV antibodies were confirmed in 15 (0·15%; 95% CI 0·09-0·24) of 10,009 people in six of the 13 provinces. The mean age of seropositive individuals was significantly younger than that of patients with reported, laboratory-confirmed, primary Middle Eastern respiratory syndrome (43·5 years [SD 17·3] vs 53·8 years [17·5]; p=0·008). Men had a higher antibody prevalence than did women (11 [0·25%] of 4341 vs two [0·05%] of 4378; p=0·028) and antibody prevalence was significantly higher in central versus coastal provinces (14 [0·26%] of 5479 vs one [0·02%] of 4529; p=0·003). Compared with the general population, seroprevalence of MERS-CoV antibodies was significantly increased by 15 times in shepherds (two [2·3%] of 87, p=0·0004) and by 23 times in slaughterhouse workers (five [3·6%] of 140; p<0·0001).Interpretation
Seroprevalence of MERS-CoV antibodies was significantly higher in camel-exposed individuals than in the general population. By simple multiplication, a projected 44,951 (95% CI 26,971-71,922) individuals older than 15 years might be seropositive for MERS-CoV in Saudi Arabia. These individuals might be the source of infection for patients with confirmed MERS who had no previous exposure to camels.Funding
European Union, German Centre for Infection Research, Federal Ministry of Education and Research, German Research Council, and Ministry of Health of Saudi Arabia.Free full text

Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study
Abstract
Background
Scientific evidence suggests that dromedary camels are the intermediary host for the Middle East respiratory syndrome coronavirus (MERS-CoV). However, the actual number of infections in people who have had contact with camels is unknown and most index patients cannot recall any such contact. We aimed to do a nationwide serosurvey in Saudi Arabia to establish the prevalence of MERS-CoV antibodies, both in the general population and in populations of individuals who have maximum exposure to camels.
Methods
In the cross-sectional serosurvey, we tested human serum samples obtained from healthy individuals older than 15 years who attended primary health-care centres or participated in a national burden-of-disease study in all 13 provinces of Saudi Arabia. Additionally, we tested serum samples from shepherds and abattoir workers with occupational exposure to camels. Samples were screened by recombinant ELISA and MERS-CoV seropositivity was confirmed by recombinant immunofluorescence and plaque reduction neutralisation tests. We used two-tailed Mann Whitney U exact tests, χ2, and Fisher's exact tests to analyse the data.
Findings
Between Dec 1, 2012, and Dec 1, 2013, we obtained individual serum samples from 10 009 individuals. Anti-MERS-CoV antibodies were confirmed in 15 (0·15%; 95% CI 0·09–0·24) of 10 009 people in six of the 13 provinces. The mean age of seropositive individuals was significantly younger than that of patients with reported, laboratory-confirmed, primary Middle Eastern respiratory syndrome (43·5 years [SD 17·3] vs 53·8 years [17·5]; p=0·008). Men had a higher antibody prevalence than did women (11 [0·25%] of 4341 vs two [0·05%] of 4378; p=0·028) and antibody prevalence was significantly higher in central versus coastal provinces (14 [0·26%] of 5479 vs one [0·02%] of 4529; p=0·003). Compared with the general population, seroprevalence of MERS-CoV antibodies was significantly increased by 15 times in shepherds (two [2·3%] of 87, p=0·0004) and by 23 times in slaughterhouse workers (five [3·6%] of 140; p<0·0001).
Interpretation
Seroprevalence of MERS-CoV antibodies was significantly higher in camel-exposed individuals than in the general population. By simple multiplication, a projected 44 951 (95% CI 26 971–71 922) individuals older than 15 years might be seropositive for MERS-CoV in Saudi Arabia. These individuals might be the source of infection for patients with confirmed MERS who had no previous exposure to camels.
Funding
European Union, German Centre for Infection Research, Federal Ministry of Education and Research, German Research Council, and Ministry of Health of Saudi Arabia.
Introduction
As of March 8, 2015, Middle East respiratory syndrome coronavirus (MERS-CoV) has caused at least 1082 mostly severe cases of respiratory infection, 439 of these fatal, since its discovery in 2012.1 Apart from its geographical focus in countries in and around the Arabian Peninsula and laboratory evidence of widespread infection of dromedary camels, little is known about the actual epidemiology of the disease in human beings.1, 2, 3, 4, 5 Few primary infections were acquired through direct camel exposure, but the relevance of this infection pattern is unclear in the absence of systematic determinations of the proportion of infections in individuals who have had contact with camels.5, 6, 7 Moreover, most reported patients with primary MERS-CoV infection have no history of camel exposure, suggesting the existence of other, as-yet-unknown sources of infection. Camel milk as a food-borne source seems unlikely.8
For secondary infections acquired through human-to-human contact, mathematical projections predicted that transmission chains in the population cannot be sustained.9, 10, 11 The apparent transmission rate in household settings is low, with fewer than 50% of index patients transmitting the infection to contacts who subsequently had no pronounced clinical symptoms.12 However, a highly fatal outbreak of MERS-CoV infection centred in Jeddah, Saudi Arabia, in March–April, 2014, was apparently caused by human-to-human transmission in several nosocomial settings, without any evidence that the causative virus differed from other MERS-CoV strains in people, in terms of replication in cell culture, immune escape, or excretion level.13 Undiscovered MERS-CoV infections might thus exist in the human population.
Serology is key to the understanding of infection statistics at a population level. However, only a few serological studies of MERS-CoV have been done.14, 15 Although none of those preliminary studies showed any evidence of previous infection with MERS-CoV in the population of Saudi Arabia, the examined cohorts have been small and methods applied in those studies have not been validated for sensitivity and specificity. We previously presented the first validated recombinant ELISA (rELISA) for MERS-CoV and combined this test with recombinant immunofluorescence assay (rIFA) and a plaque reduction virus neutralisation test (PRNT) into a staged strategy for MERS-CoV serology that was shown to identify even subclinical infections on detection of specific antibodies.12 Here, we apply the established testing algorithm on a nationwide serum collection. Additional serum samples were obtained from individuals occupationally exposed to dromedary camels.
Methods
Sample collection
For the cross-sectional serosurvey we used existing sample collections organised by the Ministry of Health of Saudi Arabia. Individual serum samples were obtained from healthy people older than 15 years, selected to represent all ages (excluding children and adolescents <15 years) in the Saudi Arabian population. Samples were obtained with written informed consent given to the Ministry of Health from participants in households randomly selected in a population-based, cross-sectional sampling frame maintained and updated by the National Census Bureau.16 Participants lived and gave samples in all 13 provinces of Saudi Arabia. Sampling was done through primary health-care centres and hospitals under the supervision of the Ministry of Health. We obtained two collections of serum samples from local primary health-care clinics for individuals selected to participate in a survey of the national burden of disease,16, 17 and a second collection of samples from individuals selected for a national study of the seroprevalence of herpes simplex virus.
Additionally, we obtained serum samples from camel shepherds who participated in an ad hoc MERS-CoV surveillance study implemented by the Ministry of Health and expatriate slaughterhouse workers in Makkah. The shepherds gave verbal consent and the slaughterhouse workers gave direct written consent in the frame of the ad hoc surveillance study.
Procedures
All serum samples were anonymised before the study and were tested for MERS-CoV antibodies without further institutional review, because the testing of all samples was done as part of a public health intervention by the Saudi Ministry of Health. We applied an anti-MERS-CoV ELISA IgG (EUROIMMUN AG, Lübeck, Germany) with minor modifications. The rELISA was based on the soluble MERS-CoV spike protein subdomain S1.18 This highly sensitive assay has been validated for use with human serum samples.12 Samples were applied at a 1/100 dilution. The cutoff optical density ratio was set to 0·3, which is three times the ratio of the mean value of all tested human serum samples without known exposure to camels.
We used rIFA to substantiate rELISA results because rIFA has been shown to be more specific than rELISA.12 rIFA was done with the full MERS-CoV spike open-reading frame expressed in VeroB4 cells.12, 19 For IgG detection, serum samples were tested at a 1/40 dilution. For IgM detection, samples were analysed at 1/20 and 1/100 dilutions.
We did plaque reduction neutralisation tests (PRNT) as previously described.20 The first applied dilution of a two-fold dilution series was 1/10. In accordance with our previous study,12 we defined a 1/20 dilution as the lowest possible diagnostically significant titre.20 We recorded serum dilutions conferring 50% and 90% plaque reduction as PRNT50 and PRNT90 titres, respectively.
We defined stage 1 seropositivity as reactivity in both rELISA (dilution 1/100) and rIFA (dilution 1/40). We defined stage 2 seropositivity as stage 1 seropositivity plus reactivity in PRNT with a reciprocal titre of at least 20 on PRNT50. We regarded stage 2 seropositive results as serological confirmation of past MERS-CoV infection.
To compare the age distribution of patients with serologically confirmed cases with that of patients with clinically apparent cases, we examined data from international case-notification statistics21 and index-case data from a study of household contact clusters.12
Statistical analysis
We did a two-tailed Mann Whitney U exact test with SPSS software (version 21). We did χ2 tests and Fisher's exact tests, and calculated CIs with OpenEpi (Open Source Epidemiologic Statistics for Public Health). We applied χ2 tests in the seroprevalence calculations of the general population versus camel-exposed individuals and for comparisons of agegroups. We used Fisher's exact tests for calculations of geographical and gender comparisons. We applied the non-parametric Mann Whitney U test to compare the mean ages of patients with MERS in different studies.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between April 1, and June 30, 2013, we obtained 5765 serum samples from local primary health-care clinics for individuals selected to participate in a survey of the national burden of disease,16, 17 and a further 4244 samples between Dec 1, 2012, and Dec 1, 2013 from primary health-care centres. Data for patient demographic characteristics (appendix) were available for 8719 (87%) of 10 009 stored serum samples.
Additionally, we obtained serum samples from 87 camel shepherds who participated in a MERS-CoV surveillance study from Feb 1, to May 31, 2014, and 140 expatriate slaughterhouse workers in Makkah during August, 2013. The camel shepherds were aged 21–84 years (mean 37·9 years, SD 13·02) and resided in six different provinces (Al-Jouf, Asir, Eastern, Madinah, Makkah, and Riyadh). The slaughterhouse workers were aged 15–62 years (mean 37·13, SD 8·64).
The mean sample size per province was 770 (range 321 to 2343 [SD 585]), representing between 19·7 and 97·1 samples per 100 000 individuals per province (mean 52·9, SD 26·7). The age distribution of samples with available age information matched that of the population of Saudi Arabia (figure 1 ). Of the 8719 individuals with available demographic information, 4341 (50%) were male.
All serum samples from the general population were first screened for MERS-CoV-specific IgG by rELISA, yielding reactive results in 152 (1·52%, 95% CI 1·29–1·78) of 10 009 samples. Subsequent testing by rIFA and PRNT50 confirmed less than 0·2% to be stage 1 and stage 2 seropositive (table 1 ; appendix). Because repeat samples were missing, the timepoint of seroconversion was established by IgM detection. Coronavirus IgM had been previously shown to be maintained for a maximum of 180 days after infection.22 Only one of 15 seropositive individuals had a reciprocal MERS-CoV IgM titre of at least 100, suggesting recent seroconversion (appendix). In accordance with our previous study,12 seropositivity with rELISA was ten times higher than with rIFA. Seroprevalence did not differ between the two subcohorts (seven [0·16%] of 4244 patients in the primary health-care study group vs eight [0·14%] of 5765 in the burden of disease study cohort group, p=0·94; appendix). Men had a higher proportion of infections (11 [0·25%] of 4341) than did women (two [0·05%] of 4378; p=0·025). Cases were identified in four of six age groups without significant age preference (appendix).
Table 1
Middle East respiratory syndrome coronavirus antibodies in the general and subpopulations of Saudi Arabia
| Year of sampling | Total number | rELISA positive | Stage 1 seropositive | Stage 2 seropositive | ||||
|---|---|---|---|---|---|---|---|---|
| n (%; 95% CI) | p value | n (%; 95% CI) | p value | n (%; 95% CI) | p value | |||
| General population | 2012–13 | 10 009 | 152 (1·5%; 1·3–1·8) | NA | 17 (0·2%; 0·1–0·3) | NA | 15 (0·2%; 0·1–0·2) | NA |
| Camel shepherds | 2014 | 87 | 6 (6·9%; 2·8–13·8) | p=0·0003 | 2 (2·3%; 0·3–7·4) | p=0·0009 | 2 (2·3%; 0·3–7·4) | p=0·0004 |
| Slaughterhouse workers | 2013 | 140 | 6 (4·3%; 1·8–8·7) | p=0·0224 | 5 (3·6%; 1·3–7·7) | p<0·0001 | 5 (3·6%; 1·3–7·7) | p<0·0001 |
Data are n (%; 95% CI) from a serosurvey, unless otherwise specified. p values refer to comparison with the general population cohort (χ2 test with Yates correction two-tail test; OpenEpi). rELISA=recombinant ELISA. NA=not applicable.
Confirmed cases of MERS-CoV infection were identified in six of the 13 administrative provinces of Saudi Arabia, with higher numbers in central provinces that generally are more rural (figure 2 ). The 13 provinces were classified into seven central and six coastal provinces, on the basis of whether they had a shoreline. Significantly more confirmed infections were in central provinces (14 [0·26%] of 5479 samples) than in coastal provinces (one [0·02%] of 4529; p=0·003).
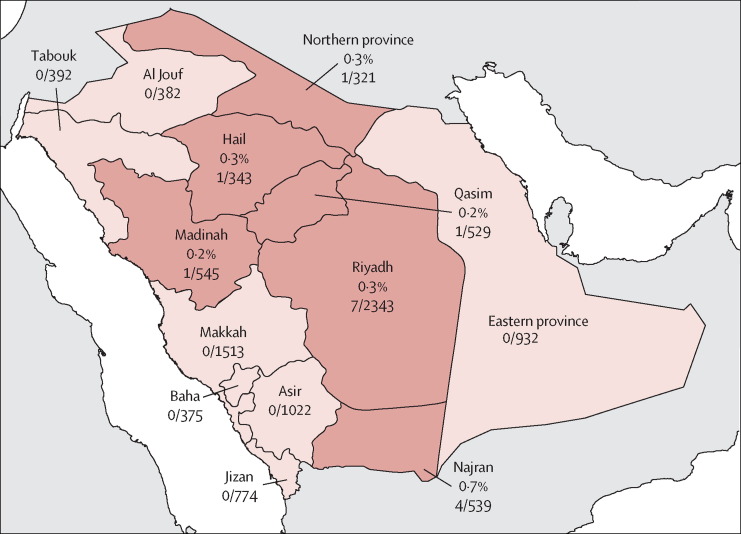
Serologically confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) in the general population of Saudi Arabia from Dec 1, 2012, to Dec 1, 2013
Red areas represent provinces that contain individuals with stage 2 seropositive MERS-CoV. Percentages show the number of stage 2 positive serum samples per number tested in each province (detailed numbers and full serological results per province in appendix).
The proportion of both camel shepherds and slaughterhouse workers who were seropositive was significantly higher than in the general population (table 1). Seropositive individuals were identified in five of six age groups with even distribution (appendix).
Both registered patients (ie, patients with confirmed MERS)21 and index patients from the household study were significantly older than the serologically confirmed individuals in our present study (table 2 ; detailed laboratory results in the appendix).
Table 2
Age profiles in groups of patients with MERS-CoV infection in Saudi Arabia
| Total | Mean age, years (SD) | Median age, years (IQR) | Reference | Date | |
|---|---|---|---|---|---|
| Patients with serological evidence of past infection | 18 | 43·50 (17·27) | 42 (30·8–50·0) | This study | 2012–13 |
| Patients with clinically apparent infection* | 482 | 53·76 (17·51)† | 55 (41·0–68·0) | MERS-CoV spatial, temporal, and epidemiological information21 | 2012–14 |
| Index patients in household-contact study | 24 | 54·29 (19·92)† | 55 (46·3–69·3) | Drosten et al, 201412 | 2013 |
Data from Saudi Arabia in 2012–14. MERS-CoV=Middle East respiratory syndrome coronavirus.
Discussion
In our study, we identified antibodies against MERS-CoV in 0·15% of samples from the general Saudi Arabian population. The true number of infections is difficult to estimate because our sample consisted of only 10 009 (0·03%) of the whole population of 29 994 300 people. However, by combining two cross-sectional subcohorts we could cover all 13 provinces and achieved an age distribution of our sample that largely matched that of the general population (panel ). All geographical regions of the country were sampled with a reasonably low variation in the proportions of population that were studied per province. To have optimum sample coverage per province, we included samples from an earlier study (unpublished report) for which detailed information about sex and age was not available for 26% of samples. Nevertheless, samples from both subcohorts should be similar in age and sex composition because both were previously obtained according to calculations by the National Census Bureau to represent the composition of the Saudi Arabian population. This similarity was lent support by the highly similar seroprevalence when we analysed both subcohorts separately. Even if we had only used data from the more thoroughly documented burden-of-disease study subcohort for which we had sex and age information for 97% of the samples, the overall seroprevalence would have been maintained (0·15% in the overall sample vs 0·14% in the subcohort).
To obtain an estimate of the total number of seropositive individuals in Saudi Arabia we did a straightforward extrapolation based on the proportion of stage-2-positive serum samples in both population subcohorts (excluding shepherd and slaughterhouse worker samples). Extrapolation by simple multiplication suggests that 44 951 seropositive individuals (95% CI 26 971–71 922) could exist in a total population of 29 994 300. Our serological assays might have missed some infections because the high specificity of rIFA and PRNT could encompass a reduction in sensitivity of the overall serological algorithm. However, even a projection based on rELISA results only would raise the estimated infection rate by about ten times, which is several orders of magnitude lower than figures expected for a respiratory disease that continuously transmits between human beings. In accordance with our previous study,12 seropositivity with rELISA was ten times higher than that with rIFA.12 This rise can be attributed to the higher sensitivity of rELISA and by putative crossreactivity of low-affinity IgG, which is raised in individuals with recent infections from the common-cold coronavirus.23
Despite low detection rates, antibodies were identified widely across Saudi Arabia, including in provinces where outbreaks in people have not been reported. Widespread acquisition of infections without subsequent causation of outbreaks corresponds to the concept of a predominantly zoonotic disease that does not readily spread between human beings. The raised prevalence in rural regions and the significantly increased proportion of infection in groups that are occupationally exposed to camels emphasises the importance of zoonotic acquisitions by dromedary camels.5, 6, 7 However, even though the analysed shepherd samples were collected in six different regions during the first months of 2014, the sampling was not randomised and samples were not strictly taken at the same time as those from the general population. Similar limitations apply for the slaughterhouse worker samples. Direct comparison of seroprevalences between these groups and the general population cohort might thus be biased. Matched case-control studies could be used to substantiate the raised seroprevalence in exposed groups.
Direct animal contact has been reported for only a few primary cases in Saudi Arabia and elsewhere. The younger age of serologically confirmed patients in our study compared with people with laboratory confirmed infection underlines the possibility that MERS-CoV might cause unrecognised infections that cause no or mild symptoms in healthy, younger (aged 15–44 years), and socially active populations. These subclinical infections could act as an unknown source for index patients who cannot recall contact with camels. Importantly, the predominance of men even within serologically confirmed cases emphasises a potential association with animal exposure because, typically, more men than women in Saudi Arabia associate with camels during leisure activities. Cases of MERS in younger people (aged <45 years) might seem mild or even subclinical, similar to cases in our study of household contact clusters,12 in which contacts were younger than index patients and mostly asymptomatic. MERS might constitute a zoonotic disease that causes enough short-transmission chains in the young male population of Saudi Arabia, for which older and predisposed individuals constitute a sentinel population.
Acknowledgments
We thank all staff of the Ministry of Health and the Ministry of Agriculture, Saudi Arabia; Sebastian Brünink and Stephan Kallies for technical assistance; and Anna Corman, Daniela Niemeyer, and the Institute for Medical Biometry, Informatics and Epidemiology of the University of Bonn Medical Centre for statistical advice. CD was supported by European Commission grant EMPERIE (contract number 223498) and ANTIGONE (contract number 278976), the German Centre for Infection Research, the Federal Ministry of Education and Research, and the German Research Council (grants 01Kl1005 and DR 772/3-1, and MU 3564/1-1).
Contributors
MAM did the experiments, designed and coordinated the study, wrote the manuscript, analysed data, and generated figures. BM did the experiments and analysed data. VMC organised sample logistics, analysed data, and generated figures. MA-M and AT organised, coordinated, and supervised the samplings. DR and AS did the laboratory tests and analysed data. SA and PW analysed data. B-JB and EL provided diagnostic reagents and analysed data. RFA, AMAs, AMAl, AMA-S, JAA-T, and AAAR designed and supervised the samplings. CD and ZAM designed and supervised the study, analysed and interpreted data, and wrote the manuscript.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/s1473-3099(15)70090-3
Read article for free, from open access legal sources, via Unpaywall:
http://www.thelancet.com/article/S1473309915700903/pdf
Citations & impact
Impact metrics
Article citations
Biphasic MERS-CoV Incidence in Nomadic Dromedaries with Putative Transmission to Humans, Kenya, 2022-2023.
Emerg Infect Dis, 30(3):581-585, 01 Mar 2024
Cited by: 1 article | PMID: 38407189 | PMCID: PMC10902546
Human Challenge Studies with Coronaviruses Old and New.
Curr Top Microbiol Immunol, 445:69-108, 01 Jan 2024
Cited by: 0 articles | PMID: 35181805
Review
Genetic diversity and molecular epidemiology of Middle East Respiratory Syndrome Coronavirus in dromedaries in Ethiopia, 2017-2020.
Emerg Microbes Infect, 12(1):e2164218, 01 Dec 2023
Cited by: 3 articles | PMID: 36620913 | PMCID: PMC9888459
MERS-CoV seroconversion amongst Malaysian Hajj pilgrims returning from the Middle East, 2016-2018: results from the MERCURIAL multiyear prospective cohort study.
Emerg Microbes Infect, 12(1):2208678, 01 Dec 2023
Cited by: 1 article | PMID: 37101375 | PMCID: PMC10208164
The spatiotemporal analysis of the population migration network in China, 2021.
Infect Dis Model, 8(4):1117-1126, 11 Oct 2023
Cited by: 1 article | PMID: 37915999 | PMCID: PMC10616395
Go to all (181) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) antibodies in dromedary camels in Israel.
Zoonoses Public Health, 65(6):749-754, 31 May 2018
Cited by: 26 articles | PMID: 29855166 | PMCID: PMC6274617
Middle East respiratory syndrome coronavirus (MERS-CoV) neutralising antibodies in a high-risk human population, Morocco, November 2017 to January 2018.
Euro Surveill, 24(48), 01 Nov 2019
Cited by: 12 articles | PMID: 31796154 | PMCID: PMC6891945
MERS-CoV exposure and risk factors for MERS-CoV ELISA seropositivity among members of livestock-owning households in southern Jordan: a population based cross-sectional study.
Lancet Microbe, 5(9):100866, 22 Jul 2024
Cited by: 0 articles | PMID: 39053480
Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission.
Lancet Infect Dis, 18(8):e217-e227, 18 Apr 2018
Cited by: 233 articles | PMID: 29680581 | PMCID: PMC7164784
Review Free full text in Europe PMC
Funding
Funders who supported this work.
ANTIGONE (1)
Grant ID: contract number 278976
European Commission grant EMPERIE (1)
Grant ID: contract number 223498