Abstract
Free full text

Unleashing the immune system: PD-1 and PD-Ls in the pre-treatment tumor microenvironment and correlation with response to PD-1/PD-L1 blockade
Abstract
Focal tumor cell PD-L1 expression adjacent to TIL can be used as a surrogate marker of an ongoing antitumor host response, which may be unleashed by PD-1 blockade. Tumor cell PD-L1 expression is superior to TIL PD-1 expression and the presence of TIL alone, when predicting response to anti-PD-1 therapy.
In addition to exciting durable tumor regressions, one of the more provocative findings associated with PD-L1/PD-1 pathway blockade involves the potential predictive value of pre-treatment specimen PD-L1 expression. We first reported a small series of nine patients from the MDX-1106/BMS-936558 trial, suggesting that tumor cell surface (membranous) PD-L1 expression may be associated with responsiveness to PD-1 blockade.1 These findings were supported in a larger series of 42 patients in the follow-up trial.2 Specifically, of the 25 patients who had a formalin-fixed paraffin-embedded (FFPE) pre-treatment specimen that was PD-L1(+), 36% had an objective response to anti-PD-1. In contrast, no patients whose tumors were PD-L1(−) demonstrated a clinical response (p = 0.006).
More recently, our group published the results of an expanded analysis conducted on 68 FFPE pre-treatment specimens from 41 patients with advanced cancers who were treated with anti-PD-1. The cohort included 16 patients with melanoma, 12 with non-small cell lung carcinoma (NSCLC), 6 with kidney cancer, 5 with colorectal carcinoma (CRC), and 2 with castration-resistant prostate cancer (CRPC).3 Fifty-three of these 68 specimens had previously been assessed for PD-L1 expression.2 The extended analysis included additional histologic and immune features in the pre-treatment tumor microenvironment, and how they related to each other and to patient outcomes. This involved a focus on infiltrating immune cell subsets, PD-1, PD-L1 and PD-L2 expression.
We found that tumor PD-L1 expression varied significantly by tumor type. Approximately 60% of the melanoma, NSCLC, and kidney cancer specimens tested demonstrated PD-L1 expression, in contrast to only one of 12 (8%) colorectal and CRPC specimens (p = 0.005). When tumor cell PD-L1 expression was observed, it was focal and seen in immediate geographic association with tumor infiltrating lymphocytes (TIL) in all but one case (33/34). Such constancy supports our hypothesis that PD-L1 expression by tumor is a mechanism of adaptive immune resistance.4 We also observed PD-L1 expression on infiltrating immune cells in the absence of tumor cell expression. For example, even though only 1 of 8 CRC cases demonstrated PD-L1+ tumor cells, 4 of the CRC cases (50%) had PD-L1 displayed on TIL and associated macrophages.
Additional histologic and immunoarchitectural features were also assessed for their relationship to PD-L1 expression. We found that the presence of TIL expressing PD-1 as well as CD20+ B-cells were both significantly associated with tumor and TIL PD-L1 expression. Features such as whether the specimen was from the primary tumor vs. a metastasis, the CD4+:CD8+ ratio, the presence or lymphoid aggregates, or tumor cell necrosis did not demonstrate a significant association. PD-L2, the second known ligand for PD-1 on T-cells, was observed to a lesser degree than PD-L1. When present, PD-L2 was observed in geographic association with PD-L1 at the interface of tumor and TIL (p = 0.05).
The finding that TIL PD-1 was displayed adjacent to PD-L1 (and sometimes with PD-L2) suggests an immunosuppressive microenvironment that may be altered by the administration of anti-PD-1 therapy. Accordingly, we examined how these factors in pre-treatment tumor specimens predicted response to anti-PD-1. We found that PD-L1 expression by tumor was the strongest single factor predicting objective response (Fig. 1), when compared to TIL PD-1 expression, or the presence of TIL alone. This is likely because focal tumor cell PD-L1 expression adjacent to TIL reflects an ongoing antitumor immune response, which may be protected by anti-PD-1. While PD-1 is the direct target of anti-PD-1, it only demonstrated a borderline association with response in our series. Similarly, the presence of TIL alone is not a significant factor predicting response to anti-PD-1. This latter finding suggests various functional states of TIL. Future studies will undoubtedly focus on further characterizing lymphocyte subsets, including regulatory T-cells, as well as other immunoactive cell types, such as myeloid-derived suppressor cells, and how these populations relate to response to anti-PD-1.
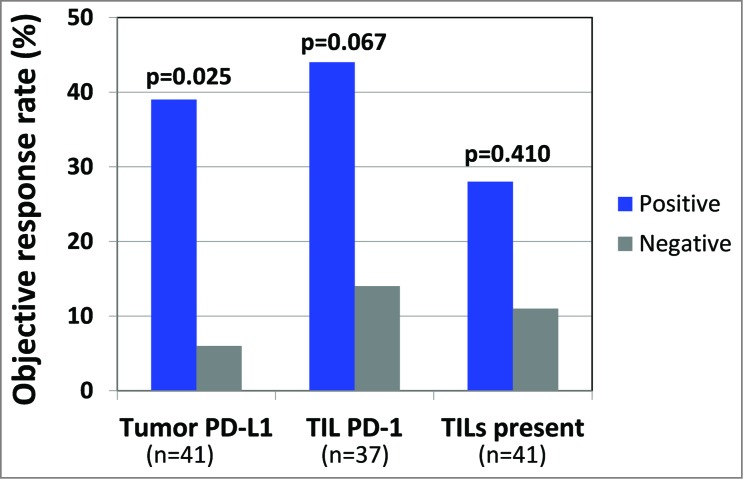
Tumor PD-L1 is the strongest single predictor of response to anti-PD-1. When analyzing either the highest scoring sample among multiple biopsies from individual patients or the specimen obtained closest to therapy, tumor cell PD-L1 expression correlated with objective response to anti-PD-1 therapy. This association was stronger than the borderline association with PD-1 expression. Simply the presence of intratumoral immune cell infiltrates did not correlate with response. Additional features examined that did not predict response to anti-PD-1 in this limited cohort included PD-L2 expression by tumor or immune cells, CD4+:CD8+ ratio, CD20+ B−cells, or the presence of lymphoid aggregates or tumor necrosis (data not shown).
A proportion of the patients in our cohort had multiple pre-treatment specimens available for testing. PD-L1 expression was also heterogeneous across different pathologic specimens from a single patient. For the purpose of the aforementioned analysis where PD-L1 expression was correlated with response to anti-PD-1, a patient was considered PD-L1(+) if any of their specimens demonstrated tumor cell PD-L1 expression. For example, one melanoma patient who demonstrated a complete response had three different pre-treatment specimens available for study. The primary melanoma was PD-L1(+), and the lymph node and subsequent subcutaneous metastases were both PD-L1(−). By our methodology, the patient was considered PD-L1(+), due to PD-L1 expression of the primary tumor. Notably, if only one of the patient's latter specimens had been tested, and PD-L1 status was used as a selection criteria for PD-1/PD-L1 blockade, the patient would have been considered ‘PD-L1(−)’ and may have missed the opportunity to receive anti-PD-1.
Identifying and validating markers that could enrich for clinical response would have great significance for optimal therapeutic development. We, and now others 5-8 have demonstrated that PD-L1 expression in the tumor microenvironment enriches for response to anti-PD-1, though the association is not absolute. Uncertainty remains as to whether PD-L1 expression in a single pathologic specimen will routinely be used to pre-select individual patients for anti-PD1 therapy. Features such as the temporal and geographic heterogeneity of PD-L1 expression across specimens from a single patient call this approach into question. Our findings support the proposed mechanism of action of anti-PD-1 and suggest that study of the pre-treatment pathologic specimens may be used to help identify tumor types likely to respond to this therapy. Pre-treatment pathologic specimens will also likely be useful in identifying additional dominant or co-dominant pathways that may be targeted in combination with anti-PD-1 to further increase the proportion of patients who benefit from these exciting agents.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
Articles from Oncoimmunology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.4161/21624011.2014.963413
Read article for free, from open access legal sources, via Unpaywall:
https://www.tandfonline.com/doi/pdf/10.4161/21624011.2014.963413?needAccess=true
Citations & impact
Impact metrics
Citations of article over time
Article citations
Aging-related biomarker discovery in the era of immune checkpoint inhibitors for cancer patients.
Front Immunol, 15:1348189, 15 Mar 2024
Cited by: 2 articles | PMID: 38590525 | PMCID: PMC11000233
Review Free full text in Europe PMC
Programmed Cell Death Ligand 1 Immunohistochemical Expression and Cutaneous Melanoma: A Controversial Relationship.
Int J Mol Sci, 25(1):676, 04 Jan 2024
Cited by: 1 article | PMID: 38203846 | PMCID: PMC10779806
Review Free full text in Europe PMC
PCSK9 regulates the efficacy of immune checkpoint therapy in lung cancer.
Front Immunol, 14:1142428, 21 Mar 2023
Cited by: 8 articles | PMID: 37025995 | PMCID: PMC10070680
Immunological and Genomic Analysis Reveals Clinically Relevant Distinctions between Angiosarcoma Subgroups.
Cancers (Basel), 14(23):5938, 30 Nov 2022
Cited by: 1 article | PMID: 36497420 | PMCID: PMC9739001
Tumor Infiltrating Lymphocyte Expression of PD-1 Predicts Response to Anti-PD-1/PD-L1 Immunotherapy.
J Immunother Precis Oncol, 5(4):90-97, 22 Sep 2022
Cited by: 15 articles | PMID: 36483582 | PMCID: PMC9714418
Go to all (55) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Programmed Death-Ligand 1 Expression in Non-Small Cell Lung Carcinoma Biopsies and Its Association with Tumor Infi ltrat ing Lymphocytes and the Degree of Desmoplasia.
Klin Onkol, 33(1):55-65, 01 Jan 2020
Cited by: 1 article | PMID: 32075390
Classifying Non-Small Cell Lung Cancer by Status of Programmed Cell Death Ligand 1 and Tumor-Infiltrating Lymphocytes on Tumor Cells.
J Cancer, 9(1):129-134, 01 Jan 2018
Cited by: 8 articles | PMID: 29290777 | PMCID: PMC5743719
Stromal PD-L1-Positive Regulatory T cells and PD-1-Positive CD8-Positive T cells Define the Response of Different Subsets of Non-Small Cell Lung Cancer to PD-1/PD-L1 Blockade Immunotherapy.
J Thorac Oncol, 13(4):521-532, 18 Dec 2017
Cited by: 88 articles | PMID: 29269008
PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions.
Oncologist, 24(suppl 1):S31-S41, 01 Feb 2019
Cited by: 198 articles | PMID: 30819829 | PMCID: PMC6394772
Review Free full text in Europe PMC




