Abstract
Free full text

Protein Tyrosine Phosphatase SHP-1 Modulates T Cell Responses by Controlling Cbl-b Degradation
Abstract
Previously, we demonstrated that CD28 and CTLA-4 signaling controls Cbl-b protein expression, which is critical for T cell activation and tolerance induction. However, the molecular mechanism(s) of this regulation remains to be elucidated. In this study, we found that Cbl-b fails to undergo tyrosine phosphorylation upon CD3 stimulation because SHP-1 is recruited to and dephosphorylates Cbl-b, while CD28 costimulation abrogates this interaction. In support of this finding, T cells lacking SHP-1 display heightened tyrosine phosphorylation and ubiquitination of Cbl-b upon TCR stimulation, which correlates with decreased levels of Cbl-b protein. The aberrant Th2 phenotype observed in T cell-specific Shp1−/− (tShp1−/−) mice is reminiscent of heightened Th2 response in Cblb−/− mice. Indeed, over-expressing Cbl-b in tShp1−/− T cells not only inhibits heightened Th2 differentiation in vitro, but also Th2 responses and allergic airway inflammation in vivo. Therefore, SHP-1 regulates Cbl-b-mediated T cell responses by controlling its tyrosine phosphorylation and ubiquitination.
INTRODUCTION
Cooperative signals from the TCR and the costimulatory molecule CD28 are essential for T cell activation. TCR ligation in the absence of CD28 co-stimulation renders T cells anergic, which represents one of the major mechanisms for peripheral T cell tolerance (1). CD28 co-stimulation reduces the number of TCRs that have to be triggered for T cell activation and allows activation of T cells by low affinity ligand (2, 3), whereas absence of CTLA-4 lowers the activation threshold (4).
The Casitas-B-lineage lymphoma (Cbl) family of proteins consists of an NH2-terminal tyrosine kinase binding (TKB) domain that encompasses a variant SH2 domain, a RING finger, a carboxyl-terminal proline-rich region with potential tyrosine phosphorylation sites, and a ubiquitin associated domain (UBA). It is now understood that Cbl functions as an E3 ubiquitin ligase with a RING finger that recruits ubiquitin-conjugating enzymes (E2), and a TKB domain that recognizes target proteins for ubiquitin conjugation (5–7). Gene targeting in mice has shown that Cbl-b is involved in pivotal events of lymphocyte activation (8, 9), indicating a critical role of Cbl-b in the maintenance of a balance between immunity and tolerance. Indeed, we demonstrated that CD28 costimulation potentiates TCR-induced Cbl-b ubiquitination and degradation, suggesting that CD28 costimulation is achieved in part by targeting Cbl-b itself for ubiquitination and degradation, whereas CTLA-4-B7 interaction enhances Cbl-b expression (4, 10). These observations indicate that CD28 and CTLA-4 tightly regulate Cbl-b protein levels, which are critical for establishing the threshold for T cell activation. This is partially achieved by negative regulation of PKC-θ and Akt signaling pathways which control NF-κB activation (11, 12). In strong support of this notion, Cbl-b plays a crucial role in the induction of T cell anergy (13, 14). Recently, we have also shown that Cbl-b is critical for maintaining Foxp3 expression in regulatory T cells (15, 16) and is crucial for controlling pro-allergic T cell development and allergic airway inflammation (17). It was reported that Nedd4 ubiquitinates Cbl-b in T cells (18, 19), and that PKC-θ phosphorylates Cbl-b at Ser282 which facilitates Cbl-b ubiquitination and degradation (20). Given that Cbl-b undergoes auto-ubiquitination in vitro (21–23), the molecular mechanism by which Cbl-b is regulated during T cell activation remains to be defined.
In this study, our aim was to define the molecular mechanism by which TCR and CD28 receptors regulate Cbl-b protein expression. Cbl-b specifically associates with SH2 domain-containing protein tyrosine phosphatase 1 (SHP-1) upon TCR stimulation, whereas CD28 costimulation abrogates this interaction. SHP-1 dephosphorylates Cbl-b and inhibits Cbl-b ubiquitination. In support of this observation, Cbl-b expression is down-regulated in T cells lacking SHP-1 due to heightened Cbl-b tyrosine phosphorylation and ubiquitination. Over-expressing Cbl-b in tShp1−/− T cells inhibits heightened Th2 responses. Therefore, our data indicate that Cbl-b-mediated inhibition of T cell response is regulated by SHP-1, a previously unappreciated mechanism.
MATERIALS AND METHODS
Mice
C57BL/6 (B6) mice and Cd4 Cre mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Cd4 Cre.Shp1f/f (tShp1−/−) mice described previously (24) were provided by Drs. Pamela S. Ohashi and Benjamin G. Neel (University of Toronto; Toronto, ON, Canada). Cblb−/− mice were provided by Dr. Josef M. Penninger (University of Toronto; Toronto, ON, Canada). Rag1−/− mice were purchased from the Jackson laboratory (Bar Harbor, ME). All experimental protocols followed NIH guidelines and were approved by the institutional animal care and use committees of the Ohio State University. All mice were used for experiments at ages of 6 to 10 weeks.
Reagents and cell lines
The following reagents were obtained from BD Biosciences (San Jose, CA): recombinant mouse IL-2 (rmIL-2), purified anti-CD3 (Clone 145-2C11), anti-mouse CD28 (37.51), hamster IgG isotypic control, FITC/PE-anti-IL-4 (11B11), and APC-anti–mouse CD4 (clone RM4-5) were purchased from BioLegend (San Diego, CA). Antibodies (Abs) against Cbl-b, SHP-1, Lck, ZAP-70, LAT, SLP-76, CD45, VHR, SHP-2, PKC-θ, and TCRζ were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-tyrosine (4G10) was purchased from EMD Millipore (Billerica, MA). Anti-phospho-PKC-θ (T538) and anti-phospho-Stat6 (Y641) were obtained from Cell Signaling, Inc. (Beverly, MA). T cell enrichment columns were obtained from R & D Systems (Minneapolis, MN). HRP-conjugated goat anti-rabbit IgG or rabbit anti-mouse IgG were purchased from Kirkegaard & Perry Laboratories (Gaithersburg, MD). Human recombinant, active SHP-1 was purchased from SignalChem (Burlington, NC). His-tagged ubiquitin, E1, and E2 Ubc5 were purchased from Boston Biochem, Inc. (Cambridge, MA). Rabbit anti-hamster IgG, rabbit anti-mouse IgG was purchased from Sigma (St. Louis, MO). Protein A-Sepharose was purchased from Amersham Biosciences. (Piscataway, NJ). The plasmids encoding HA-tagged Cbl-b and its mutants were kindly provided by Dr. Stanley Lipkowitz (NCI/NIH; Bethesda, MD). Wild-type (WT) SHP-1 and its mutants (25) were obtained from Dr. Richard A. Anderson (University of Wisconsin Medical School; Madison, WI). JCaM1.6 cell line (Lck deficient Jurkat cell line) and P116 cell line (ZAP-70-deficient Jurkat cell line) were obtained from Dr. Weiguo Zhang (Duke University; Durham, NC). Recombinant, active Lck and ZAP-70, C8863 (Lck inhibitor) and PF-06465469 (ITK inhibitor) were purchased from Sigma-Aldrich (St. Louis, MO).
T cell isolation and activation
Splenic T cells from naive WT and tShp1−/− mice were isolated (purity ≥ 95% as determined by FACS analysis of CD3+ cell surface expression) on T cell enrichment columns. For in vitro activation, T cells (1×107/ml) were incubated with anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) mAbs on ice, followed by crosslinking with rabbit-anti-hamster IgG (10 μg/ml). The cells were lysed in 0.5 % NP-40 lysis buffer or in radioimmunoprecipitation assay (RIPA) buffer containing 1 % SDS (17) where indicated.
Immunoprecipitation and Western blotting
Protein concentrations in the cell lysates were determined using a bicinchoninic acid assay kit (Pierce, Rockford, IL). Cell lysates were precleared, postnuclear cell lysates were normalized for protein concentration levels, and immunoprecipitated (3 h, 4°C) with the specific polyclonal Abs or control isotype-matched preimmune immunoglobulin coupled to protein A CL-4B Sepharose. The immunoprecipitates were resolved on SDS-PAGE and transferred to nitrocellulose membranes (Hybond C Super, Amersham). Blots were blocked for 1 h at room temperature in PBS containing 2% BSA and 0.05% Tween-20. Membranes were incubated overnight with specific Abs, then washed 3x in PBS containing 0.05% Tween-20, and detected using HRP-conjugated goat anti-rabbit IgG or rabbit anti-mouse IgG. After 3 washes in PBS containing 0.05% Tween-20, signals were revealed by enhanced chemiluminescence detection system (Amersham) and visualized by autoradiography. The fold changes of protein bands indicated in arbitrary densitometric units were determined by the ImageJ 1.48 (NIH; Bethesda, MD).
Cbl-b autoubiquitination assay
T cells were treated with pervanadate for 5 and 15 min which allowed cellular proteins to be tyrosine phosphorylated, and lysed in RIPA buffer which contains 1% SDS to disrupt non-specific proteins binding to Cbl-b. The cell lysates were immunoprecipitated with anti-Cbl-b, incubated with recombinant, active SHP-1 for 30 min, and extensively washed. Ubiquitination assays were performed on the immune complexes using His-tagged ubiquitin, E1, and E2 Ubc5. Cbl-b phosphorylation and ubiquitination were determined by anti-pTyr (4G10) and anti-ubiquitin immunoblottings, respectively.
Plasmids and transfection
cDNAs encoding full-length (FL) Cbl-b or different Cbl-b mutants with an HA epitope in pCEFL were described previously (22). His6-tagged ubiquitin plasmid was a gift from Dr. Dirk Bohmann (University of Rochester, Rochester, NY). SHP-1 plasmids were obtained from Dr. Richard A. Anderson (University of Wisconsin-Madison; Madison, WI). 0.75 × 106 HEK293T cells on 6-well tissue culture plates were transfected with 4 μg total DNA using CaCl2 as described (17). For purification of HA-tagged Cbl-b protein, Cbl-b overexpressing HEK293T cells were lysed, and purified by a HA-tagged protein purification kit (Life Technologies; Grand Island, NY).
In vitro kinase assay
In vitro kinase assay was performed as previously described (26, 27) with some modifications. In brief, recombinant, active Lck or ZAP-70 protein was incubated in 25 μl kinase buffer (25 mM Hepes, pH 7.4, 5 mM MnCl2, and 500 μM ATP) in the presence of purified 1 μg HA-tagged Cbl-b at 30°C. Reactions were stopped by addition of an ice-cold buffer containing 50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM MnCl2, 20 mM EDTA, 0.2% NP-40, and 10 mM NaF. Proteins were resolved by SDS-PAGE. The membranes were blotted with anti-phospho-tyrosine antibody.
Retrovirus-mediated gene transfer and in vitro Th2 cell differentiation assay
The cDNA encoding human Cbl-b and Cbl-b C373A mutant were subcloned into the plasmid MIGR1 (28). Calcium Phosphate transfection of HEK293T cells with MIGR1 was carried out as previously described (17). Naïve CD4+CD25−CD62Lhi CD44lo T cells from tShp1−/− mice were activated with anti-CD3 (0.5 μg/ml) and anti-CD28 (0.5 μg/ml) in the presence IL-4 (5 ng/ml), IL-2 (5 U/ml), anti-IL-12 (10 μg/ml), and anti-IFN-γ (10 μg/ml), and infected with retroviruses expressing HA-Cbl-b-GFP, HA-Cbl-b C373A-GFP or control empty vector. Three days after infection, the cells were restimulated with PMA and ionomycin in the presence of Golgi-stop for 5 h, after which IL-4-producing cells were analyzed with intracellular staining on a CD4+ gate (17, 29).
Generation of chimeric mice by bone marrow reconstitution
To generate WT or tShp1−/− bone marrow (BM) chimeric mice expressing WT Cbl-b or Cbl-b C373A mutant in tShp1−/− BM cells, mature T-cell–depleted BM cells from tShp1−/− mice were cultured for 24 h in IL-3 (10 ng/mL), IL-6 (10 ng/mL), and stem cell factor (100 ng/mL) (all from Peptrotech) containing complete DMEM before initial retroviral infection (30). Mature T-cell–depleted BM cells were infected with retroviruses expressing HA-Cbl-b-GFP, HA-Cbl-b C373A-GFP or control empty vector together with 5 μg/mL polybrene by centrifuging cells at 420 × g for 60 min at room temperature. At 2 d after infection, retrovirally transduced BM cells were injected into lethally irradiated (950 rad) Rag1−/− recipient mice. Eight weeks later, recipient mice receiving tShp1−/− BM cells overexpressing HA-Cbl-b-GFP, HA-Cbl-b C373A-GFP, or control empty vector were used for asthma induction.
Asthma induction
tShp1−/− BM chimeric mice over-expressing Cbl-b or Cbl-b C373A (5 mice/group) were immunized by intraperitoneal (i.p.) injection of OVA (100 μg/ml; Sigma-Aldrich) adsorbed to 2 mg of an aqueous solution of aluminum hydroxide and magnesium hydroxide (Alum; Fischer Scientific International) on day 0 and day 14 as previously described (16, 17). After 21 days, the mice were challenged intranasally with OVA (50 μg/10 μl) for three consecutive days. The mice were sacrificed and assessed for allergic inflammation of the lungs 24 hr after the last intranasal challenge. Sera and BAL fluid were collected at the sacrifice, and serum IgE and cytokine concentrations in the BAL fluid were measured by ELISA (17). WT BM chimeric mice over-expressing Cbl-b or Cbl-b C373A were also generated to determine whether over-expressing Cbl-b inhibited T cell proliferation, Th2 cell differentiation, and Stat6 phosphorylation.
Induction of experimental autoimmune encephalomyelitis (EAE)
Cd4 Cre and tShp1−/− mice (8–10 wk of age) were immunized by s.c. injection over four sites in the flank with 200 μl myelin oligodendrocyte glycoprotein (MOG) peptide (33–55) (MOG33–55) in an emulsion with CFA (Difco). 200 ng pertussis toxin per mouse in PBS was injected intraperitoneally at the time of immunization and 48 h later. Mice were scored on scale of 0 to 6: 0, no clinical disease; 1, limp/flaccid tail; 2, moderate hind limb weakness; 3, severe hind limb weakness; 4, complete hind limb paralysis; 5 quadriplegia or premoribund state; and 6, death (31). For assessment of Th1 and Th17 responses, splenocytes were cultured with MOG33–55 for 48 h. For the last 4–5 h of the incubation, 50 ng/ml PMA and 750 ng/ml ionomycin were added to cells. The splenocytes were surface-stained with anti-CD4, and intracellularly stained with anti-IFN-γ or anti-IL-17, respectively. The CD4+IFN-γ+ and CD4+IL-17+ cells were determined by flow cytometry as previously described (17, 29).
Statistical analysis
A two-tailed Student’s t-test was applied for statistical comparison of two groups or, where appropriate and a Mann-Whitney test for nonparametric data (airway inflammation scoring). A P value of 0.05 or less was considered significant.
RESULTS
Cbl-b tyrosine phosphorylation and ubiquitination requires CD28 co-stimulation in primary T cells
A recurring theme in the interplay between phosphorylation and ubiquitination is that phosphorylation often influences the ubiquitination and thus degradation of the modified protein (32). Cbl-b contains several tyrosine residues in its TKB domain and C-terminal region, as well as the linker region that separates the TKB and RING finger domains (7). Several reports indicate that Y363, located in linker region, is critical for its E3 ubiquitin ligase activity (21, 33). However, the regulation of Cbl-b tyrosine phosphorylation and ubiquitination during T cell activation is poorly defined. To determine the relationship between Cbl-b tyrosine phosphorylation and ubiquitination T cells from C57BL/6 (B6) mice were stimulated with anti-CD3 or anti-CD3 plus anti-CD28, or treated with a pan protein tyrosine phosphatase (PTPase) inhibitor pervanadate as a positive a control. The cell lysates were immunoprecipitated with anti-Cbl-b antibody and blotted with anti-phospho-tyrosine (pTyr) and anti-ubiquitin to determine Cbl-b tyrosine phosphorylation and ubiquitination. The cell lysates were also blotted directly with phospho-antibodies against TCRζ, Lck, ZAP-70, LAT, and SLP76. As shown in Figure 1A, although TCR stimulation induced phosphorylation of Lck, ZAP-70, SLP76, LAT, and TCRζ, Cbl-b phosphorylation was undetectable. Cbl-b tyrosine phosphorylation was only detected when CD28 costimulation was provided, whereas treating primary T cells with pervanadate favored Cbl-b phosphorylation (Fig. 1A). Interestingly, Cbl-b tyrosine phosphorylation positively correlated with its ubiquitination (Fig. 1A). These data raised the possibility that one or more PTPases may associate with Cbl-b and prevent Cbl-b phosphorylation. Previous studies showed that Cbl-b is phosphorylated in Jurkat T cells upon TCR stimulation (34), and consistent with this we similarly found Cbl-b to be tyrosine phosphorylated upon TCR stimulation of Jurkat T cells (Fig. 1B). These data indicate that the failure of Cbl-b to undergo TCR-induced phosphorylation in primary mouse T cells is a physiologically relevant observation.
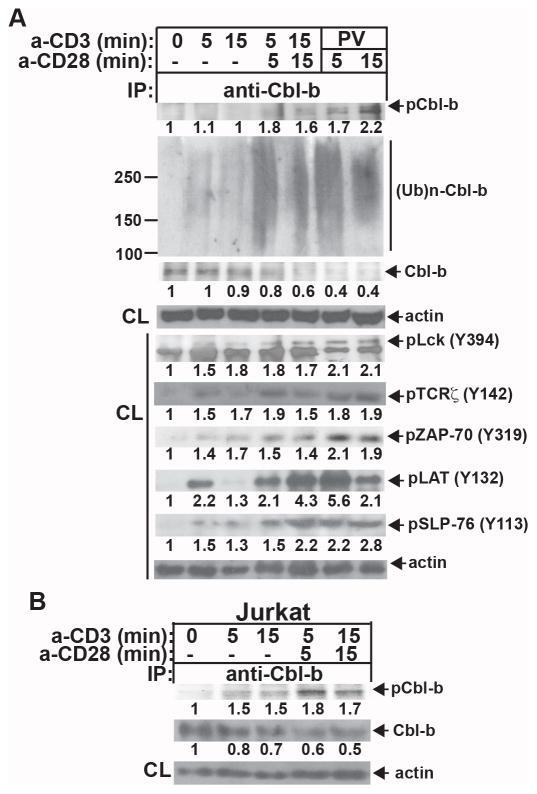
Tyrosine phosphorylation of Cbl-b requires CD28 costimulation in primary T cells. (A) B6 T cells were stimulated with anti-CD3 or anti-CD3 plus anti-CD28, or treated with pervanadate, and lysed in RIPA buffer containing 1% SDS. The cell lysates were immunoprecipitated with anti-Cbl-b, and blotted with anti-pTyr and anti-ubiquitin, respectively, or blotted with phospho-specific Abs against Lck (Y394), TCRζ (Y142) ZAP-70 (Y319), LAT (Y132), and SLP-76 (Y113). Aliquots of cell lysates from each sample were blotted with anti-actin as a loading control. (B) Jurkat T cells were stimulated with anti-CD3 or anti-CD3 and anti-CD28, and lysed. The cell lysates were immunoprecipitated with anti-anti-Cbl-b, and blotted with anti-pTyr. Aliquots of cell lysates from each sample were blotted with anti-actin as a loading control. The fold changes of protein bands indicated in arbitrary densitometric units were determined by the ImageJ 1.48. Data are representative of at least four independent experiments.
Cbl-b associates with SHP-1 via its TKB domian
The failure of Cbl-b to undergo tyrosine phosphorylation upon TCR stimulation led us to hypothesize that a PTPase(s) may associate with Cbl-b. To test this hypothesis, B6 T cells were stimulated with anti-CD3 or anti-CD3 and anti-CD28, and lysed in 0.5% NP-40 lysis buffer. The cell lysates were immunoprecipitated with anti-Cbl-b and blotted with anti-SHP-1, anti-SHP-2, anti-CD45, anti-TcPTP, and anti-VHR. Cbl-b was found to specifically associate with SHP-1 and SHP-2 upon TCR stimulation, and CD28 co-stimulation abrogated this association (Fig. 2A). Given that SHP-2 plays a positive role in T cell development and function (35), we decided to focus on whether Cbl-b expression and function is regulated by SHP-1.
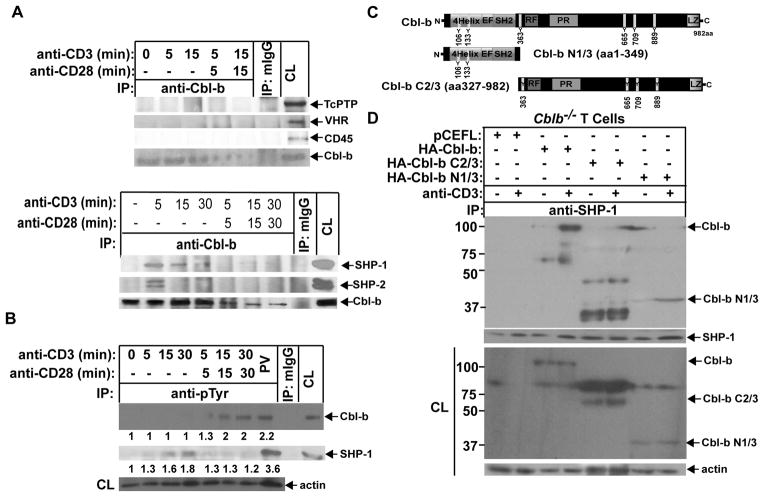
SHP-1 binds to Cbl-b upon TCR stimulation via its TKB domain. (A) B6 T cells were stimulated with anti-CD3 or anti-CD3 plus anti-CD28 for times indicated, and lysed in 0.5% NP-40 lysis buffer. The cell lysates were immunoprecipitated with anti-Cbl-b and blotted with anti-TcPTP, anti-CD45, anti-VHR, anti-SHP-1, and anti-SHP-2. (B) B6 T cells were stimulated with anti-CD3 or anti-CD3 plus anti-CD28 for times indicated, and lysed in RIPA buffer. The cell lysates were immunoprecipitated with anti-pTyr, and blotted with anti-anti-Cbl-b and anti-SHP-1, respectively. The equal loading was confirmed by immunolblotting the cell lysates with anti-actin. B6 T cells treated with PV for 15 min were used as positive control. (C) Schematic design of Cbl-b mutants. (D) Cblb−/− T cells were retrovirally transfected with HA-FL Cbl-b, Cbl-b N1/3, and Cbl-b C2/3. The transfected cells were stimulated with anti-CD3, and lysed. The cell lysates were immunoprecipitated with anti-SHP-1 and blotted with anti-HA. The fold changes of protein bands are indicated in arbitrary densitometric units. Data are representative of at least three independent experiments.
Cbl-b does not undergo tyrosine phosphorylation upon TCR stimulation, whereas SHP-1 is phosphorylated following TCR stimulation, and this response is down-regulated by CD28 costimulation (Fig. 2B). We therefore speculated that the Cbl-b TKB domain binds to phosphotyrosine residues of SHP-1 upon TCR stimulation, and CD28 costimulation weakens this interaction. To test this possibility, we first used a set of Cbl-b mutants as shown in Figure 2C. Cblb−/− T cells were transiently transfected with HA-tagged full-length (FL) Cbl-b, Cbl-b N1/3 and C2/3 constructs, and treated or untreated with anti-CD3. We found that FL Cbl-b and Cbl-b N1/3, but not Cbl-b C2/3 bound to SHP-1 (Fig. 2D), suggesting that the TKB domain of Cbl-b is required for its binding to SHP-1, possibly through phospho-tyrosine residues of SHP-1. Note that SHP-1 does not undergo degradation as shown previously (4, 10), suggesting that SHP-1 is not a substrate of Cbl-b.
SHP-1 prevents Cbl-b phosphorylation and subsequent ubiquitination
To further determine whether SHP-1 indeed prevented Cbl-b phosphorylation and ubiquitination, we employed a cell-free system. Since GST-Cbl-b fusion protein is not soluble, we isolated Cbl-b from primary T cells by immunoprecipitation. To obtain tyrosine-phosphorylated Cbl-b, B6 T cells were first treated with pervanadate which allows the accumulation of cellular proteins including Cbl-b. The cells were lysed in RIPA buffer, immunoprecipitated with anti-Cbl-b, and incubated with or without recombinant, active SHP-1, and extensively washed. As shown in Figure 3A, the presence of SHP-1 significantly abrogated Cbl-b phosphorylation and auto-ubiquitination.
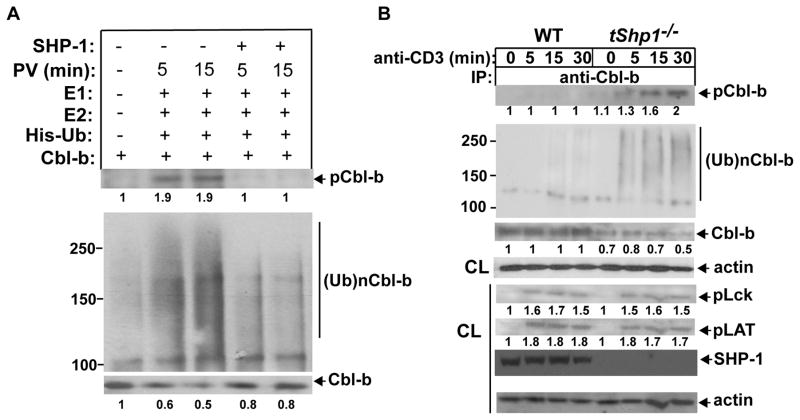
SHP-1 prevents Cbl-b tyrosine phosphorylation and ubiquitination. (A) B6 T cells pretreated with pervanadate or left untreated were lysed in RIPA buffer, and immunoprecipitated with anti-Cbl-b. Cbl-b immunoprecipitates were incubated with recombinant, active SHP-1 for 30 min, and extensively washed with RIPA buffer to remove SHP-1 which may potentially bind to Cbl-b. SHP-1-treated or untreated Cbl-b immunoprecipitates were incubated with E1, Ubc5, and His-tagged ubiquitin, and blotted with anti-pTyr and anti-His. (B) Naïve CD4+ T cells from Cd4 Cre and tShp1−/− mice were stimulated with anti-CD3, and lysed in 1% RIPA buffer. The cell lysates were immunoprecipitated with anti-Cbl-b, and blotted with anti-pTyr, anti-ubiquitin, and anti-Cbl-b, respectively (upper panel). The lysates from Cd4 Cre and tShp1−/− T cells were blotted with anti-phospho-Lck, anti-phospho-LAT, anti-SHP-1, and anti-actin (lower panel). The fold changes of protein bands are indicated in arbitrary densitometric units. Data are representative of three independent experiments.
To verify the role of SHP-1 in Cbl-b expression, tShp1−/− mice were used. Naïve WT and tShp1−/− CD4+ T cells were stimulated with anti-CD3, and lysed. Although Cbl-b phosphorylation was difficult to detect in WT T cells upon TCR stimulation alone, SHP-1 deficiency in T cells resulted in rapid Cbl-b phosphorylation and ubiquitination, both of which occurred at 5 min after stimulation (Fig. 3B). Accordingly, Cbl-b expression was decreased in CD4+ T cells lacking SHP-1 (Fig. 3B). These data suggest that Cbl-b tyrosine phosphorylation may be a prerequisite for Cbl-b ubiquitination. We did not observe a significant increase in tyrosine phosphorylation of Lck and LAT (Fig. 3B), suggesting that Cbl-b, but not Lck or LAT, is the physiological substrate of SHP-1. Taken together, our data indicate that SHP-1 regulates Cbl-b expression by binding to Cbl-b and preventing its phosphorylation and subsequent ubiquitination.
It has previously been shown that Nedd4 targets Cbl-b for ubiquitination (19), and that PKC-θ phosphorylates Cbl-b at Ser282, thus facilitating Cbl-b ubiquitination (20). To determine whether SHP-1 deficiency affects PKC-θ activation, which potentially regulates Cbl-b ubiquitination, we measured PKC-θ phosphorylation at Thr508 in T cells from Cd4 Cre and tShp1−/− mice in response to TCR and TCR/CD28 stimulation. We found that loss of SHP-1 in T cells did not affect PKC-θ activity (Supplemental Fig. 1). Our data suggest that SHP-1 regulates Cbl-b ubiquitination via a PKC-θ-independent mechanism.
Lck is the PTK responsible for phosphorylating Cbl-b in T cells
To identify the potential PTK(s) that phosphorylates Cbl-b, we utilized several chemical inhibitors that have been shown to specifically inhibit Lck and ITK. Inhibition of Lck but not ITK significantly inhibited Cbl-b tyrosine phosphorylation (Fig. 4A). To further verify that Lck is the PTK for Cbl-b, we used an Lck-deficient Jurkat T cell line (JCaM1.6) and a ZAP-70-deficient Jurkat T cell line (P116). Cbl-b tyrosine phosphorylation was compromised in response to TCR stimulation in Jurkat T cells lacking Lck but not ZAP-70 (Fig. 4B and C). To directly prove that Cbl-b is the substrate of Lck, we performed an in vitro kinase assay by incubating recombinant, active Lck with affinity-purified HA-Cbl-b in the presence of ATP. We also used recombinant, active ZAP-70 as a control. As shown in Figure 4D, recombinant, active Lck, but not ZAP-70, phosphorylated Cbl-b in vitro. Taken together, these data collectively support the notion that Lck is the PTK responsible for phosphorylating Cbl-b in T cells.
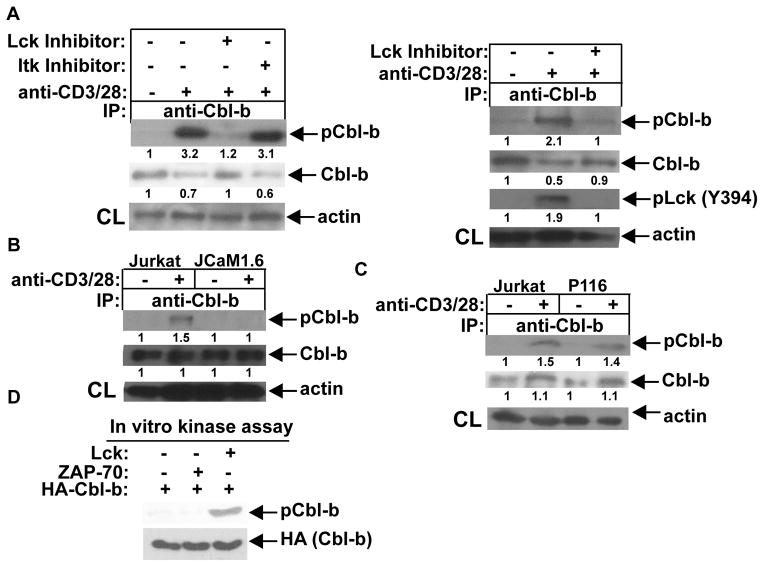
Lck is the PTK that phosphorylates Cbl-b. (A) B6 T cells were pretreated with specific inhibitors of Lck or ITK (left panel), or Lck inhibitor (right panel), for 30 min, stimulated with anti-CD3 and anti-CD28 for 15 min, and lysed. The cell lysates were immunoprecipitated with anti-Cbl-b, and blotted with anti-pTyr (left panel). The cell lysates were blotted with anti-pLck (Y394) and anti-Cbl-b, and reprobed with anti-actin (right panel). (B and C) Parental Jurkat cells, JCaM1.6 cells, or P116 cells were stimulated with anti-CD3 and anti-CD28, and lysed. Cbl-b tyrosine phosphorylation was determined. (D) Recombinant, active Lck and ZAP-70 were incubated with HA-Cbl-b in the kinase buffer in the presence of ATP. After stopping the reaction, the mixture was blotted with anti-pTyr. The fold changes of protein bands are indicated in arbitrary densitometric units. Data are representative of two independent experiments.
Cbl-b phosphorylation at Y106, Y133, and Y363 is critical for its E3 ubiquitin ligase activity
Previously, it has been shown that Cbl-b Y363 is critical for its E3 ubiquitin activity (21, 33). To further verify the role of other tyrosine residues in the regulation of Cbl-b E3 ubiquitin ligase activity, we mutated individual tyrosine residues by site-directed mutagenesis based upon the NetPhos 2.0 with the scores >0.80 (very high confidence) and generate Cbl-b Y106F, Y133F, Y363F, Y665F, Y709F, and Y889F (Fig. 5A). To define the biological relevance of these Cbl-b tyrosine mutants on its activity, we transfected HEK293 cells with HA-tagged WT Cbl-b or Cbl-b Y mutants, and pretreated them with pervanadate to induce Cbl-b tyrosine phosphorylation. As shown in Figure 5B, the mutations of tyrosine residues 106, 133, and 363 of Cbl-b abrogated Cbl-b auto-ubiquitination, suggesting that the tyrosine residues located in TKB domain and linker region are required for Cbl-b E3 ubiquitin ligase activity. To further confirm this data, we purified HA-tagged Cbl-b, and Cbl-b Y106F, Y133F, and Y363F proteins from HEK293 cells using a HA-tagged protein isolation kit, and performed an in vitro Cbl-b autoubiquitination assay. As expected, Y106F, Y133F, and Y363F of Cbl-b abrogated its E3 ubiquitin ligase activity (Fig. 5C). This data therefore provides additional evidence for the regulation of Cbl-b E3 ubiquitin ligase activity by its tyrosine phosphorylation.
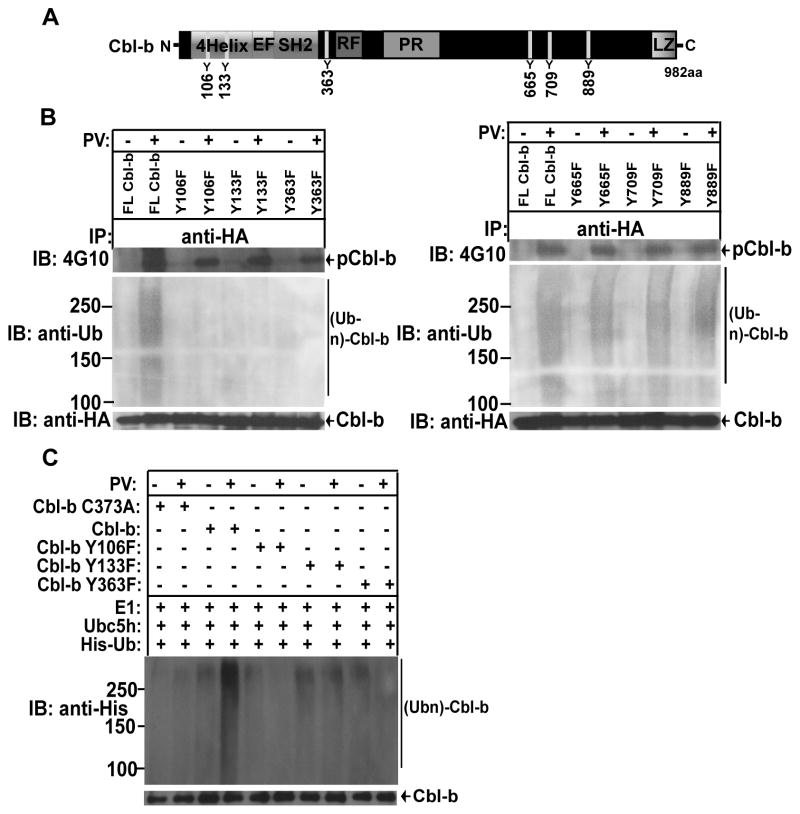
(A) Schematic model of Cbl-b structure. (B) HEK293 cells transfected with HA-Cbl-b, or Cbl-b Y106F, Y133F, Y363F, Y665F, Y709F, and 889F, and treated with PV, and lysed in RIPA buffer. The cell lysates were immunoprecipitated with anti-HA, and blotted with 4G10. The membranes were stripped, and reprobed with anti-pTyr, anti-ubiquitin, and anti-HA. (C) Affinity-purified HA-Cbl-b, or Cbl-b Y106F, Y133F, Y363F, Y665F, Y709F, and 889F were incubated with E1, Ubc5, and His-tagged ubiquitin, and blotted with anti-His and anti-HA. Data are representative of two independent experiments.
Over-expression of Cbl-b attenuates heightened Th2 responses in tShp1−/− mice
It was reported that SHP-1 deficiency in T cells leads to an aberrant Th2 phenotype (24). However, whether SHP-1 regulates Th1 or Th17 response in vivo is unknown. To further assess whether SHP-1 deficiency in T cells specifically affects Th2 response in vivo, we utilized EAE as a model which is mediated by both Th1 and Th17 (36). We immunized Cd4 Cre and tShp1−/− mice with MOG33–55 in CFA, and the disease severity and Th1/Th17 responses were monitored. SHP-1 deficiency in T cells did not lead to the development of severe disease, and heightened Th1/17 responses (Supplemental Fig. 2A and B).
We have recently shown that Cbl-b deficiency leads to aberrant Th2 responses in vitro and in vivo (17), which correlate with the phenotype of tShp1−/− mice (24), further suggesting that Cbl-b may be a substrate of SHP-1. To determine the biological relevance of Cbl-b as a substrate of SHP-1, we retrovirally transfected naive CD4+ T cells from tShp1−/− mice with WT Cbl-b or Cbl-b C373A mutant, and differentiated them into the Th2 lineage. Over-expression of WT Cbl-b, but not Cbl-b C373A, mutant inhibited the biased Th2 phenotype in tShp1−/− mice, further supporting the notion that Cbl-b is a substrate of SHP-1 (Fig 6A). To verify this finding in vivo, tShp1−/− bone marrow (BM) cells were transfected with GFP-tagged WT Cbl-b or Cbl-b C373A mutant, and GFP-positive cells were sorted and injected in lethally-irradiated Rag1−/− mice. Eight weeks later, the BM chimeric mice were immunized with OVA in alum, and challenged intranasally with OVA for three consecutive days. The extent of airway inflammation was then determined. Expression of WT Cbl-b, but not the Cbl-b C373A mutant, in tShp1−/− BM cells significantly inhibited heightened airway inflammation and mucous production in irradiated Rag1−/− mice (Fig. 6B). Consistent with this data, serum IgE, IL-4, IL-5 and IL-13, but not IL-17 and IFN-γ in branchoalveolar lavage (BAL) fluid, Th2 cell development, and also frequencies of memory phenotype of T cells were significantly reduced in tShp1−/− BM chimeric mice overexpressing WT Cbl-b but not Cbl-b C373A mutant (Fig. 6C–D; Supplemental Fig. 3). These data strongly indicate that Cbl-b is the downstream target of SHP-1, and that the aberrant Th2 response observed in tShp1−/− mice is mediated by diminished expression of Cbl-b. To assess the effect of over-expression of Cbl-b on T cell proliferation and differentiation in general, we generated WT BM chimeric mice overexpressing Cbl-b or Cbl-b C373A. We performed in vitro T cell proliferation and Th2 cell differentiation assays. Over-expressing Cbl-b but not the Cbl-b C373A mutant significantly inhibited TCR/CD28-induced T cell proliferation and Th2 cell differentiation as well as IL-4-induced Stat6 phosphorylation (Supplemental Fig. 4). Therefore, our data collectively indicate that SHP-1 specifically regulates Th2 but not Th1 and Th17 responses in vivo.
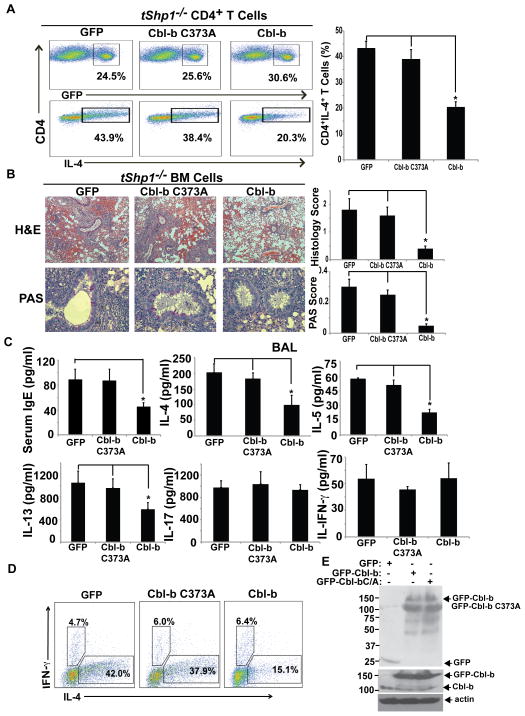
Over-expression of Cbl-b in T cells inhibits heightened Th2 response in tShp1−/− mice. (A) Naïve CD4+CD25− T cells from tShp1−/− mice were transfected with HA-tagged Cbl-b or Cbl-b C373A mutant by retroviral infection, and cultured under Th2-biased condition. Three days later, the cells were surface-stained with anti-CD4, and intracellularly stained with anti-IL-4 and anti-IFN-γ. (B) Bone-marrow cells from tShp1−/− mice were transfected with GFP-tagged Cbl-b or Cbl-b C373A mutant, and injected into lethally-irradiated Rag1−/− mice. Eight weeks later, the recipients were immunized with OVA in alum, challenged at day 21 for three consecutive days, and sacrificed on day 24. Lung histology was determined by H&E and PAS staining. (C) IgE in the serum and cytokines (IL-4, IL-5, IL-13, and IFN-γ) in the BAL fluid were determined. (D) The spleen cells from B were surface-stained with anti-CD4, and intracellularly stained with anti-IL-4 and anti-IFN-γ. (E) T cell lysates from irradiated Rag1−/− mice receiving tShp1−/− BM cells overexpressing HA-Cbl-b-GFP, HA-Cbl-b C373A-GFP or a control empty vector were blotted with anti-GFP, anti-Cbl-b, and anti-actin, respectively. Data are representative of two independent experiments. *p<0.05; Student t test.
DISCUSSION
Although it has been well documented that Cbl-b is a check-point regulator of lymphocyte activation, tolerance induction, and Th2/9 cell differentiation (7), the regulation of Cbl-b expression and E3 ubiquitin ligase activity is poorly understood. In this study, we found that SHP-1 is specifically recruited to Cbl-b upon TCR stimulation, and CD28 costimulation disrupts this association. SHP-1 dephosphorylates Cbl-b, and inhibits its E3 ubiquitin ligase activity. Consistent with this data, the absence of SHP-1 results in heightened tyrosine phosphorylation of Cbl-b and down-regulation of Cbl-b protein expression. Furthermore, we identified Y106 and Y133, together with previously described Y363, as key tyrosine residues that are crucial for Cbl-b E3 ubiquitin ligase activity. We also identified Lck as the PTK responsible for phosphorylating Cbl-b. Over-expressing Cbl-b but not Cbl-b C373A mutant in T cells lacking SHP-1 inhibits aberrant Th2 phenotype in vitro and in vivo. Our data therefore indicate that SHP-1 controls Cbl-b expression and E3 ubiquitin ligase activity in T cells, thus regulating the T cell responses mediated by Cbl-b.
We have previously demonstrated that Cbl-b protein levels are tightly controlled by CD28 and CTLA-4. CD28 costimulation potentiates Cbl-b auto-ubiquitination and degradation, whereas CTLA-4-B7 interaction enhances Cbl-b protein expression (4, 10). In keeping with this data, Cbl-b expression is significantly increased in anergic T cells, and loss of Cbl-b results in the resistance to T cell anergy induction in vitro and in vivo (13, 14). Several studies reported that Nedd4 targets Cbl-b for ubiquitination and degradation in T cells (18, 19). Furthermore, PKC-θ has been shown to phosphorylate Cbl-b at Ser282, thus facilitating Cbl-b ubiquitination and degradation (20). However, we have also shown that Cbl-b inhibits Nedd4-mediated inactivation of Pten in T cells, and loss of Nedd4 attenuates hyper-T cell response in Cblb−/− mice (12), suggesting that Cbl-b and Nedd4 regulate each other. It is widely accepted that Cbl-b undergoes autoubiquitination which reflects its E3 ubiquitin ligase activity (21–23). Cbl-b tyrosine residue within the linker region (Y363) has been shown to be critical for its E3 ubiquitin ligase activity (21, 33). Therefore, the regulation of Cbl-b ubiquitination and degradation is still not fully understood. Interestingly, we found that although Cbl-b contains many tyrosine residues they are not phosphorylated upon TCR stimulation alone and require CD28 costimulation (Fig 1A). Further analysis revealed that SHP-1 rapidly associates with Cbl-b upon CD3 stimulation, raising the possibility that SHP-1 may prevent Cbl-b tyrosine phosphorylation. Indeed, CD28 costimulation dissociates SHP-1 from Cbl-b (Fig. 2A), allowing Cbl-b to be phosphorylated by Lck (Fig. 4). Using a cell-free system and T cells from tShp1−/− mice we further determined that SHP-1 dephosphorylates Cbl-b, thus regulating its ubiquitin ligase activation and degradation (Fig. 3). In addition to Cbl-b Y363, we also identified two tyrosine residues located in the TKB domain (Y106 and Y133) that are also critical for Cbl-b E3 ubiquitin ligase activity. In contrast, the well-characterized C-terminal tyrosine residues are not required for its E3 ubiquitin ligase activity (Fig. 5B and C). Thus, we demonstrate that Cbl-b tyrosine phosphorylation at N-terminal tyrosine residues regulates its E3 ubiquitin ligase activity. Taken together, we have provided ample evidence for the regulation of Cbl-b tyrosine phosphorylation, E3 ubiquitin ligase activity and expression by SHP-1. It has been shown that the Cbl-b UBA domain binds to ubiquitin chains which mediates dimerization, thus enhancing its E3 ubiquitin ligase activity (23, 37). However, without the Cbl-b UBA domain, Cbl-b tyrosine phosphorylation and autoubiquitination still exist, albeit significantly decreased (23). Therefore, it is possible that Nedd4 may directly target Cbl-b for ubiquitination via a PKC-θ-dependent mechanism by adding ubiquitin chains to its UBA domain.
SHP-1 was originally thought to be essential for thymic positive selection, and negatively regulates T cell activation possibly by dephosphorylating several key molecules in the TCR signaling pathway including TCRζ, Lck, LAT, and ZAP-70 (38–42). However, using tShp1−/− mice, we confirmed that SHP-1 deficiency in T cells is not responsible for the phenotype observed in motheaten (me/me) mice. tShp1−/− mice display normal thymocyte development, and do not develop fatal systemic autoinflammation (24). Therefore, it is possible that the lethal systemic autoimmune inflammation observed in me/me mice may result from SHP-1 mutations in cell types other than T cells. Indeed, mice carrying an Y208N mutation in the C-terminal SH2 domain of SHP-1 develop a severe inflammatory syndrome that resembles neutrophilic dermatosis in humans (43, 44). Furthermore, mice with SHP-1 specifically deleted from B cells or dendritic cells develop systemic lupus-like autoimmunity (45–47), while mice deficient for SHP-1 in neutrophils result in cutaneous inflammation, but not autoimmunity (47).
It is well documented that Cbl-b regulates the threshold of TCR activation (16, 48) which appears to be contradicted by the data showing that tShp1−/− T cell proliferation did not differ in response to various doses of anti-CD3 stimulation (24). One possibility is that the increased TCR affinity resulting from loss of Cbl-b, and a decline of Cbl-b expression caused by SHP-1 deficiency, may have different effects on in vitro T cell sensitivity when analyzed by an in vitro T cell proliferation assay. Of note is a recent report using human CD8+ T cells engineered with TCRs of incremental affinity for the tumor antigen HLA-A2/NY-ESO-1 showed that SHP-1 phosphatase activity counteracts increased TCR affinity (49), which is consistent with a previous report indicating that SHP-1 is involved in suppression by antagonists (50). Possibly, a more complete understanding of the role of SHP-1 in TCR affinity will require the generation of TCR transgenic/tShp1−/− mice to test T cell proliferation in response to agonist, partial agonist, and antagonist in vitro and in vivo.
Our in vitro biochemical data are supported by the phenotype of tShp1−/− mice which display a Th2-biased response (24), reminiscent of our recent observation that Th2 responses are heightened in Cblb−/− mice (17). In support of a critical role for SHP-1 in controlling Cbl-b expression, Cbl-b in naïve T cells lacking SHP-1 undergoes rapid ubiquitination and degradation compared to WT T cells (Fig. 3B). Furthermore, over-expressing WT Cbl-b but not a Cbl-b C373A mutant inhibits heightened Th2 cell differentiation in vitro (Fig. 6A). This data is further supported by the fact that chimeric mice over-expressing WT Cbl-b in tShp1−/− BM cells inhibits OVA-induced allergic airway inflammation and Th2 responses in vivo (Fig. 6B–D). These data are supported by the evidence that T cells from WT BM chimeric mice overexpressing Cbl-b but not the Cbl-b C373A mutant inhibited TCR-induced T cell proliferation, Th2 cell differentiation, and IL-4-induced Stat6 phosphorylation (Supplemental Fig. 4). Consistent with these findings T cells lacking Lck, which phosphorylates Cbl-b, display defective Th2 cell response (51). Cblb−/− mice are highly susceptible to Th17-mediated autoimmune diseases such as experimental autoimmune encephalomyelitis and collagen-induced arthritis (9, 13, 20). Intriguingly, although Cblb−/− mice develop severe myocarditis, the Cbl-b deficiency in T cells may not be the primary cause for the severe disease (our unpublished data). Further analysis indicates that Cbl-b does not regulate Th17 cell differentiation, but inhibits Th2/Th9 cell differentiation (17). It is possible that Cbl-b may affect innate immune responses which indirectly regulates Th17 cell response. This hypothesis is currently under investigation.
In conclusion, we have found that TCR stimulation induces the recruitment of Cbl-b to SHP-1 which prevents Cbl-b tyrosine phosphorylation by Lck, while CD28 costimulation disrupts the Cbl-b-SHP-1 interaction, and allows Cbl-b to be phosphorylated. We also demonstrate that Cbl-b phosphorylation at Y106, Y133, and Y363 is critical for its E3 ubiquitin ligase activity. Furthermore, SHP-1 limits T cell responses by controlling Cbl-b phosphorylation and ubiquitination. Therefore, we identified a previously unappreciated mechanism by which SHP-1 regulates T cell activation and response.
Acknowledgments
We thank Drs. Pamela S. Ohashi, Benjiamin G. Neel, Josef M. Penninger, Stanley Lipkowitz, Richard A. Anderson, Dirk P. Bohmann, and Weiguo Zhang for providing Cd4 Cre.Shp1f/f, Cblb−/− mice, Cbl-b, SHP-1, and ubiquitin constructs, and JCaM1.6 cell line and P116 cell line that made this study possible.
This study was supported by the US National Institutes of Health (R01 AI090901 to J.Z.) and a Start-up fund from the Ohio State University College of Medicine (to J.Z.).
Footnotes
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
Full text links
Read article at publisher's site: https://doi.org/10.4049/jimmunol.1501200
Read article for free, from open access legal sources, via Unpaywall:
https://www.jimmunol.org/content/jimmunol/195/9/4218.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
The pathogenic response of cytotoxic T‑lymphocytes, a common therapeutic target for cancer, has a direct impact on treatment outcomes (Review).
Oncol Rep, 52(1):98, 21 Jun 2024
Cited by: 0 articles | PMID: 38904200 | PMCID: PMC11200153
Review Free full text in Europe PMC
Regulation of PPARγ2 Stability and Activity by SHP-1.
Mol Cell Biol, 44(7):261-272, 03 Jun 2024
Cited by: 0 articles | PMID: 38828991 | PMCID: PMC11253886
Cbl-b restrains priming of pathogenic Th17 cells via the inhibition of IL-6 production by macrophages.
iScience, 25(10):105151, 15 Sep 2022
Cited by: 2 articles | PMID: 36185364 | PMCID: PMC9523381
Protein phosphatases regulate the liver microenvironment in the development of hepatocellular carcinoma.
Exp Mol Med, 54(11):1799-1813, 15 Nov 2022
Cited by: 4 articles | PMID: 36380016 | PMCID: PMC9722691
Review Free full text in Europe PMC
Targeting the Cbl-b-Notch1 axis as a novel immunotherapeutic strategy to boost CD8+ T-cell responses.
Front Immunol, 13:987298, 26 Aug 2022
Cited by: 5 articles | PMID: 36090975 | PMCID: PMC9459147
Go to all (30) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
E3 ubiquitin ligase Cbl-b suppresses proallergic T cell development and allergic airway inflammation.
Cell Rep, 6(4):709-723, 06 Feb 2014
Cited by: 40 articles | PMID: 24508458 | PMCID: PMC3969736
Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination.
J Immunol, 169(5):2236-2240, 01 Sep 2002
Cited by: 106 articles | PMID: 12193687
PKC-theta modulates the strength of T cell responses by targeting Cbl-b for ubiquitination and degradation.
Sci Signal, 2(76):ra30, 23 Jun 2009
Cited by: 56 articles | PMID: 19549985
Activation of T cells: releasing the brakes by proteolytic elimination of Cbl-b.
Sci Signal, 2(76):pe38, 23 Jun 2009
Cited by: 16 articles | PMID: 19549983
Review
Funding
Funders who supported this work.
NIAID NIH HHS (3)
Grant ID: R01 AI090901
Grant ID: R21AI117547
Grant ID: R21 AI117547





