Abstract
Free full text

A meta‐analysis of nutrition interventions on mental development of children under‐two in low‐ and middle‐income countries
Associated Data
Abstract
Interventions to improve nutritional status of young children in low‐ and middle‐income countries (LMIC) may have the added benefit of improving their mental and motor development. This meta‐analysis updates and goes beyond previous ones by answering two important questions: (1) do prenatal and postnatal nutritional inputs improve mental development, and (2) are effects on mental development associated with two theoretically interesting mediators namely physical growth and motor development? The meta‐analysis of articles on Medline, PsycINFO, Global Health and Embase was limited to randomized trials in LMICs, with mental development of children from birth to age two years as an outcome. The initial yield of 2689 studies was reduced to 33; 12 received a global quality rating of strong. Of the 10 prenatal and 23 postnatal nutrition interventions, the majority used zinc, iron/folic acid, vitamin A or multiple micronutrients, with a few evaluating macronutrients. The weighted mean effect size, Cohen's d (95% CI) for prenatal and postnatal nutrition interventions on mental development was 0.042 (−0.0084, 0.092) and 0.076 (0.019, 0.13), respectively. Postnatal supplements consisting of macronutrients yielded an effect size d (95% CI) of 0.14 (0.0067, 0.27), multiple micronutrients 0.082 (−0.012, 0.18) and single micronutrients 0.058 (−0.0015, 0.12). Motor development, but not growth status, effect sizes were significantly associated with mental development in postnatal interventions. In summary, nutrition interventions had small effects on mental development. Future studies might have greater effect if they addressed macronutrient deficiencies combined with child stimulation and hygiene and sanitation interventions.
Abbreviations
- RCT
- randomized controlled trial
- LMIC
- low‐ and middle‐income countries
- DHA
- docosahexaenoic acid
- EPHPP
- Effective Public Health Practice Project
- BSID
- Bayley Scales of Infant and Toddler Development
- HAZ
- height‐for‐age z‐score
- ERP
- event‐related brain potential
Introduction
Malnourished children consistently perform poorly on tests of mental development in both cross‐sectional and longitudinal studies (Grantham‐McGregor et al. 2007). The impact of nutrition on cognitive and language development is particularly important in low‐ and middle‐income countries (LMIC) where many children are affected by both macronutrient and micronutrient deficiencies. Consequently a number of recent nutrition interventions have examined cognitive benefits along with physical growth. The first objective of this review is to examine accumulating evidence for the effects of both macro and micronutrient supplements on mental development in young children less than two years of age.
The secondary aim is to examine the proposed pathways by which nutrition may impact mental development. It is critical to examine mediators in the pathways showing how nutritional status/physical growth leads to mental development. Previous meta‐analyses have not examined these mediators. Postnatally, better nutrition may influence mental development through several pathways. In addition to direct effects on brain development, another is through motor development: children with better nutrition may walk at an earlier age (Dewey et al. 2001), leading to increased interaction with, and exploration of, their environment. Adolph and Tamis‐LeMonda argue that infants willingly abandon their status as expert crawlers to become unsteady walkers, in part because it leads to richer experiences, more ground to cover and objects to play with, and a different type of interaction with others (2014). Height and weight may also influence the caregiver's behaviour toward the child, such as providing more sophisticated stimulation to a child who appears more mature physically and less to a malnourished child (Brown & Pollitt 1996). Both pathways enhance stimulation and may in turn affect children's overall cognitive performance (Prado & Dewey 2014). This review examines mental development and motor development outcomes of children under‐24 months receiving a nutrition intervention. It also investigates whether children's mental development (mainly cognitive) is associated with greater growth and motor development, thus supporting explanations of the link between nutrition and mental development.
months receiving a nutrition intervention. It also investigates whether children's mental development (mainly cognitive) is associated with greater growth and motor development, thus supporting explanations of the link between nutrition and mental development.
Two previous systematic reviews examined the effects of multiple micronutrients on cognitive outcomes in children, together including only two randomized controlled trials (RCT) of children under‐two years (Ramakrishnan et al. 2009; Eilander et al. 2010). Several studies assessed motor development only. Eilander et al. (2010) included one study on mental development with no significant effects (Dhingra et al. 2004). Two other trials included outcomes of motor development, specifically the age of walking unassisted, where multiple micronutrients had significantly positive effects (Faber et al. 2005; Olney et al. 2006). In Ramakrishnan et al.'s review (2009) only one study, different than the one noted by Eilander and colleagues, looked at mental development and found no significant effects because of multiple micronutrients (Black et al. 2004a). These reviews were limited to postnatal micronutrient interventions only with few assessing child cognition outcomes; therefore, the effect of micronutrient supplementation on children's cognition as well as the comparison with macronutrient supplementation requires further investigation.
A third systematic review examined the effects of a single micronutrient, namely iron supplements, in seven RCTs where there was a non‐significant effect on mental and motor development of children under‐24 months of age (Pasricha et al. 2013). The effect of iron, a single micronutrient, compared with multiple micronutrient supplementation can be further analysed. Finally, a fourth systematic review found a small effect of postnatal nutrition interventions on mental development but was confined to studies between 2000 and 2012, and so excluded many studies conducted before that date when macronutrients were more likely to be studied (Aboud & Yousafzai 2015). Further, this review did not examine possible explanations for the small overall effect seen.
months of age (Pasricha et al. 2013). The effect of iron, a single micronutrient, compared with multiple micronutrient supplementation can be further analysed. Finally, a fourth systematic review found a small effect of postnatal nutrition interventions on mental development but was confined to studies between 2000 and 2012, and so excluded many studies conducted before that date when macronutrients were more likely to be studied (Aboud & Yousafzai 2015). Further, this review did not examine possible explanations for the small overall effect seen.
A systematic review looking at prenatal micronutrient supplementation and its effect on children's mental development found no significant results (Leung et al. 2011). However, psychomotor outcomes improved in two studies using multiple micronutrients and one study using fish oil (Joos et al. 1983; Tofail et al. 2006; Li et al. 2009). Several other fatty acid reviews have been conducted and found non‐significant overall effects on mental and motor development (Smithers et al. 2008; Beyerlein et al. 2010; Qawasmi et al. 2012; Gould et al. 2013; Qawasmi et al. 2013), but their findings are largely from RCTs in high‐income countries limiting the interpretation of these data for LMICs where maternal malnutrition is highly prevalent.
In recent years, the number of nutrition intervention studies assessing development as a primary or secondary outcome has increased making it timely for current review. The primary objective of this meta‐analysis is to examine the effect of nutrition supplementation on mental development in LMICs. This meta‐analysis extends upon previous systematic reviews by addressing effects of pre and postnatal interventions, as well as micro and macronutrition supplementation and their effects on mental development. The secondary objective is to examine the potential pathways by which nutritional inputs may affect mental development, namely nutritional status and motor development. Motor development has been used to explain how nutritional inputs affect mental development (Brown & Pollitt 1996; Prado & Dewey 2014); for example, by enhancing activity and exploration with length and weight underpinning this explanation. We examine these associations in order to identify key mediators, but also potential barriers to why mental development may not be affected. This review is limited to RCTs in LMICs to study the effect of nutrition in resource‐poor settings and is also limited to children under the age of two years. The first 1000 days is now of greatest concern to nutritionists and is an age of rapid brain development (Werker & Tees 2005). As a result, improved conditions before the age of two years may have greater benefits on mental and motor development than at a later age. Therefore, this review looks at the effect of nutrition interventions in pregnant and lactating mothers on their children's mental development before the age of two years, and also the effect of nutrition interventions in children on their mental development until the age of two years.
days is now of greatest concern to nutritionists and is an age of rapid brain development (Werker & Tees 2005). As a result, improved conditions before the age of two years may have greater benefits on mental and motor development than at a later age. Therefore, this review looks at the effect of nutrition interventions in pregnant and lactating mothers on their children's mental development before the age of two years, and also the effect of nutrition interventions in children on their mental development until the age of two years.
Methods
Study search
A search of four databases, Global Health, Medline, PsycINFO and Embase, was conducted to identify articles on nutrition interventions and mental development. The search strategy included topics related to nutrition, mental development, and evaluated interventions, using the following terms: nutrient requirements, infant foods, feeding behaviour, food supplements, nutrients, micronutrient, diet, iodine, iron, stunting, height, malnutrition, Bayley, PPVT, language, cognitive, trial, intervention, programme and RCT. The search was limited to years January 1970 to September 2014, and to English language publications. In Medline, it was possible to limit the age from birth to 24 months. The references from the identified articles were also searched for any additional studies. The PRISMA guidelines were followed (Moher et al. 2009). The clinical trials registry (http://www.clinicaltrials.gov) was searched for relevant trials in the same study period that were not captured in the peer reviewed literature search. However, no additional trials were found.
months. The references from the identified articles were also searched for any additional studies. The PRISMA guidelines were followed (Moher et al. 2009). The clinical trials registry (http://www.clinicaltrials.gov) was searched for relevant trials in the same study period that were not captured in the peer reviewed literature search. However, no additional trials were found.
Inclusion and exclusion criteria
Inclusion criteria were listed as: LMIC, RCT, mental development outcome measured in children from birth to 24 months and an empirical analysis of the data. Fine and gross motor, morbidity, mortality, growth and other nutritional outcomes were recorded if they were analysed in the article. Authors were contacted to obtain outcome statistics if they were not included in the published article. Both prenatal and postnatal nutritional supplementation trials were included. Samples of preterm children were excluded because the degree of prematurity cannot be reliably assessed in many LMICs, particularly with home births. Studies with supplementation periods shorter than two months were excluded. Other exclusion criteria included: no specific child‐ or prenatal‐based intervention, such as screening or cash transfer interventions; hospital‐based studies for children with a major disease or disorder, such as cancer or diabetes; autistic children and reviews or secondary analyses of studies that were already included.
months and an empirical analysis of the data. Fine and gross motor, morbidity, mortality, growth and other nutritional outcomes were recorded if they were analysed in the article. Authors were contacted to obtain outcome statistics if they were not included in the published article. Both prenatal and postnatal nutritional supplementation trials were included. Samples of preterm children were excluded because the degree of prematurity cannot be reliably assessed in many LMICs, particularly with home births. Studies with supplementation periods shorter than two months were excluded. Other exclusion criteria included: no specific child‐ or prenatal‐based intervention, such as screening or cash transfer interventions; hospital‐based studies for children with a major disease or disorder, such as cancer or diabetes; autistic children and reviews or secondary analyses of studies that were already included.
Study selection and data extraction
A first pass of the articles yielded by the search strategy examined the country of data, age of children and nature of the sample. This yielded still a large number of citations, which were further examined in a second pass that considered all inclusion criteria. These two passes were done independently by two reviewers. Data extraction was also completed independently by these two reviewers. Discrepancies were resolved through discussion. Data extraction tables were created with the following information: (1) reference and country; (2) sample size analysed, ages at baseline and endpoint, baseline height‐for‐age z‐score (HAZ) or body mass index (BMI); (3) study design; (4) intervention including nutrients, duration; (5) main mental development outcomes, nutritional outcomes, motor outcomes and effect size Cohen's d; and (6) quality assessment. The effect size was the main summary measure. All mental development test scores were retrieved, whether they included separate or combined cognitive and language subtest scores. Most studies had groups that were comparable at baseline on variables that correlated with the outcome. For this reason, the outcome mean and standard deviation at the study endpoint were used to calculate the effect size for each comparison. Effect sizes were calculated for all group comparisons in a single study.
Quality assessment of RCTs
The Effective Public Health Practice Project (EPHPP) quality assessment tool was used to assign a global rating to each study (Jackson & Waters 2005). Quality is rated according to selection bias, study design, confounders, blinding, data collection methods, withdrawals and dropouts, intervention integrity and analysis. Ratings of prenatal and postnatal studies were assigned by two independent reviewers to ensure reliability (kappa =
= 0.69 for prenatal studies and kappa
0.69 for prenatal studies and kappa =
= 0.72 for postnatal studies). We used funnel plots to assess potential publication bias.
0.72 for postnatal studies). We used funnel plots to assess potential publication bias.
Analysis
The effect size Cohen's d for each group comparison was calculated by dividing the difference in the mean endpoint scores for the intervention and control group by the pooled standard deviation. These effect sizes were then weighted by the inverse variance of the endpoint scores. The overall effect size was calculated by taking the mean of these weighted individual trial effect sizes. In order to appropriately assess for statistical heterogeneity among trials, we ran a chi squared test on the Cochrane's heterogeneity statistic Q, and calculated the I
2 statistic (calculated as I
2 =
= (Q
(Q −
− df)
df) /
/ Q, where df is the degree of freedom). For prenatal trials, the Q‐statistic was 15.46 with a P‐value of 0.22 and I
2 of 22.39; postnatal trials resulted in a Q‐statistic of 41.39 with a P‐value of 0.08 and I
2 of 25.11. These I
2 values represent moderate heterogeneity, and therefore random effects models were used. The statistical software SAS version 9.4 was used for the analysis.
Q, where df is the degree of freedom). For prenatal trials, the Q‐statistic was 15.46 with a P‐value of 0.22 and I
2 of 22.39; postnatal trials resulted in a Q‐statistic of 41.39 with a P‐value of 0.08 and I
2 of 25.11. These I
2 values represent moderate heterogeneity, and therefore random effects models were used. The statistical software SAS version 9.4 was used for the analysis.
Specific to our secondary objective, we used PROC MIXED to run a random effects meta‐regression model to examine whether study quality (its global rating), intervention type, sample size, baseline HAZ (for postnatal studies) or baseline maternal BMI (for prenatal studies), motor development effect size and endline HAZ effect size were significantly associated with mental development effect size. The study was included in the model as a random effect and study quality, intervention type, sample size, baseline HAZ or BMI, motor development effect size and endline HAZ effect size were used as fixed effects in the model. We used the empirical sandwich estimator to account for covariance correlation matrix between and within studies. This analysis adjusts for correlations among multiple effects derived from the different interventions provided within specific studies.
Results
Search flow
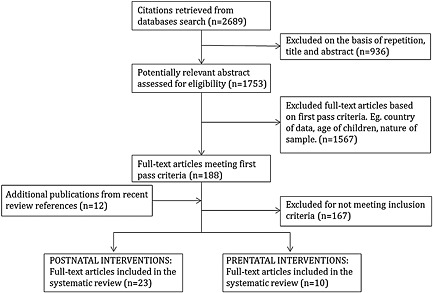
The original search of all four databases yielded 2689 citations with 936 excluded because of duplicates. The first pass reduced the number of studies to 188, and the second pass left 33 RCTs of nutrition interventions analysing mental development in children aged less than two years in LMICs (Fig. (Fig.1).1). An additional 12 studies were identified from recent reviews that appeared in the database search, of which none was included in the final sample after full text review.
Study characteristics
The studies included in the current meta‐analysis were classified into two main categories (see Tables Tables11 and and2):2): (1) those where nutrition was given prenatally and children under the age of two were followed‐up after birth to test mental development; and (2) those where supplementation or some other type of nutrition intervention was given to children under the age of two years and their mental development was assessed shortly after. Ten studies fit the former category and 23 met the latter category. All but one had samples that ranged from well nourished to moderately malnourished on average. Prenatal interventions included 5352 children; postnatal intervention included 6485 children.
Table 1
Prenatal intervention studies
| Reference | Sample size analysed, ages at base and endline | Design | Main development outcomes | Quality | ||||
|---|---|---|---|---|---|---|---|---|
Mental development (mean ± ± SD) SD) | d | Motor development (mean ± ± SD) SD) | d | Item ratings | Global rating | |||
| Thilly et al. 1980a DR Congo HDI .304 (Thilly, 1980a,1980b; Thilly, 1981) |
N Mothers enrolled in 2nd and 3rd trimester Infants tested at 23 | RCT where Intervention received a single dose of 475‐mg iodine in oil and Control received a placebo. |
Brunet‐Lézine: INT (115 ± ± 18) 18) > > CTRL (103 CTRL (103 ± ± 24)** 24)**
| 0.57 | W, S, S, S, W, W, S, M | Weak | ||
| Joos et al. 1983 Taiwan HDI .699 (Joos et al. 1983) |
N Mothers enrolled during lactation of first pregnancy. Infants from second pregnancy tested at 8 | RCT where Intervention received a high calorie and protein supplement (800 kcal and 40‐g protein per day). Control received placebo with 6 kcal and 40‐g protein per day). Control received placebo with 6 kcal or 80 kcal or 80 kcal per day. They were in liquid form. A vitamin and mineral pill was given to all women. Supplementation during lactation of first child and continued through interpregnancy, pregnancy and lactation of second child. This analysis used children from the second pregnancy only. kcal per day. They were in liquid form. A vitamin and mineral pill was given to all women. Supplementation during lactation of first child and continued through interpregnancy, pregnancy and lactation of second child. This analysis used children from the second pregnancy only. |
BSID: INT (4.48 ± ± 1.8) 1.8) = = CTRL (4.39 CTRL (4.39 ± ± 1.8) 1.8) | 0.05 |
BSID: INT (3.8 ± ± 1.9) 1.9) = = CTRL (3.31 CTRL (3.31 ± ± 1.71) 1.71) | 0.27 | M, S, S, S, W, S, M, S | Moderate |
| Hamadani et al. 2002 Bangladesh HDI .515 (Hamadani et al. 2002) |
N Women started supplementation at 4‐m gestation Infants tested at 13 | RCT where Intervention received daily zinc supplementation (30‐mg zinc acetate tablets) and Control received a placebo. |
BSID II: INT (99.34 ± ± 11.2) 11.2) < < CTRL (102.64 CTRL (102.64 ± ± 10.0)* 10.0)*
| −0.31 |
BSID II: INT (88.7 ± ± 17.4) 17.4) < < CTRL (95.7 CTRL (95.7 ± ± 15.0)** 15.0)**
| −0.43 | M, S, S, S, S, M, S, S | Strong |
| Schmidt et al. 2004 Indonesia HDI .629 (Schmidt et al. 2004) |
N Women enrolled at 16–20‐wk gestation Infants measured at 12 | RCT where Intervention received weekly supplementation of 120‐mg Fe + + 500‐mcg FA 500‐mcg FA + + 4800‐mcg retinol in the form of retinyl acetate and Control received 120‐mg Fe 4800‐mcg retinol in the form of retinyl acetate and Control received 120‐mg Fe + + 500‐mcg FA. 500‐mcg FA. |
BSID: INT (105.4 ± ± 22.3) 22.3) = = CTRL (104.0 CTRL (104.0 ± ± 27.1) 27.1) | 0.06 |
BSID: INT (98.3 ± ± 32.0) 32.0) = = CTRL (102.3 CTRL (102.3 ± ± 36.8)*** 36.8)***
| −0.12 | M, S, S, S, W, S, S, S | Moderate |
| Tofail et al. 2006 Bangladesh HDI .515 (Tofail et al. 2006) |
N Mothers supplemented in last trimester Infants tested at 10 | RCT where Intervention received daily supplementation of 4 g of fish oil (containing 1.2 g of fish oil (containing 1.2 g of docosahexaenoic acid and 1.8 g of docosahexaenoic acid and 1.8 g of eicosapentaenoic acid) and Control received 4 g of eicosapentaenoic acid) and Control received 4 g of daily soy‐oil (containing 2.25 g of daily soy‐oil (containing 2.25 g of linoleic acid and 0.27 g of linoleic acid and 0.27 g of α‐linolenic acid). g of α‐linolenic acid). |
BSID II: INT (102.5 ± ± 8.0) 8.0) = = CTRL (101.5 CTRL (101.5 ± ± 7.8) 7.8) | 0.13 |
BSID II: INT (101.7 ± ± 10.9) 10.9) = = CTRL (100.5 CTRL (100.5 ± ± 10.1) 10.1) | 0.11 | M, S, S, S, S, S, S, S | Strong |
| McGrath et al. 2006 Tanzania HDI .476 (McGrath et al. 2006) |
N Mothers enrolled at 12–27‐wk gestation Infants measured at 18 | RCT where Interventions received daily vitamin A (30 mg of β‐carotene mg of β‐carotene + + 5000 5000 IU preformed vitamin A), multivitamins with no vitamin A (20‐mg B1, 20‐mg B2, 25‐mg B6, 100‐mg niacin, 50‐mcg B12, 500‐mg vitamin C, 30‐mg vitamin E and 0.8‐mg FA), or multivitamins IU preformed vitamin A), multivitamins with no vitamin A (20‐mg B1, 20‐mg B2, 25‐mg B6, 100‐mg niacin, 50‐mcg B12, 500‐mg vitamin C, 30‐mg vitamin E and 0.8‐mg FA), or multivitamins + + vitamin A, and Control received placebo. vitamin A, and Control received placebo. |
BSID II: INT MV (78.4 ± ± 13.8) 13.8) = = CTRL No MV (82.0 CTRL No MV (82.0 ± ± 13.5) INT Vit A (80.6 13.5) INT Vit A (80.6 ± ± 12.5) 12.5) = = CTRL No vit A (79.3 CTRL No vit A (79.3 ± ± 15.4) 15.4) | −0.26 0.09 |
BSID II: INT MV (86.2 ± ± 14.3) 14.3) = = CTRL No MV (88.5 CTRL No MV (88.5 ± ± 14.8) INT Vit A (87.4 14.8) INT Vit A (87.4 ± ± 12.2) 12.2) = = CTRL No vit A (87.0 CTRL No vit A (87.0 ± ± 15.1) 15.1) | −0.16 0.03 | M, S, S, S, W, S, S, S | Moderate |
| Tofail et al. 2008 Bangladesh HDI .515 (Tofail et al. 2008) |
N Infants measured at 7m Mean base maternal BMI | RCT where Intervention received daily MMN and Control received iron and folate. MMN included 150‐mcg I (potassium iodide), 15‐mg Zn (sulphate), 65‐mcg Se (sodium selenite), 2‐mg Cu (sulphate), 800‐mcg retinyl acetate (RE) vitamin A, 1.4‐mg thiamine mononitrate, 1.4‐mg vitamin riboflavin, 18‐mg vitamin B3 (niacin), 1.9‐mg vitamin B6 (pyridoxine hydrochloride), 2.6‐mcg vitamin B12 (cyanocobalmin), 70‐mg vitamin C, 200 IU vitamin D (vitamin D3) and 10‐mcg vitamin E (α‐tocopherol acetate) in the recommended dietary allowance dose in addition to 60‐mg Fe (fumarate) and 400‐mcg folate. Control received 30‐mg Fe (fumarate) and 400‐mcg folate. IU vitamin D (vitamin D3) and 10‐mcg vitamin E (α‐tocopherol acetate) in the recommended dietary allowance dose in addition to 60‐mg Fe (fumarate) and 400‐mcg folate. Control received 30‐mg Fe (fumarate) and 400‐mcg folate. |
Problem solving test: Support: INT MM (11.3 ± ± 7.9) 7.9) = = CTRL (11.1 CTRL (11.1 ± ± 7.5) 7.5) | 0.03 |
BSID II:INT MM (103.66 ± ± 16.6) 16.6) = = CTRL (102.42 CTRL (102.42 ± ± 15.4) 15.4) | 0.08 | S, S, S, W, M, S, S | Moderate |
| Li et al. 2009 China HDI .699 (Li et al. 2009) |
N Mean gestation age of mothers at enrollment | Cluster RCT where Interventions received MMN, iron/folic acid or folic acid supplementation. Assume two trimesters of supplementation. Control received folic acid. MMN included 30‐mg iron, 400‐mcg folate, 15‐mg zinc, 2‐mg copper, 65‐mcg selenium, 150‐mcg iodine, 800‐mcg vitamin A, 1.4‐mg vitamin B1, 1.4‐mg vitamin B2, 1.9‐mg vitamin B6, 2.6‐mcg vitamin B12, 5‐mcg vitamin D, 70‐mg vitamin C, 10‐mg vitamin E and 18‐mg niacin. FE/FA included 60‐mg iron and 400‐mcg folic acid. FA included 400‐mcg folic acid. |
BSID: INT Fe/FA (102.44 ± ± 45.26) 45.26) = = CTRL (102.65 CTRL (102.65 ± ± 49.21) INT MMN (103.65 49.21) INT MMN (103.65 ± ± 42.11) 42.11) = = CTRL (102.65 CTRL (102.65 ± ± 49.21) 49.21) | 0.04 0.02 |
BSID: INT Fe/FA (45.3 ± ± 22.15) 22.15) = = CTRL (45.39 CTRL (45.39 ± ± 22.40) INT MMN (45.64 22.40) INT MMN (45.64 ± ± 19.83) 19.83) = = CTRL (45.39 CTRL (45.39 ± ± 22.40) 22.40) | −0.004 0.01 | S, S, S, S, W, M, S, S | Moderate |
| Chang et al. 2013 China HDI .699 (Chang et al. 2013) |
N Mean gestation age of mothers at enrollment | Cluster RCT where Interventions received MMN, iron/folic acid or folic acid supplementation. Assume two trimesters of supplementation. Control received folic acid. MMN included 30‐mg iron, 400‐mcg folate, 15‐mg zinc, 2‐mg copper, 65‐mcg selenium, 150‐mcg iodine, 800‐mcg vitamin A, 1.4‐mg vitamin B1, 1.4‐mg vitamin B2, 1.9‐mg vitamin B6, 2.6‐mcg vitamin B12, 5‐mcg vitamin D, 70‐mg vitamin C, 10‐mg vitamin E and 18‐mg niacin. FE/FA included 60‐mg iron and 400‐mcg folic acid. FA included 400‐mcg folic acid. |
BSID II: INT Fe/FA (90.31 ± ± 16.1) 16.1) = = CTRL (88.78 CTRL (88.78 ± ± 17.3) INT MMN (89.67 17.3) INT MMN (89.67 ± ± 19.5) 19.5) = = CTRL (88.78 CTRL (88.78 ± ± 17.3) 17.3) | 0.09 0.05 |
BSID II: INT Fe/FA (104.47 ± ± 11.7) 11.7) = = CTRL FA (103.89 CTRL FA (103.89 ± ± 12.8) INT MMN (103.13 12.8) INT MMN (103.13 ± ± 13.0) 13.0) = = CTRL FA (103.89 CTRL FA (103.89 ± ± 12.8) 12.8) | 0.05–0.06 | S, S, S, W, M, S, S | Moderate |
| Hanieh et al. 2013 Viet Nam HDI .617 (Hanieh et al. 2013) |
N Mothers enrolled if <16‐wk gestation Infants tested at 6m Mean base maternal BMI | Cluster RCT where Intervention received MMN twice per week and Control received 60‐mg elemental iron plus 0.4‐mg folic acid daily. MMN contained 15 micronutrients, including 60‐mg iron, 20‐mg zinc, 300‐mcg iodine, 4‐mg copper, 130‐mcg selenium, 1.6‐mg vitamin A, 2.8‐mg thiamine, 2.8‐mg riboflavin, 36‐mg niacin, 3.8‐mg vitamin B6, 5.2‐mcg vitamin B12, 1.5‐mg folic acid, 140‐mg vitamin C, 400 IU vitamin D and 20‐mg vitamin E. IU vitamin D and 20‐mg vitamin E. |
BSID III:INT (101.2 ± ± 9.9) 9.9) = = CTRL (100.2 CTRL (100.2 ± ± 11.4) 11.4) | 0.09 | S, S, S, S, W, S, S, S | Moderate | ||
Note: Assessment of Quality (Item Ratings) with following categories in order: selection bias, study design, confounders, blinding, data collection methods, withdrawals and dropouts, intervention integrity, analysis; HDI, Human Development Index; RCT, Randomized Controlled Trial; m, month; wk, week; INT, Intervention group; CTRL, Control group; BMI, body mass index; BSID, Bayley Scales of Infant Development; effect size d, standardized mean difference; SD, standard deviation; W, weak; M, moderate; S, strong; MMP, multiple micronutrient powder; MMN, multiple micronutrients; MV, multivitamin; Zn, zinc; Fe, iron; FA, folic acid.
 <
< 0.05;
0.05; <
< 0.01;
0.01; <
< 0.001.
0.001.Table 2
Postnatal intervention studies
| Reference | Sample size analysed, ages at base and endline | Design | Duration (m) | Main developmental outcomes | Effect size d | Growth parameters (mean ± ± SD) SD) | Effect size d | Quality | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
Mental development (mean ± ± SD) SD) | Effect size d | Motor development (mean ± ± SD) SD) | Item ratings | Global rating | |||||||
| Grantham‐McGregor et al. 1991 Jamaica HDI .731 (Grantham‐McGregor et al. 1991) |
N = = 65 INT n 65 INT n = = 32 CTRL n 32 CTRL n = = 33 Base age 33 Base age = = 9–24 9–24 m with mean m with mean = = 18 18 m End age m End age = = 33–48 33–48 m with mean m with mean = = 24 24 m Mean base HAZ m Mean base HAZ = = −2.9 −2.9 | RCT where Intervention received milk‐based formula 750 kcal and 20 kcal and 20 g protein/day and Control received no supplement. g protein/day and Control received no supplement. | 24 |
Griffiths Mental Scales: INT (86 ± ± 10) 10) = = CTRL (83 CTRL (83 ± ± 10) 10) | 0.30 |
Griffiths Mental Scales: INT (97 ± ± 13) 13) = = CTRL (95 CTRL (95 ± ± 11) 11) | 0.17 | M, S, S, S, S, S, M, M | Strong | ||
| Idjradinata & Pollitt 1993 Indonesia HDI .629 (Idjradinata & Pollitt 1993) |
N = = 44 INT n 44 INT n = = 22 CTRL n 22 CTRL n = = 22 Base age 22 Base age = = 12–18 12–18 m End age m End age = = 16–22 16–22 m Iron‐sufficient infants m Iron‐sufficient infants | RCT where Intervention received ferrous sulphate 3 mg/kg per day in syrup form and Control received a placebo syrup. mg/kg per day in syrup form and Control received a placebo syrup. | 4 |
BSID: INT (109.1 ± ± 2.2) 2.2) = = CTRL (106.8 CTRL (106.8 ± ± 2.3) 2.3) | 1.02 |
BSID: INT (108.7 ± ± 2.1) 2.1) = = CTRL (108.3 CTRL (108.3 ± ± 2.1) 2.1) | 0.19 | M, S, S, S, W, S, S, M | Moderate | ||
| Ashworth et al. 1998 Brazil HDI .730 (Ashworth et al. 1998) |
N = = 138 INT 1mgZn n 138 INT 1mgZn n = = 48 INT 5‐mg Zn 48 INT 5‐mg Zn = = 48 CTRL n 48 CTRL n = = 44 LBW term infants. Base age 44 LBW term infants. Base age = = 0 0 m End age m End age = = 12 12 m m | Prospective double‐blind part‐RCT where two Intervention groups received 1‐mg or 5‐mg Zn (as zinc sulphate) daily (except Sundays) and Control received placebo. Injected liquid Zn/placebo with syringe. | 2 |
BSID: INT 1‐mg Zn (101.1 ± ± 11.0) 11.0) = = CTRL(100.4 CTRL(100.4 ± ± 11.3) INT 5‐mg Zn (100.0 11.3) INT 5‐mg Zn (100.0 ± ± 11.6) 11.6) = = CTRL(100.4 CTRL(100.4 ± ± 11.3) 11.3) | 0.06–0.03 |
BSID: INT 1‐mg Zn (106.7 ± ± 11.1) 11.1) = = CTRL(109.1 CTRL(109.1 ± ± 12.2) INT 5‐mg Zn (106.9 12.2) INT 5‐mg Zn (106.9 ± ± 12.1) 12.1) = = CTRL(109.1 CTRL(109.1 ± ± 12.2) 12.2) | −0.21 −0.18 | M, M, S, S, W, M, S, S | Moderate | ||
| Pollitt et al. 2000 Indonesia HDI .629 (Beckett et al. 2000; Pollitt 2000; Pollitt et al. 2000) |
N = = 75 INT n 75 INT n = = 38 CTRL n 38 CTRL n = = 37 Base age 37 Base age = = 12 12 m End age m End age = = 24 24 m Base HAZ m Base HAZ ≤ ≤ −1 −1 | Cluster RCT where Intervention given daily high energy + + Fe (E) (1171 Fe (E) (1171 kJ kJ + + 12‐mg iron) or given Fe with low energy (M) (12‐mg iron 12‐mg iron) or given Fe with low energy (M) (12‐mg iron + + 209 209 kJ). Control given skim milk only (209 kJ). Control given skim milk only (209 kJ). MMN tablet included eight micronutrients, in tablet form (doses of micronutrients other than iron not identified). High energy drink was condensed milk vs low energy skim milk. kJ). MMN tablet included eight micronutrients, in tablet form (doses of micronutrients other than iron not identified). High energy drink was condensed milk vs low energy skim milk. | 12 |
BSID I: INT M + + E (151.3; E (151.3; ± ± 6.0) 6.0) = = CTRL (149.3 CTRL (149.3 ± ± 8.0) 8.0) | 0.28 | W, S, W, M, W, W, S, M | Weak | ||||
| Castillo‐Duran et al. 2001 Chile HDI .819 (Castillo‐Duran et al. 2001) |
N = = 112 term neonates; INT n 112 term neonates; INT n = = 57 CTRL n 57 CTRL n = = 55 Base age 55 Base age = = newborn End age newborn End age = = 12 12 m m | RCT where intervention group was given 5 mg/d supplemental zinc within 20 mg/d supplemental zinc within 20 days of birth, and then monthly until 1 days of birth, and then monthly until 1 year. Control group was given a lactose placebo. All received iron 1 to 2 year. Control group was given a lactose placebo. All received iron 1 to 2 mg/kg/d after 5 mg/kg/d after 5 mo. mo. | 12 |
BSID II: At 12 m: INT (90.9 m: INT (90.9 ± ± 10.5) 10.5) = = CTRL (88.9 CTRL (88.9 ± ± 9.1) 9.1) | 0.20 |
BSID II: At 12 m: INT (84.5 m: INT (84.5 ± ± 11.5) 11.5) = = CTRL (87.6 CTRL (87.6 ± ± 9.9) 9.9) | −0.29 |
WAZ: At 12 m: INT (−0.23 m: INT (−0.23 ± ± 1.04) 1.04) = = CTRL (0.22 CTRL (0.22 ± ± 0.86) HAZ: At 12 0.86) HAZ: At 12 m: INT (−0.44 m: INT (−0.44 ± ± 0.94) 0.94) = = CTRL (−0.07 CTRL (−0.07 ± ± 0.75) 0.75) | −0.47 −0.60 | M, S, S, S, W, M, S, M | Moderate |
| Hamadani et al. 2001 Bangladesh HDI .515 (Hamadani et al. 2001) |
N = = 198 INT n 198 INT n = = 97 CTRL n 97 CTRL n = = 101 Base age mean 101 Base age mean = = 1.4 1.4 m End age mean m End age mean = = 13.6 13.6 m Mean base HAZ m Mean base HAZ = = −1.1 −1.1 | RCT where Intervention received daily 5‐mg Zn and Control received placebo syrup. | 6 |
BSID II: INT (103.1 ± ± 11.0) 11.0) < < CTRL (106.4 CTRL (106.4 ± ± 9.3)* 9.3)*
| −0.33 |
BSID II: INT (88.0 ± ± 18.9) 18.9) = = CTRL (90.6 CTRL (90.6 ± ± 18.9) 18.9) | −0.14 |
WAZ: INT (−2.4 ± ± 0.9) 0.9) = = CTRL (−2.5 CTRL (−2.5 ± ± 0.9) HAZ: INT (−2.3 0.9) HAZ: INT (−2.3 ± ± 1.0) 1.0) = = CTRL (−2.4 CTRL (−2.4 ± ± 1.0) 1.0) | 0.11 0.10 | M, S, S, S, S, M, S, S | Strong |
| Black et al. 2004a Bangladesh HDI .515 (Black et al. 2004a) |
N = = 221 INT MMN n 221 INT MMN n = = 35 INT Zn 35 INT Zn + + Fe n Fe n = = 43 INT Fe n 43 INT Fe n = = 47 INT Zn n 47 INT Zn n = = 49 CTRL (riboflavin) n 49 CTRL (riboflavin) n = = 45 Base age 6.5 45 Base age 6.5 m End age 12.7 m End age 12.7 m Mean base HAZ m Mean base HAZ = = −1.2 −1.2 | RCT where Intervention received 16 MMN compared with Control (riboflavin) and with iron and zinc separately and together. INT MMN: 2xRDA thiamine, niacin, FA, pantothenic acid, iodine, copper, manganese, selenium and Vitamins C, D, E, B6, B12 + + 20‐mg Fe 20‐mg Fe + + 20‐mg Zn INT Zn 20‐mg Zn INT Zn + + Fe: 20‐mg Zn sulphate Fe: 20‐mg Zn sulphate + + 20‐mg Fe sulphate INT Fe: 20‐mg Fe sulphate INT Zn: 20‐mg Zn acetate CTRL: 1‐mg riboflavin 20‐mg Fe sulphate INT Fe: 20‐mg Fe sulphate INT Zn: 20‐mg Zn acetate CTRL: 1‐mg riboflavin | 6 |
BSID II: Fe (104.3 ± ± 9.5) 9.5) = = CTRL (102.7 CTRL (102.7 ± ± 13.5) Zn (104.7 13.5) Zn (104.7 ± ± 8.3) 8.3) = = CTRL (102.7 CTRL (102.7 ± ± 13.5) Fe 13.5) Fe + + Zn (105.4 Zn (105.4 ± ± 11.6) 11.6) = = CTRL (102.7 CTRL (102.7 ± ± 13.5) MMN (104.3 13.5) MMN (104.3 ± ± 13.0) 13.0) = = CTRL (102.7 CTRL (102.7 ± ± 13.5) 13.5) | 0.14 0.18 0.12 0.22 |
BSID II: Fe (99.5 ± ± 16.6) 16.6) = = CTRL (95.4 CTRL (95.4 ± ± 16.3) Zn (101.2 16.3) Zn (101.2 ± ± 16.6) 16.6) = = CTRL (95.4 CTRL (95.4 ± ± 16.3) Fe 16.3) Fe + + Zn (103.7 Zn (103.7 ± ± 16.2) 16.2) = = CTRL (95.4 CTRL (95.4 ± ± 16.3) MMN (104.5 16.3) MMN (104.5 ± ± 16.5) 16.5) = = CTRL (95.4 CTRL (95.4 ± ± 16.3) 16.3) | 0.25 0.35 0.51 0.55 |
WAZ: Fe (−2.2 ± ± 1.0) 1.0) = = CTRL (−2.0 CTRL (−2.0 ± ± 1.2) Zn (−2.0 1.2) Zn (−2.0 ± ± 1.2) 1.2) = = CTRL (−2.0 CTRL (−2.0 ± ± 1.2) Fe 1.2) Fe + + Zn (−2.2 Zn (−2.2 ± ± 1.3) 1.3) = = CTRL (−2.0 CTRL (−2.0 ± ± 1.2) MMN (−2.1 1.2) MMN (−2.1 ± ± 1.0) 1.0) = = CTRL (−2.0 CTRL (−2.0 ± ± 1.2) HAZ: Fe (−1.6 1.2) HAZ: Fe (−1.6 ± ± 0.9) 0.9) = = CTRL (−2.0 CTRL (−2.0 ± ± 1.2) Zn (−1.6 1.2) Zn (−1.6 ± ± 0.9) 0.9) = = CTRL (−2.0 CTRL (−2.0 ± ± 1.2) Fe 1.2) Fe + + Zn (−1.8 Zn (−1.8 ± ± 0.9) 0.9) = = CTRL (−1.7 CTRL (−1.7 ± ± 1.0) MMN (−1.7 1.0) MMN (−1.7 ± ± 0.8) 0.8) = = CTRL (−1.7 CTRL (−1.7 ± ± 1.0) 1.0) | −0.18 0 −0.16 −0.09 0.38 0.38 −0.11 0 | S, S, S, S, S, M, S, M | Strong |
| Black 2004 India HDI .554 (Black et al. 2004b) |
N = = 162 INT MMN 162 INT MMN + + Zn n Zn n = = 85 CTRL MM n 85 CTRL MM n = = 77 Base age 77 Base age = = 1 1 m End age m End age = = 10 10 m Mean base HAZ m Mean base HAZ = = −1.9 All newborns small‐for‐gestational age, <10th percentile weight, term −1.9 All newborns small‐for‐gestational age, <10th percentile weight, term | RCT where 5 MMN + + Zn group compared with 5 MMN group. Syrup with MMN fed directly to children daily. MMN includes 0.5 Zn group compared with 5 MMN group. Syrup with MMN fed directly to children daily. MMN includes 0.5 mg/d riboflavin, 180 mg/d riboflavin, 180 mg/d calcium, 90 mg/d calcium, 90 mg/d phosphorus, 60 mg/d phosphorus, 60 mol/d folate and 10 mol/d folate and 10 mg/d iron, with 5 mg/d iron, with 5 mg of zinc sulphate. mg of zinc sulphate. | 9 |
BSID II: INT MMN + + Zn (86.2 Zn (86.2 ± ± 4.9) 4.9) = = CTRL MMN (86.4 CTRL MMN (86.4 ± ± 5.1) 5.1) | 0.04 |
BSID II: INT MMN + + Zn (91.7 Zn (91.7 ± ± 9.8) 9.8) = = CTRL MMN (91.5 CTRL MMN (91.5 ± ± 14.2) 14.2) | 0.020 | S, S, S, S, S, S, S, S | Strong | ||
| Lind et al. 2004 Indonesia HDI .629 (Lind et al. 2004) |
N = = 650 INT Fe n 650 INT Fe n = = 163 INT Zn n 163 INT Zn n = = 162 INT Fe 162 INT Fe + + Zn n Zn n = = 161 CTRL n 161 CTRL n = = 164 Base age 164 Base age = = 6 6 m End age m End age = = 12 12 m Mean base HAZ m Mean base HAZ = = −0.3 −0.3 | RCT where Intervention received daily supplementation with 10‐mg Fe, or 10‐mg Zn, or both, and Control received placebo syrup. | 6 |
BSID II: INT Fe (101 ± ± 9.7) 9.7) = = CTRL (99 CTRL (99 ± ± 10.0) INT Zn (101 10.0) INT Zn (101 ± ± 9.3) 9.3) = = CTRL (99 CTRL (99 ± ± 10.0) INT Zn 10.0) INT Zn + + Fe (100 Fe (100 ± ± 9.8) 9.8) = = CTRL (99 CTRL (99 ± ± 10.0) 10.0) | 0.20 0.21 0.10 |
BSID II: INT Fe (106 ± ± 11.0) 11.0) > > CTRL (103 CTRL (103 ± ± 10.8)* INT Zn (105 10.8)* INT Zn (105 ± ± 10.6) 10.6) = = CTRL (99 CTRL (99 ± ± 10.0) INT Zn 10.0) INT Zn + + Fe (103 Fe (103 ± ± 10.3) 10.3) = = CTRL (99 CTRL (99 ± ± 10.0) 10.0) | 0.27 0.58 0.39 |
WAZ: INT Zn (−1.46 ± ± 1.8) 1.8)  < < CTRL (−1.72 CTRL (−1.72 ± ± 1.00)* INT Fe (−1.65 1.00)* INT Fe (−1.65 ± ± 1.08) 1.08)  = = CTRL (−1.72 CTRL (−1.72 ± ± 1.00) INT Fe 1.00) INT Fe + + Zn (−1.68 Zn (−1.68 ± ± 1.02) 1.02)  = = CTRL (−1.72 CTRL (−1.72 ± ± 1.00) HAZ: INT Fe (−0.66 1.00) HAZ: INT Fe (−0.66 ± ± 0.91) = 0.91) = CTRL (−0.81 CTRL (−0.81 ± ± 0.86) INT Zn (−0.77 0.86) INT Zn (−0.77 ± ± 0.92) 0.92) = = CTRL (−0.81 CTRL (−0.81 ± ± 0.86) INT Fe 0.86) INT Fe + + Zn (−0.90 Zn (−0.90 ± ± 0.90) 0.90) = = CTRL (−0.81 CTRL (−0.81 ± ± 0.86) 0.86) | 0.18 0.06 0.04 0.17 0.04 −0.10 | S, S, S, S, W, S, S, S | Moderate |
| Taneja et al. 2005 India HDI .554 (Taneja et al. 2005) |
N = = 571 INT n 571 INT n = = 283 CTRL n 283 CTRL n = = 288 Base age 288 Base age = = 12–18 12–18 m with mean m with mean = = 14.9 14.9 m End age m End age = = 16–24 16–24 m with mean m with mean = = 21 21 m Base stunting m Base stunting = = 36.8% 36.8% | RCT where Intervention received daily 20‐mg zinc (10‐mg for infants) and Control received placebo. | 4 |
BSID II: INT (92.8 ± ± 10.9) 10.9) = = CTRL (91.3 CTRL (91.3 ± ± 10.8) 10.8) | 0.14 |
BSID II: INT (93.9 ± ± 11.8) 11.8) = = CTRL (92.2 CTRL (92.2 ± ± 11.4) 11.4) | 0.15 | S, S, S, S, S, S, M, M | Strong | ||
| Gardner et al. 2005 Jamaica HDI .731 (Gardner et al. 2005) |
N = = 114 INT n 114 INT n = = 55 CTRL n 55 CTRL n = = 59 Base age 59 Base age = = 9–30 9–30 m End age m End age = = 15–36 15–36 m Mean base HAZ m Mean base HAZ = = −1.4 −1.4 | Cluster RCT where Intervention received 10‐mg Zn daily with/out stimulation and Control received placebo with/out stimulation, All children received 0.5 ml of 10 MMN including 1500 ml of 10 MMN including 1500 IU vitamin A, 400 IU vitamin A, 400 IU vitamin D, 0.5‐mg vitamin B1, 0.8‐mg riboflavin, 7‐mg nicotinamide, 1‐mg vitamin B6, 30‐mg vitamin C, 8‐mg iron, 1‐mg folic acid and 2‐mg vitamin B12. IU vitamin D, 0.5‐mg vitamin B1, 0.8‐mg riboflavin, 7‐mg nicotinamide, 1‐mg vitamin B6, 30‐mg vitamin C, 8‐mg iron, 1‐mg folic acid and 2‐mg vitamin B12. | 6 |
Griffiths Mental Scales: Cognitive INT (89.45 ± ± 12.8) 12.8) = = CTRL (89.85 CTRL (89.85 ± ± 11.8) 11.8) | −0.03 | Griffiths Mental Scales: Locomotor INT (97.4 ± ± 12.7) 12.7) = = CTRL (102.6 CTRL (102.6 ± ± 9.9) 9.9) | −0.46 | WAZ: INT (−2.04 ± ± 0.56) 0.56) = = CTRL (−2.03 CTRL (−2.03 ± ± 0.58) HAZ: INT (−1.26 0.58) HAZ: INT (−1.26 ± ± 0.71) 0.71) = = CTRL (−1.08 CTRL (−1.08 ± ± 0.80) 0.80) | −0.02 −0.24 | M, S, S, S, S, S, S, S | Strong |
| Aboud & Akhter 2011 Bangladesh HDI .515 (Aboud & Akhter 2011) |
N = = 186 INT n 186 INT n = = 99 CTRL n 99 CTRL n = = 85 Base age mean 85 Base age mean = = 14 14 m End age mean m End age mean = = 21 21 m Mean base HAZ m Mean base HAZ = = −1.6 −1.6 | Cluster RCT where Intervention received MMP (containing 12.5 mg of iron, 300 mg of iron, 300 mcg of vitamin A, 150 mcg of vitamin A, 150 mcg of folic acid, 50 mcg of folic acid, 50 mg of vitamin C and 5 mg of vitamin C and 5 mg of zinc) plus six‐session parenting programme on stimulation and Control received parenting programme but no MMP. mg of zinc) plus six‐session parenting programme on stimulation and Control received parenting programme but no MMP. | 7 |
BSID II: Language: INT (30.89 ± ± 21.2) 21.2) = = CTRL (32.7 CTRL (32.7 ± ± 21.3) 21.3) | −0.09 |
WAZ: INT (−1.87 ± ± 1.0) 1.0) > > CTRL (−2.03 CTRL (−2.03 ± ± 1.0)*
HAZ: INT (−1.89 1.0)*
HAZ: INT (−1.89 ± ± 1.1) 1.1) = = CTRL (−1.99 CTRL (−1.99 ± ± 1.1) 1.1) | 0.16 0.09 | M, S, S, M, S, S, S, S | Strong | ||
| Rosado et al. 2011 Mexico HDI .775 (Rosado et al. 2011) |
N = = 186 INT n 186 INT n = = 55 CTRL n 55 CTRL n = = 61 Base age 61 Base age = = 12–24 12–24 m with mean m with mean = = 22.4 22.4 m End age18–30 m End age18–30 m with mean m with mean = = 28 28 m Mean base HAZ m Mean base HAZ = = −1.2 −1.2 | RCT where Intervention received daily supplement (44‐g MMP daily, consisting of 194 kcal, 815 kcal, 815 kJ, 6‐g protein, 7‐g fat, 28‐g carbohydrates, 25‐mg sodium, 10‐mg iron, 10‐mg zinc, 400‐mcg vitamin A, 6‐mg vitamin E, 40‐mg vitamin C, 0.7‐mcg vitamin B12, 50‐mcg folic acid, 0.8‐mg riboflavin) and Control received placebo (174 kJ, 6‐g protein, 7‐g fat, 28‐g carbohydrates, 25‐mg sodium, 10‐mg iron, 10‐mg zinc, 400‐mcg vitamin A, 6‐mg vitamin E, 40‐mg vitamin C, 0.7‐mcg vitamin B12, 50‐mcg folic acid, 0.8‐mg riboflavin) and Control received placebo (174 kcal, 731 kcal, 731 kJ, 30‐g carbohydrates). Half‐supplement group excluded here. kJ, 30‐g carbohydrates). Half‐supplement group excluded here. | 6 |
BSID II: INT (135.9 ± ± 13.7) 13.7) = = CTRL (137.6 CTRL (137.6 ± ± 12.6) 12.6) | −0.13 |
BSID II: INT (88.6 ± ± 9.2) 9.2) = = CTRL (90.0 CTRL (90.0 ± ± 9.3) 9.3) | −0.15 |
WAZ: INT (−0.7 ± ± 0.9) 0.9) = = CTRL(−0.7 CTRL(−0.7 ± ± 1.0)**
HAZ: INT (−1.1 1.0)**
HAZ: INT (−1.1 ± ± 1.0) 1.0) = = CTRL (−1.0 CTRL (−1.0 ± ± 1.1) 1.1) | 0 −0.10 | S, S, S, M, W, S, S, M | Moderate |
| Siegel et al. 2011 Nepal HDI .463 (Siegel et al. 2011) |
N = = 325 INT Zn n 325 INT Zn n = = 80 INT Fe 80 INT Fe + + FA n FA n = = 80 INT MMN n 80 INT MMN n = = 80 CTRL n 80 CTRL n = = 81 Base age 1–35 81 Base age 1–35 wk with mean wk with mean = = 7 7 m End age mean m End age mean = = 19 19 m Base stunting m Base stunting = = 16.7% 16.7% | Cluster RCT with 3 INTs (daily supplement with Zn, Fe or both) and Control received a placebo. INT Zn: 5 mg/d Zn INT Fe mg/d Zn INT Fe + + FA: 6.25 FA: 6.25 mg/d Fe; 25 mg/d Fe; 25 mcg/d FA INT MMN: 5 mcg/d FA INT MMN: 5 mg/d Zn mg/d Zn + + 6.25 6.25 mg/d Fe mg/d Fe + + 25 25 mc/d FA mc/d FA | 12 |
Fagen Test of Infant Intelligence: Fixation time: INT Zn (1.31 ± ± 0.2) 0.2) = = CTRL (1.30 CTRL (1.30 ± ± 0.21) INT Fe 0.21) INT Fe + + FA (1.30 FA (1.30 ± ± 0.4) 0.4) = = CTRL (1.30 CTRL (1.30 ± ± 0.21) INT MMN (1.32 0.21) INT MMN (1.32 ± ± 0.2) 0.2) = = CTRL (1.30 CTRL (1.30 ± ± 0.21) 0.21) | 0.05 0 0.10 | WAZ: INT Zn (−1.52 ± ± 1.04) 1.04) = = CTRL (−1.99 CTRL (−1.99 ± ± 1.00) INT Fe 1.00) INT Fe + + FA (−1.83 FA (−1.83 ± ± 0.92) 0.92) = = CTRL (−1.99 CTRL (−1.99 ± ± 1.00) INT MMN (−1.75 1.00) INT MMN (−1.75 ± ± 1.10) 1.10) = = CTRL (−1.99 CTRL (−1.99 ± ± 1.00) HAZ: INT Zn (−0.95 1.00) HAZ: INT Zn (−0.95 ± ± 0.98) 0.98) = = CTRL (−1.64 CTRL (−1.64 ± ± 0.93) INT Fe 0.93) INT Fe + + FA (−1.34 FA (−1.34 ± ± 0.93) 0.93) = = CTRL (−1.64 CTRL (−1.64 ± ± 0.93) INT MMN (−1.40 0.93) INT MMN (−1.40 ± ± 1.09) 1.09) = = CTRL (−1.64 CTRL (−1.64 ± ± 0.93) 0.93) | 0.46 0.17 0.23 0.72 0.32 0.24 | M, S, S, S, W, M, S, S | Moderate | ||
| Gurnida et al. 2012 Indonesia HDI .629 (Gurnida et al. 2012) |
N = = 59 INT n 59 INT n = = 29 CTRL n 29 CTRL n = = 30 Base age 2–8 30 Base age 2–8 wk with mean wk with mean = = 3.8 3.8 wk End age 24 wk End age 24 wk Mean base HAZ wk Mean base HAZ = = −0.4 −0.4 | RCT where Intervention given formula with 9 mg/100 mg/100 g gangliosides for up to 6 g gangliosides for up to 6 months. Controls given standard formula milk with 6 months. Controls given standard formula milk with 6 mg/100 mg/100 g. g. | 6 |
Griffiths Mental Development Scale: Cognitive: INT (131.1 ± ± 14.8) 14.8) > > CTRL (123.2 CTRL (123.2 ± ± 16.0)*** 16.0)***
| 0.53 |
Griffiths Mental Development Scale: Gross (Locomotive) motor: INT (120.0 ± ± 12.23) 12.23) = = CTRL (117. CTRL (117. ± ± 16.91) 16.91) | 0.20 | M, S, S, S, W, S, S, M | Moderate | ||
| Manno et al. 2012 Zambia HDI .448 (Growth 2010; Gibson et al. 2011; Manno et al. 2012) |
N = = 335 INT n 335 INT n = = 160 CTRL n 160 CTRL n = = 175 Base age 175 Base age = = 6 6 m End age m End age = = 18 18 m Mean base HAZ m Mean base HAZ = = −0.8 38% of mothers were HIV+; 3.9% of children were HIV+ −0.8 38% of mothers were HIV+; 3.9% of children were HIV+ | RCT where Intervention given richly fortified porridge flour and Controls given standard fortified flour. INT flour was fortified with 18 micronutrients (6.5‐mg vitamin A, 2‐g vitamin c, 0.1‐mg vitamin D, 9‐mg thiamin, 11‐mg riboflavin, 140‐mg niacin, 9‐mg pyridoxine, 2‐mg folate, 10‐mcg vitamin B12, 40‐mg pantothenic acid, 1‐g magnesium oxide, 250‐mg iron, 200‐mg zinc, 3‐mg copper, 12‐mg manganese, 0.2‐mg selenium, 7‐g calcium and 5‐g phosphorus). | 12 |
BSID II: INT (89.8 ± ± 7.4) 7.4) = = CTRL (88.4 CTRL (88.4 ± ± 7.4) 7.4) | 0.19 |
BSID II: INT (90.0 ± ± 6.2) 6.2) < < CTRL (91.4 CTRL (91.4 ± ± 6.1)* 6.1)*
| −0.23 |
HAZ: INT (−1.05 ± ± 1.2) 1.2) = = CTRL (−1.12 CTRL (−1.12 ± ± 1.11) 1.11) | 0.06 | M, S, S, S, S, M, S, S | Strong |
| Nahar et al. 2012 Bangladesh HDI .515 (Nahar et al. 2012) |
N = = 136 INT n 136 INT n = = 77 CTRL n 77 CTRL n = = 59 Base age 59 Base age = = 6–24 6–24 m End age m End age = = 12−30 12−30 m Mean base HAZ m Mean base HAZ = = −3.5 −3.5 | RCT where Intervention received food supplementation (FS) and control received growth monitoring, health education and micronutrient supplementation. FS consisted of roasted rice powder 20 g, roasted lentil powder 10 g, roasted lentil powder 10 g, molasses 5 g, molasses 5 g and soya oil 3 g and soya oil 3 g, to provide 150 g, to provide 150 kcal (~630 kcal (~630 kJ) of energy with 11% of the energy derived from protein. Both groups received multivitamin drops with a daily dose of 1 kJ) of energy with 11% of the energy derived from protein. Both groups received multivitamin drops with a daily dose of 1 ml providing vitamin‐A, vitamin‐D, thiamin, riboflavin, pyridoxine, nicotinamide, calcium, ascorbic acid and zinc sulphate, and from weeks 2 to 12, iron and folic acid were provided as standard treatment of severe malnutrition (micronutrient doses were not specified). ml providing vitamin‐A, vitamin‐D, thiamin, riboflavin, pyridoxine, nicotinamide, calcium, ascorbic acid and zinc sulphate, and from weeks 2 to 12, iron and folic acid were provided as standard treatment of severe malnutrition (micronutrient doses were not specified). | 6 |
BSID II: INT (67.9 ± ± 14.4) 14.4) = = CTRL (66.7 CTRL (66.7 ± ± 13.6) 13.6) | 0.09 |
BSID II: INT (66.2 ± ± 15.2) 15.2) = = CTRL (69.4 CTRL (69.4 ± ± 16.4) 16.4) | −0.20 |
WAZ: INT (−3.4 ± ± 0.8) 0.8) = = CTRL (−3.2 CTRL (−3.2 ± ± 1.1) HAZ: INT (−3.9 1.1) HAZ: INT (−3.9 ± ± 1.1) 1.1) = = CTRL (−4.1 CTRL (−4.1 ± ± 1.0) 1.0) | −0.21 0.40 | W, S, S, S, S, M, S, S | Moderate |
| Phuka et al. 2012 Malawi HDI .418 (Phuka et al. 2009; Phuka et al. 2012) |
N = = 163 INT n 163 INT n = = 51 CTRL n 51 CTRL n = = 56 Base age 56 Base age = = 6 6 m End age m End age = = 18 18 m Mean base HAZ m Mean base HAZ = = −1.6 −1.6 | RCT where Intervention given daily 50‐g lipid‐based spread fortified with 17 vitamins and minerals (256 kcal, 7‐g protein, 14‐g carbohydrate, 17‐g fat, 400‐mcg retinol, 160‐mcg folate, 6‐mg niacin, 2‐mg pantothenic acid, 0.5‐mg riboflavin, 0.5‐mg thiamin, 0.5‐mg vitamin B6, 0.9‐mcg vitamin B12, 30‐mg vitamin C, 5‐mcg vitamin D, 283‐mg calcium, 0.4‐mg copper, 135‐mcg iodine, 8‐mg iron, 60‐mg magnesium, 17‐mcg selenium and 8‐mg zinc). Controls given daily 71‐g corn‐soy flour with fewer micronutrients. kcal, 7‐g protein, 14‐g carbohydrate, 17‐g fat, 400‐mcg retinol, 160‐mcg folate, 6‐mg niacin, 2‐mg pantothenic acid, 0.5‐mg riboflavin, 0.5‐mg thiamin, 0.5‐mg vitamin B6, 0.9‐mcg vitamin B12, 30‐mg vitamin C, 5‐mcg vitamin D, 283‐mg calcium, 0.4‐mg copper, 135‐mcg iodine, 8‐mg iron, 60‐mg magnesium, 17‐mcg selenium and 8‐mg zinc). Controls given daily 71‐g corn‐soy flour with fewer micronutrients. | 12 |
Griffiths Mental Development: Cognitive: INT (18.25 ± ± 2.41) 2.41) = = CTRL (18.41 CTRL (18.41 ± ± 2.31) 2.31) | −0.07 |
Griffiths Mental Development: Gross motor (Locomotive): INT (15.81 ± ± 0.78) 0.78) = = CTRL (15.91 CTRL (15.91 ± ± 0.98) 0.98) | −0.11 |
WAZ: INT (−0.62 ± ± 1.04) 1.04) = = CTRL (−1.74 CTRL (−1.74 ± ± 1.07) HAZ: INT (−1.57 1.07) HAZ: INT (−1.57 ± ± 1.01) 1.01) = = CTRL(−1.64 CTRL(−1.64 ± ± 0.82) 0.82) | 1.06 0.08 | M, S, S, M, S, S, M, S | Strong |
| Surkan et al. 2013 Nepal HDI .463 (Surkan et al. 2013) |
N = = 569 INT Zn n 569 INT Zn n = = 127 INT Fe 127 INT Fe + + FA n FA n = = 129 INT 3 MMN n 129 INT 3 MMN n = = 161 CTRL n 161 CTRL n = = 152 Base age 152 Base age = = 4–17 4–17 m End age m End age = = 16–29 16–29 m m | Cluster randomized trial with three Interventions with daily supplement with Zn, Fe or both, and Control received placebo. INT Zn: 10 mg/d zinc INT Fe mg/d zinc INT Fe + + FA: 12.5 FA: 12.5 mg/d Fe; 50 mg/d Fe; 50 mcg/d FA INT 3 MMN (Zn mcg/d FA INT 3 MMN (Zn + + Fe Fe + + FA) Children <1 FA) Children <1 yr received half‐dose of supplement. yr received half‐dose of supplement. | 12 |
Fagen Test of Infant Intelligence: Language score: INT Zn (10.2 ± ± 3.39) 3.39) = = CTRL No Zn (10.35 CTRL No Zn (10.35 ± ± 2.92) INT Fe (10.2 2.92) INT Fe (10.2 ± ± 3.38) 3.38) = = CTRL No Fe (10.25 CTRL No Fe (10.25 ± ± 2.93) 2.93) | −0.05 −0.02 |
Griffiths Mental Development Scale and the MacArthur Communicative Development Inventory: INT Zn (23.8 ± ± 6.5) 6.5) = = CTRL No Zn (24.2 CTRL No Zn (24.2 ± ± 6.5) INT Fe (24.1 6.5) INT Fe (24.1 ± ± 6.5) 6.5) = = CTRL No Fe (24.0 CTRL No Fe (24.0 ± ± 6.5) 6.5) | −0.06 0.02 | S, S, S, S, W, S, S, M | Moderate | ||
| Yousafzai et al. 2014 Pakistan HDI .515 (Yousafzai et al. 2014) |
N = = 680 INT n 680 INT n = = 334 CTRL n 334 CTRL n = = 346 Base age 346 Base age = = 0–2.5 0–2.5 m End age m End age = = 24 24 m Mean base HAZ m Mean base HAZ = = −1.0 −1.0 | Cluster RCT, factorial design where Intervention received nutrition education and MMP and Control received standard care. MMP comprised of iron, folate, vitamin A and vitamin C (doses not specified). | 24 |
BSID III: Cognition: INT (76.5 ± ± 22.2) 22.2) > > CTRL (71.9 CTRL (71.9 ± ± 18.0)*** 18.0)***
| 0.20 |
BSID III: INT 87.8 (22.6) > > CTRL 81.9 (20.7)*** CTRL 81.9 (20.7)***
| d = = 0.20 0.20 | S, S, S, S, W, S, S, S | Moderate | ||
| Singla et al. 2014 Bangladesh HDI .515 (Singla et al. 2014) |
N = = 186 INT n 186 INT n = = 99 CTRL n 99 CTRL n = = 87 Base age 87 Base age = = 7–12 7–12 m End age m End age = = 16–22 16–22 m LBW children Mean base HAZ m LBW children Mean base HAZ = = −2.0 −2.0 | RCT where Intervention received daily 22‐element MMP plus education and control received education only. MMP contained 300‐mcg vitamin A, 5‐mcg vitamin D, 6‐mg vitamin E, 30‐mg vitamin C, 0.5‐mg vitamin B1, 0.5‐mg vitamin B2, 0.5‐mg vitamin B6, 0.5‐mcg vitamin B12, 6‐mg niacin, 160‐mcg FA, 10‐mg Fe, 10‐mg Zn, 0.5‐mg copper, 20‐mcg selenium, 90‐mcg iodine, 100‐mg calcium, 20‐mg magnesium, 100‐mg phosphorus, 0.6‐mg manganese, 20‐mcg vitamin K, 1.8‐mg pantothenic acid and 6‐mcg biotin. | 6 |
BSID III: Cognition: INT (51.2 ± ± 4.66) 4.66) = = CTRL (50.67 CTRL (50.67 ± ± 4.31) 4.31) | 0.08 | S, S, S, S, S, S, S, S | Strong | ||||
| Attanasio et al. 2014 Colombia HDI .719 (Attanasio et al. 2014) |
N = = 626 INT n 626 INT n = = 308 CTRL n 308 CTRL n = = 318 Base age 318 Base age = = 12–24 12–24 m End age m End age = = 20–42 20–42 m Base stunting m Base stunting = = 13.0% 13.0% | Cluster RCT where Intervention group received micronutrient sprinkles and Control group received nothing. Sprinkles contained 12.5‐mg iron, 5‐mg zinc, vitamin A 300‐mcg retinol equivalents, 160‐mcg folic acid and 30‐mg vitamin C. | 18 |
BSID III: Cognition: INT (71.63 ± ± 4.26) 4.26) = = CTRL (71.68 CTRL (71.68 ± ± 4.38) 4.38) | −0.01 |
BSID III: Gross Motor: INT (63.19 ± ± 2.99) 2.99) = = CTRL (63.31 CTRL (63.31 ± ± 2.79) 2.79) | −0.04 | M, S, S, S, W, S, S, S | Moderate | ||
| Colombo et al. 2014 Peru HDI .741 (Colombo et al. 2014) |
N = = 249 INT n 249 INT n = = 128 CTRL n 128 CTRL n = = 121 Base age 121 Base age = = 6 6 m End age m End age = = 18 18 m Mean base HAZ m Mean base HAZ = = −0.5 −0.5 | RCT where Intervention group received a daily liquid supplement containing 10 mg/d of zinc (zinc sulphate), 10 mg/d of zinc (zinc sulphate), 10 mg/d of iron (ferrous sulphate) and 0.5 mg/d of iron (ferrous sulphate) and 0.5 mg/d of copper (copper oxide), and Control group received an identical daily liquid supplement containing only 10 mg/d of copper (copper oxide), and Control group received an identical daily liquid supplement containing only 10 mg/d of iron and 0.5 mg/d of iron and 0.5 mg/d of copper. mg/d of copper. | 12 |
BSID II: INT (94.98 ± ± 6.95) 6.95) = = CTRL (94.43 CTRL (94.43 ± ± 6.76) 6.76) | 0.08 |
BSID II: INT (104.10 ± ± 5.77) 5.77) = = CTRL (103.92 CTRL (103.92 ± ± 6.10) 6.10) | 0.03 |
WAZ: INT (0.1 ± ± 0.9) 0.9) = = CTRL (0.1 CTRL (0.1 ± ± 0.8) HAZ: INT (−0.5 0.8) HAZ: INT (−0.5 ± ± 1.0) 1.0) = = CTRL (−0.6 CTRL (−0.6 ± ± 0.9) 0.9) | 0 0.11 | S, S, S, S, W, S, S, S | Moderate |
Note: Assessment of Quality (Item Ratings) with following categories in order: selection bias, study design, confounders, blinding, data collection methods, withdrawals and dropouts, intervention integrity, analysis; HDI, Human Development Index; RCT, Randomized Controlled Trial; m, month; wk, week; INT, Intervention group; CTRL, Control group; HAZ, height‐for‐age z‐score; BSID, Bayley Scales of Infant Development; effect size d, standardized mean difference; SD, standard deviation; RDA, recommended dietary allowance; W, weak; M, moderate; S, strong; MMP, multiple micronutrient powder; MMN, multiple micronutrients; MV, multivitamin; Zn, zinc; Fe, iron; FA, folic acid.
 <
< 0.05;
0.05; <
< 0.01;
0.01; <
< 0.001.
0.001.Most studies were conducted in low‐income countries from Africa and South Asia. Some were conducted in Latin America, where countries have higher Human Development Indexes; nonetheless samples included malnourished children from urban slums or rural sites.
The prenatal studies used supplementation in the second and/or third trimesters of pregnancy. Most of the postnatal nutrition interventions started when the child was six months of age. However, six trials began within the first two months after birth. The duration of the interventions ranged from two months to 24 months, with a mode of six months.
months, with a mode of six months.
The majority of studies used zinc, iron/folic acid, vitamin A, iodine or multiple micronutrients (n =
= 24). Others looked at the effect of supplementation with fatty acids or food supplements (n
24). Others looked at the effect of supplementation with fatty acids or food supplements (n =
= 8), and one gave a calorie‐ and protein‐dense milk supplement. With respect to the comparison group, the majority of studies provided a placebo or nothing (n
8), and one gave a calorie‐ and protein‐dense milk supplement. With respect to the comparison group, the majority of studies provided a placebo or nothing (n =
= 19), and the remainder provided either fewer micronutrients or lower energy supplements.
19), and the remainder provided either fewer micronutrients or lower energy supplements.
Mental development tests used
Almost all of the studies included in this meta‐analysis used a direct assessment of the child, where a sequence of tasks, ordered in terms of level of difficulty, is given to the child and scored as pass or fail. Items involve measuring competencies related to cognition, expressive and receptive language and fine motor skills.
Twenty‐four studies used the Bayley Scales of Infant Development (BSID‐I, ‐II or ‐III)—Mental Scale (Bayley 2006). Four studies instead administered the Griffiths Mental Developmental Scale (Griffiths 1996), while single studies used the Fagan Test of Infant Intelligence (Fagan & Shepard 1986), a language test derived from BSID II, language milestones or a two‐item problem‐solving test included in the BSID II. Most studies were unable to separate language and cognition subscores and so the effect of the intervention on these distinct outcomes is not clear. When language and cognition were measured separately, cognition was used to calculate the effect size for mental development. The Bayley, Griffith and milestones were used to measure gross motor development if included as an outcome in the study.
Effects of nutrition interventions on mental development
Regarding prenatal supplementation interventions, the mean effect size for mental development was very small and non‐significant at d =
= 0.042 (95% CI: −0.0084, 0.092) (n
0.042 (95% CI: −0.0084, 0.092) (n =
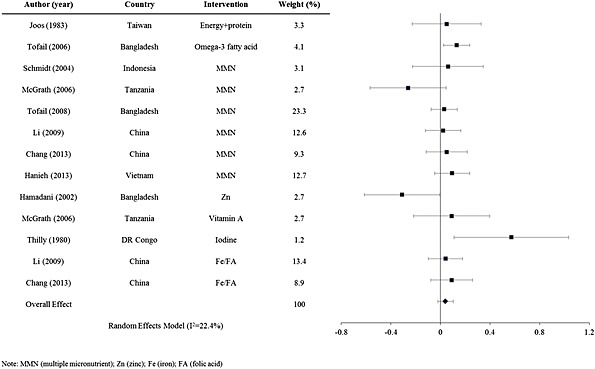
= 10 studies, 5352 participants), ranging from −0.31 to 0.57. The forest plot for mental scores from prenatal supplementation interventions is shown in Fig. Fig.2.2. Most of the interventions had very little positive effect on mental development, and some even had significant negative effects. However, the 10 studies in this group were quite heterogeneous with respect to intervention, preventing analysis of trends among similar studies.
10 studies, 5352 participants), ranging from −0.31 to 0.57. The forest plot for mental scores from prenatal supplementation interventions is shown in Fig. Fig.2.2. Most of the interventions had very little positive effect on mental development, and some even had significant negative effects. However, the 10 studies in this group were quite heterogeneous with respect to intervention, preventing analysis of trends among similar studies.
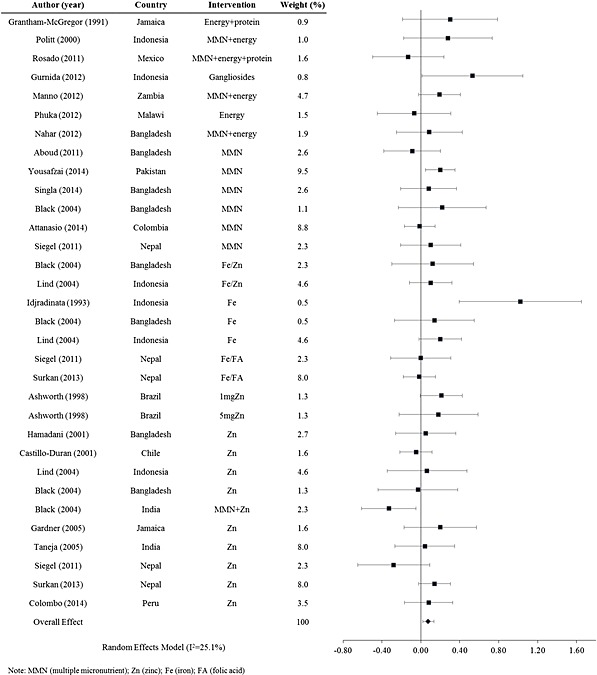
Looking next at postnatal trials, nutrition interventions resulted in a significant mean effect size Cohen's d for mental development of d =
= 0.076 (95% CI: 0.019, 0.13) (n
0.076 (95% CI: 0.019, 0.13) (n =
= 23 studies, 6485 participants) with a range from −0.33 to 1.022. The forest plot for mental development scores from postnatal supplementation trials is shown in Fig. Fig.3.3. Excluding the outlier, the study by Idjradinata & Pollitt (1993), the effect size remained similar at d
23 studies, 6485 participants) with a range from −0.33 to 1.022. The forest plot for mental development scores from postnatal supplementation trials is shown in Fig. Fig.3.3. Excluding the outlier, the study by Idjradinata & Pollitt (1993), the effect size remained similar at d =
= 0.069 (95% CI: 0.021, 0.12) (n
0.069 (95% CI: 0.021, 0.12) (n =
= 22 studies, 6441 participants).
22 studies, 6441 participants).
Positive outcomes on mental development in postnatal interventions are seen with the use of calorie‐ and protein‐dense milk, gangliosides added to milk and the use of fortified porridge or a rice and lentil mixture (Grantham‐McGregor et al. 1991; Pollitt et al. 2000; Gurnida et al. 2012; Manno et al. 2012; Nahar et al. 2012). Multiple micronutrients, iron and folic acid and zinc interventions showed mixed results. Multiple micronutrient supplementation of children seems to have a slightly higher positive effect on mental development when compared with supplementation with only one micronutrient. When stratified by supplementation type, the weighted effect sizes for multiple micronutrient interventions (Fig. (Fig.4)4) and for single micronutrient interventions (Fig. (Fig.5)5) are d =
= 0.082 (95% CI: −0.012, 0.18) (n
0.082 (95% CI: −0.012, 0.18) (n =
= 6 interventions, 1915 participants) and d
6 interventions, 1915 participants) and d =
= 0.058 (95% CI: −0.0015, 0.12) (n
0.058 (95% CI: −0.0015, 0.12) (n =
= 19 interventions, 3803 participants), respectively.
19 interventions, 3803 participants), respectively.
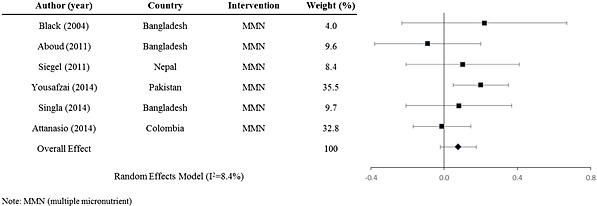
Forest plot for of mental development effect sizes for postnatal multiple micronutrient interventions.
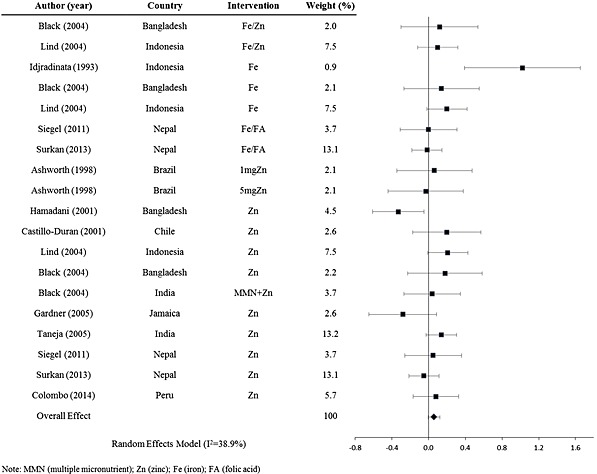
Forest plot for of mental development effect sizes for postnatal single micronutrient interventions.
There could be some added benefit from the provision of fats, energy and protein, When stratifying the analysis by interventions with energy, fat or protein compared with those giving one or more micronutrients, we see a slightly higher benefit from the former. The weighted effect size for interventions giving energy, fat, omega‐3 fatty acid or protein (Fig. 6) is d =
= 0.14 (95% CI: 0.0067, 0.27) (n
0.14 (95% CI: 0.0067, 0.27) (n =
= 7 interventions, 893 participants) and for interventions giving one or multiple micronutrients, it is d
7 interventions, 893 participants) and for interventions giving one or multiple micronutrients, it is d =
= 0.066 (95% CI: 0.016, 0.12) (n
0.066 (95% CI: 0.016, 0.12) (n =
= 25 interventions, 5592 participants).
25 interventions, 5592 participants).
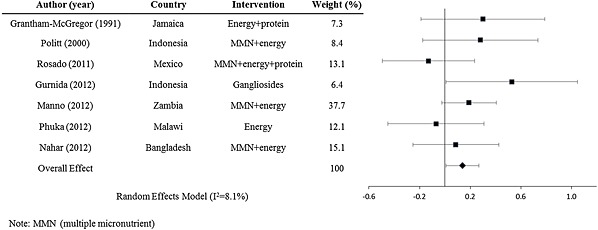
Forest plot for of mental development effect sizes for postnatal energy, protein and fat interventions.
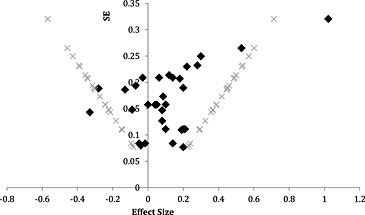
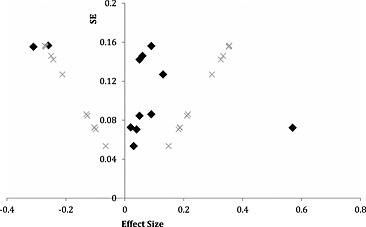
The funnel plot for postnatal interventions looks symmetrical (Fig. (Fig.7).7). Consequently we ruled out bias in publishing only studies with significant effects. Publication bias seems to be more of an issue with prenatal supplementation interventions. The funnel plot shown in Fig. Fig.88 is asymmetrical, with more studies with positive effect sizes. It should be noted that there are fewer prenatal supplementation trials compared with postnatal supplementation trials.
Using the random effects meta‐regression on mental development, we found that quality of the study, sample size and intervention type (micronutrient or energy given) were not significant predictors of either postnatal or prenatal effect size. Further, baseline HAZ (n =
= 19 interventions) was not a significant predictor of postnatal effect size and baseline maternal BMI (n
19 interventions) was not a significant predictor of postnatal effect size and baseline maternal BMI (n =
= 10 interventions) was not a significant predictor of prenatal effect size. A random effects model was fit to look at the association between motor and mental development and found that motor development effect size (n
10 interventions) was not a significant predictor of prenatal effect size. A random effects model was fit to look at the association between motor and mental development and found that motor development effect size (n =
= 11 prenatal interventions, n
11 prenatal interventions, n =
= 22 postnatal interventions) was significantly associated with mental development effect size (regression coefficient
22 postnatal interventions) was significantly associated with mental development effect size (regression coefficient =
= 0.32, 95% CI: 0.087, 0.54) in postnatal but not prenatal interventions. End‐line HAZ effect size (n
0.32, 95% CI: 0.087, 0.54) in postnatal but not prenatal interventions. End‐line HAZ effect size (n =
= 19 postnatal interventions), however, was not significantly associated with mental development.
19 postnatal interventions), however, was not significantly associated with mental development.
Discussion
Taken together, nutrition interventions did not seem to have a significant effect on mental (cognitive) development of children under‐two years in LMICs. The effect sizes were d =
= 0.042 for prenatal nutrition and d
0.042 for prenatal nutrition and d =
= 0.076 for postnatal nutrition. This meta‐analysis comparing interventions between studies, although not statistically significant, showed a trend toward more benefit on cognitive development from the provision of postnatal multiple micronutrients compared with a single micronutrient, and also for provision of fats, energy and protein with micronutrients compared with micronutrients alone. This suggests that the most promising approach is a combination of macro‐ and micronutrients; however, future work would need to examine these approaches further to determine significant benefits of combined macro and micronutrient supplementation with single approaches.
0.076 for postnatal nutrition. This meta‐analysis comparing interventions between studies, although not statistically significant, showed a trend toward more benefit on cognitive development from the provision of postnatal multiple micronutrients compared with a single micronutrient, and also for provision of fats, energy and protein with micronutrients compared with micronutrients alone. This suggests that the most promising approach is a combination of macro‐ and micronutrients; however, future work would need to examine these approaches further to determine significant benefits of combined macro and micronutrient supplementation with single approaches.
Several lines of evidence support the need for both macro and micronutrients in brain development and particularly in LMICs samples. For example, during infancy 20% of the body's energy is used to support brain structure and function (Raichle 2010). Furthermore, specific macro‐ and micronutrients gain added importance when considering how widespread is their presence in the brain. Fats have important functions in synaptogenesis, membrane function and the synthesis of myelin that coats neurons and are thought to speed processing (Georgieff 2007). Iron plays a role in myelination, transmitter synthesis and hippocampal energy metabolism in the neonatal period (Georgieff 2007). However, the relation between brain and behaviour is not always straightforward; a lack of correspondence may occur if secondary sites of the brain compensate for deficits in the primary site. For example, visual sites of the brain may compensate for deficits in the more efficient language sites for reading (Parviainen et al. 2006). The trend toward a larger impact of fats, calories and protein could be a function of the setting of these studies, all being in food insecure areas where both macro and micronutrients are lacking in diets. There is a need to explore the effect of fat, energy and protein provision in addition to micronutrients on cognitive outcomes of children in resource poor areas.
The effect sizes of motor development are significantly associated with those of mental development in postnatal interventions. One explanation offered by Brown and Pollitt and elaborated by Prado and Dewey is that better‐nourished children's motor ability (fine and gross) to interact with and explore their environment could positively affect their cognitive development (Brown & Pollitt 1996; Prado & Dewey 2014). Nutrition supplements did increase exploration and activity in one study (Aburto et al. 2010) but not in another (Meeks Gardner et al. 1995). However, the benefits of exploration and activity accrue only if they lead to mentally challenging stimulation, which was the case in the Aburto study when the combination of macro and micronutrients enhanced exploration (fine motor manipulation of play objects) but not activity (gross body movements) (2010). In sum, nutrition has the opportunity to enhance cognitive development when it supports fine motor skills that can be applied to stimulating play materials rather than gross motor skills that generate activity (Aburto et al. 2010). Motor development as a mediator in the relationship between nutrition and mental development cannot be conclusively established in this review because of their concurrent measurement, and future work would need to examine the temporality of this relationship; however, their significant relationship is indicative of this possibility and should be further investigated in appropriately designed interventions.
This review found no association between HAZ and mental scores effect sizes in postnatal interventions. However, a recent meta‐analysis of 68 observational studies in LMIC found an association of better HAZ with earlier walking and better motor scores, and for every unit increase of HAZ prior to two years of age, an improved +0.22 standard deviation unit increase of prospective mental development was observed (Sudfeld et al. 2015). Therefore, despite common cross‐sectional findings that height and mental development are strongly correlated (Grantham‐McGregor et al. 2007; Hadley et al. 2008; Olney et al. 2009; Barros et al. 2010; Servili et al. 2010), there is no evidence from these studies to explain why and the mechanisms warrant future investigation.
The degree of variation between studies and the limited number of prenatal trials makes it difficult to identify trends other than by intervention type. Out of all the studies included, 36% had a quality rating of strong. The majority of those rated as moderate or weak decreased their quality by not reporting validity and reliability of their developmental measure in their context, not including study participation rates, and because of high drop‐out rates.
We have added to previous systematic reviews by including more studies for pre‐ and postnatal nutrition, and by examining evidence for two explanatory variables, namely motor development and nutritional outcomes. This is a comprehensive meta‐analysis that compiles studies using single and multiple micronutrients, as well as various fats, energy and protein. Mean effect sizes for these different nutrition interventions could be calculated for only three categories (fats/energy/protein, multiple micronutrients and single micronutrient) and suggest a trend toward greater benefit for multiple micronutrients and fats/energy/protein.
There are a number of limitations to this analysis. The control, or comparison, groups of these studies did not all receive a placebo; some received calories, multiple micronutrients or a single nutrient. The effect size from the latter type of studies is likely smaller than what it would be had the control group received a placebo. Furthermore, not all studies looked at the nutritional outcomes and motor development of the children, so sufficient evidence for this explanation is still lacking. To see an effect on mental development in children aged under‐two years, supplementation may need to be provided over a longer duration. Being restricted to LMICs only, the overall small effect size could also be because of the children's lack of protein and energy, in general. This could influence growth, which would affect motor development and the children's ability to explore their environment, thus affecting their mental development (Prado & Dewey 2014).
Previous reviews have likewise not identified significant effects of nutrition interventions on mental development in children aged under‐two years (Ramakrishnan et al. 2009; Eilander et al. 2010; Leung et al. 2011; Pasricha et al. 2013; Aboud & Yousafzai 2015). Only two studies in this meta‐analysis were appropriately sampled for analysis of development outcomes using the Bayley test (Attanasio et al. 2014; Yousafzai et al. 2014); to detect an effect size of 0.25 or larger (a meaningful change in development scores), a power of 0.8 and an alpha of 0.05, a sample size of 175 per group is required. Second, the tests used to measure mental development in young children could be insensitive to the changes observed through a nutrition intervention and future studies could benefit from using tests and instruments that measure brain development and function on a finer scale (Cheatham et al. 2006; Colombo & Carlson 2012), such as functional near‐infrared spectroscopy and event‐related brain potential (ERP). For example, one ERP study identified memory deficits specific to infants of diabetic mothers that were not picked up by the Bayley mental test (Nelson et al. 2000). A constraint in the current available data from nutrition interventions in LMICs is the lack of outcome measures outside of the traditional cognitive assessments (e.g. BSID or Griffiths Mental Development Scales). Although we limited this meta‐analysis to studies in children under‐two, measuring the longitudinal effect of early nutrition intervention on mental development in older ages may capture benefits not observed earlier (Colombo & Carlson 2012). Only a few studies such as the Jamaican and Guatemalan cohorts have longitudinal measures of mental development (Stein et al. 2008; Martorell et al. 2010; Walker et al. 2011). In the case of the Jamaican cohort, the high‐energy nutrition intervention in children under two years did not confer a benefit on intelligence at 22 years of age (Walker et al. 2011). However, in the Guatemalan cohort, those exposed to a high‐energy intervention in the prenatal period and the first two years of life experienced improved intellectual functioning at 27 to 33
years of age (Walker et al. 2011). However, in the Guatemalan cohort, those exposed to a high‐energy intervention in the prenatal period and the first two years of life experienced improved intellectual functioning at 27 to 33 years of age (Stein et al. 2008). It would be important for future studies to measure development over time including after the age of two years, so that this data can be included in other meta‐analyses. In such studies, it would be important to do repeat measures to analyse and understand the pathways between early nutrition and subsequent development. Currently these data are highly limited in the literature from LMICs. There is also interest in combining nutrition with stimulation interventions to see whether additive or synergistic effects might be observed on mental development. Only a few studies have been appropriately designed to address this question and currently there is limited evidence on additive benefits, but more research is required on how to optimize integrated nutrition and stimulation packages of intervention (Grantham‐McGregor et al. 2014). Other combined packages of care with nutrition also warrant further investigation (e.g. nutrition and water, sanitation and hygiene).
years of age (Stein et al. 2008). It would be important for future studies to measure development over time including after the age of two years, so that this data can be included in other meta‐analyses. In such studies, it would be important to do repeat measures to analyse and understand the pathways between early nutrition and subsequent development. Currently these data are highly limited in the literature from LMICs. There is also interest in combining nutrition with stimulation interventions to see whether additive or synergistic effects might be observed on mental development. Only a few studies have been appropriately designed to address this question and currently there is limited evidence on additive benefits, but more research is required on how to optimize integrated nutrition and stimulation packages of intervention (Grantham‐McGregor et al. 2014). Other combined packages of care with nutrition also warrant further investigation (e.g. nutrition and water, sanitation and hygiene).
This meta‐analysis was done in response to the growing amount of literature and uncertainty around the effect of nutrition supplementation interventions on mental development in young children. Three promising avenues to pursue in terms of future research are identified. First, the combination of micro and macronutrients appear to be the most promising supplement in terms of its effect on mental development of young children, yet more needs to be done to investigate how variations in the study design and implementation influence the outcome. Second, the connection between nutrients and mediators of mental development, such as length, illness, temperament and motor development, needs to be examined more carefully. Third, more prenatal supplementation trials are needed to establish the effect of in‐utero nutritional gains on mental development. In all cases, early nutrition interventions warrant further investigation, beyond two years of age, to identify whether there is an impact in later childhood or adult functioning.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
L.M.L. and A.K.Y. performed the literature review and compiled the data. LML performed the analyses and wrote the manuscript. Both authors read and approved the final manuscript. We would like to acknowledge Frances E. Aboud for her help in revising this manuscript and Emory University's Nutrition and Health Sciences Program, and the Laney Graduate School for L.M.L.'s support in pursuing her PhD.
Notes
Larson L. M., and Yousafzai A. K. (2017) A meta‐analysis of nutrition interventions on mental development of children under‐two in low‐ and middle‐income countries, Maternal & Child Nutrition, 13, e12229. 10.1111/mcn.12229. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
References
- Aboud F.E. & Akhter S. (2011) A cluster‐randomized evaluation of a responsive stimulation and feeding intervention in Bangladesh. Pediatrics 127, e1191–e1197. [Abstract] [Google Scholar]
- Aboud F.E. & Yousafzai A.K. (2015) Global health and development in early childhood. Annual Review of Psychology 66, 433–457. [Abstract] [Google Scholar]
-
Aburto N.J., Ramirez‐Zea M., Neufeld L.M. & Flores‐Ayala R. (2010) The effect of nutritional supplementation on physical activity and exploratory behavior of Mexican infants aged 8–12
 months. European Journal of Clinical Nutrition
64, 644–651. [Abstract] [Google Scholar]
months. European Journal of Clinical Nutrition
64, 644–651. [Abstract] [Google Scholar] - Adolph K.E. & Tamis–LeMonda C.S. (2014) The costs and benefits of development: the transition from crawling to walking. Child Development Perspectives 8, 187–192. [Europe PMC free article] [Abstract] [Google Scholar]
- Ashworth A., Morris S.S., Lira P.I. & Grantham‐McGregor S.M. (1998) Zinc supplementation, mental development and behaviour in low birth weight term infants in northeast Brazil. European Journal of Clinical Nutrition 52, 223–227. [Abstract] [Google Scholar]
- Attanasio O.P., Fernández C., Fitzsimons E.O.A., Grantham‐McGregor S.M., Meghir C. & Rubio‐Codina M. (2014) Using the infrastructure of a conditional cash transfer program to deliver a scalable integrated early child development program in Colombia: cluster randomized controlled trial. BMJ 349, g5785. [Europe PMC free article] [Abstract] [Google Scholar]
- Barros A.J., Matijasevich A., Santos I.S. & Halpern R. (2010) Child development in a birth cohort: effect of child stimulation is stronger in less educated mothers. International Journal of Epidemiology 39, 285–294. [Europe PMC free article] [Abstract] [Google Scholar]
- Bayley N. (2006) Bayley Scales of Infant Development Manual, 3rd edn The Psychological Corporation: Antonio, TX. [Google Scholar]
- Beckett C., Durnin J.V., Aitchison T.C. & Pollitt E. (2000) Effects of an energy and micronutrient supplement on anthropometry in undernourished children in Indonesia. European Journal of Clinical Nutrition 54(Suppl 2), S52–S59. [Abstract] [Google Scholar]
-
Beyerlein A., Hadders‐Algra M., Kennedy K., Fewtrell M., Singhal A., Rosenfeld E., et al (2010) Infant formula supplementation with long‐chain polyunsaturated fatty acids has no effect on Bayley developmental scores at 18
 months of age—IPD meta‐analysis of 4 large clinical trials. Journal of Pediatrics Gastroenterology and Nutrition
50, 79–84. [Abstract] [Google Scholar]
months of age—IPD meta‐analysis of 4 large clinical trials. Journal of Pediatrics Gastroenterology and Nutrition
50, 79–84. [Abstract] [Google Scholar] - Black M.M., Baqui A.H., Zaman K., Ake Persson L., El Arifeen S., Le K., et al (2004a) Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. American Journal of Clinical Nutrition 80, 903–910. [Abstract] [Google Scholar]
- Black M.M., Sazawal S., Black R.E., Khosla S., Kumar J. & Menon V. (2004b) Cognitive and motor development among small‐for‐gestational‐age infants: impact of zinc supplementation, birth weight, and caregiving practices. Pediatrics 113, 1297–1305. [Europe PMC free article] [Abstract] [Google Scholar]
- Brown J.L. & Pollitt E. (1996) Malnutrition, poverty and intellectual development. Scientific American 274, 38–43. [Abstract] [Google Scholar]
- Castillo‐Duran C., Perales C.G., Hertrampf E.D., Marin V.B., Rivera F.A. & Icaza G. (2001) Effect of zinc supplementation on development and growth of Chilean infants. Journal of Pediatrics 138, 229–235. [Abstract] [Google Scholar]
- Chang S., Zeng L., Brouwer I.D., Kok F.J. & Yan H. (2013) Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics 131, e755–e763. [Abstract] [Google Scholar]
- Cheatham C.L., Colombo J. & Carlson S.E. (2006) N‐3 fatty acids and cognitive and visual acuity development: methodologic and conceptual considerations. American Journal of Clinical Nutrition 83, 1458s–1466s. [Abstract] [Google Scholar]
- Colombo J. & Carlson S.E. (2012) Is the measure the message: the BSID and nutritional interventions. Pediatrics 129, 1166–1167. [Europe PMC free article] [Abstract] [Google Scholar]
-
Colombo J., Zavaleta N., Kannass K.N., Lazarte F., Albornoz C., Kapa L.L., et al (2014) Zinc supplementation sustained normative neurodevelopment in a randomized, controlled trial of Peruvian infants aged 6–18
 months. Journal of Nutrition. 10.3945/jn.113.189365
[Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
months. Journal of Nutrition. 10.3945/jn.113.189365
[Europe PMC free article] [Abstract] [CrossRef] [Google Scholar] - Dewey K.G., Cohen R.J., Brown K.H. & Rivera L.L. (2001) Effects of exclusive breastfeeding for four versus six months on maternal nutritional status and infant motor development: results of two randomized trials in Honduras. Journal of Nutrition 131, 262–267. [Abstract] [Google Scholar]
- Dhingra P., Menon V.P., Sazawal S., Dhingra U., Marwah D., Sarkar A., et al (2004) Effect of fortification of milk with zinc and iron along with vitamins C, E, A and selenium on growth, iron status and development in preschool children—a community‐based double‐masked randomized trial. In: Report from the 2nd World Congress of Pediatric Gastroenterology, Hepatology and Nutrition.
- Eilander A., Gera T., Sachdev H.S., Transler C., van der Knaap H.C., Kok F.J., et al (2010) Multiple micronutrient supplementation for improving cognitive performance in children: systematic review of randomized controlled trials. American Journal of Clinical Nutrition 91, 115–130. [Abstract] [Google Scholar]
- Faber M., Kvalsvig J.D., Lombard C.J. & Benade A.J. (2005) Effect of a fortified maize‐meal porridge on anemia, micronutrient status, and motor development of infants. American Journal of Clinical Nutrition 82, 1032–1039. [Abstract] [Google Scholar]
- Fagan J.F. & Shepard P. (1986) The Fagan Test of Infant Intelligence. Infantest Corporation: Cleveland, OH: 87. [Google Scholar]
- Gardner J.M., Powell C.A., Baker‐Henningham H., Walker S.P., Cole T.J. & Grantham‐McGregor S.M. (2005) Zinc supplementation and psychosocial stimulation: effects on the development of undernourished Jamaican children. American Journal of Clinical Nutrition 82, 399–405. [Abstract] [Google Scholar]
- Georgieff M.K. (2007) Nutrition and the developing brain: nutrient priorities and measurement. American Journal of Clinical Nutrition 85, 614s–620s. [Abstract] [Google Scholar]
- Gibson R.S., Kafwembe E., Mwanza S., Gosset L., Bailey K.B., Mullen A., et al (2011) A micronutrient‐fortified food enhances iron and selenium status of Zambian infants but has limited efficacy on zinc. Journal of Nutrition 141, 935–943. [Abstract] [Google Scholar]
- Gould J.F., Smithers L.G. & Makrides M. (2013) The effect of maternal omega‐3 (n‐3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta‐analysis of randomized controlled trials. American Journal of Clinical Nutrition 97, 531–544. [Abstract] [Google Scholar]
-
Grantham‐McGregor S., Cheung Y.B., Cueto S., Glewwe P., Richter L. & Strupp B. (2007) Developmental potential in the first 5
 years for children in developing countries. Lancet
369, 60–70. [Europe PMC free article] [Abstract] [Google Scholar]
years for children in developing countries. Lancet
369, 60–70. [Europe PMC free article] [Abstract] [Google Scholar] - Grantham‐McGregor S.M., Fernald L.C., Kagawa R.M. & Walker S. (2014) Effects of integrated child development and nutrition interventions on child development and nutritional status. Annals of the New York Academy of Sciences 1308, 11–32. [Abstract] [Google Scholar]
- Grantham‐McGregor S.M., Powell C.A., Walker S.P. & Himes J.H. (1991) Nutritional supplementation, psychosocial stimulation, and mental development of stunted children: the Jamaican Study. Lancet 338, 1–5. [Abstract] [Google Scholar]
-
Griffiths R. (1996) GMDS 0‐2. Griffiths Mental Development Scales—Revised: Birth to 2
 Years. Hogrefe: Oxford. [Google Scholar]
Years. Hogrefe: Oxford. [Google Scholar] - Gurnida D.A., Rowan A.M., Idjradinata P., Muchtadi D. & Sekarwana N. (2012) Association of complex lipids containing gangliosides with cognitive development of 6‐month‐old infants. Early Human Development 88, 595–601. [Abstract] [Google Scholar]
- Hadley C., Tegegn A., Tessema F., Asefa M. & Galea S. (2008) Parental symptoms of common mental disorders and children's social, motor, and language development in sub‐Saharan Africa. Annals of Human Biology 35, 259–275. [Abstract] [Google Scholar]
- Hamadani J.D., Fuchs G.J., Osendarp S.J., Huda S.N. & Grantham‐McGregor S.M. (2002) Zinc supplementation during pregnancy and effects on mental development and behaviour of infants: a follow‐up study. Lancet 360, 290–294. [Abstract] [Google Scholar]
- Hamadani J.D., Fuchs G.J., Osendarp S.J., Khatun F., Huda S.N. & Grantham‐McGregor S.M. (2001) Randomized controlled trial of the effect of zinc supplementation on the mental development of Bangladeshi infants. American Journal of Clinical Nutrition 74, 381–386. [Abstract] [Google Scholar]
- Hanieh S., Ha T.T., Simpson J.A., Casey G.J., Khuong N.C., Thoang D.D., et al (2013) The effect of intermittent antenatal iron supplementation on maternal and infant outcomes in rural Viet Nam: a cluster randomised trial. PLoS Medicine 10, e1001470. [Europe PMC free article] [Abstract] [Google Scholar]
- Idjradinata P. & Pollitt E. (1993) Reversal of developmental delays in iron‐deficient anaemic infants treated with iron. Lancet 341, 1–4. [Abstract] [Google Scholar]
- Jackson N. & Waters E. (2005) Criteria for the systematic review of health promotion and public health interventions. Health Promotion International 20, 367–374. [Abstract] [Google Scholar]
- Joos S.K., Pollitt E., Mueller W.H. & Albright D.L. (1983) The Bacon Chow study: maternal nutritional supplementation and infant behavioral development. Child Development 54, 669–676. [Abstract] [Google Scholar]
- Leung B.M., Wiens K.P. & Kaplan B.J. (2011) Does prenatal micronutrient supplementation improve children's mental development? A systematic review. BMC Pregnancy and Childbirth 11, 12. [Europe PMC free article] [Abstract] [Google Scholar]
- Li Q., Yan H., Zeng L., Cheng Y., Liang W., Dang S., et al (2009) Effects of maternal multimicronutrient supplementation on the mental development of infants in rural western China: follow‐up evaluation of a double‐blind, randomized, controlled trial. Pediatrics 123, e685–e692. [Abstract] [Google Scholar]
- Lind T., Lonnerdal B., Stenlund H., Gamayanti I.L., Ismail D., Seswandhana R., et al (2004) A community‐based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. American Journal of Clinical Nutrition 80, 729–736. [Abstract] [Google Scholar]
- Manno D., Kowa P.K., Bwalya H.K., Siame J., Grantham‐McGregor S., Baisley K., et al (2012) Rich micronutrient fortification of locally produced infant food does not improve mental and motor development of Zambian infants: a randomised controlled trial. British Journal of Nutrition 107, 556–566. [Europe PMC free article] [Abstract] [Google Scholar]
- Martorell R., Melgar P., Maluccio J.A., Stein A.D. & Rivera J.A. (2010) The nutrition intervention improved adult human capital and economic productivity. Journal of Nutrition 140, 411–414. [Europe PMC free article] [Abstract] [Google Scholar]
- McGrath N., Bellinger D., Robins J., Msamanga G.I., Tronick E. & Fawzi W.W. (2006) Effect of maternal multivitamin supplementation on the mental and psychomotor development of children who are born to HIV‐1‐infected mothers in Tanzania. Pediatrics 117, e216–e225. [Abstract] [Google Scholar]
- Meeks Gardner J., Grantham‐McGregor S.M., Chang S.M., Himes J.H. & Powell C.A. (1995) Activity and behavioral development in stunted and nonstunted children and response to nutritional supplementation. Child Development 66, 1785–1797. [Abstract] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D.G. (2009) Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 151, 264–269. [Abstract] [Google Scholar]
- Nahar B., Hossain M.I., Hamadani J.D., Ahmed T., Huda S.N., Grantham‐McGregor S.M., et al (2012) Effects of a community‐based approach of food and psychosocial stimulation on growth and development of severely malnourished children in Bangladesh: a randomised trial. European Journal of Clinical Nutrition 66, 701–709. [Abstract] [Google Scholar]
- Nelson C.A., Wewerka S., Thomas K.M., de Regnier R.‐a., Tribbey‐Walbridge S. & Georgieff M. (2000) Neurocognitive sequelae of infants of diabetic mothers. Behavioral Neuroscience 114, 950. [Abstract] [Google Scholar]
- Olney D.K., Kariger P.K., Stoltzfus R.J., Khalfan S.S., Ali N.S., Tielsch J.M., et al (2009) Development of nutritionally at‐risk young children is predicted by malaria, anemia, and stunting in Pemba, Zanzibar. Journal of Nutrition 139, 763–772. [Abstract] [Google Scholar]
- Olney D.K., Pollitt E., Kariger P.K., Khalfan S.S., Ali N.S., Tielsch J.M., et al (2006) Combined iron and folic acid supplementation with or without zinc reduces time to walking unassisted among Zanzibari infants 5‐ to 11‐mo old. Journal of Nutrition 136, 2427–2434. [Abstract] [Google Scholar]
- Parviainen T., Helenius P., Poskiparta E., Niemi P. & Salmelin R. (2006) Cortical sequence of word perception in beginning readers. Journal of Neuroscience 26, 6052–6061. [Europe PMC free article] [Abstract] [Google Scholar]
-
Pasricha S.‐R., Hayes E., Kalumba K. & Biggs B.‐A. (2013) Effect of daily iron supplementation on health in children aged 4–23
 months: a systematic review and meta‐analysis of randomised controlled trials. The Lancet Global Health
1, e77–e86. [Abstract] [Google Scholar]
months: a systematic review and meta‐analysis of randomised controlled trials. The Lancet Global Health
1, e77–e86. [Abstract] [Google Scholar] - Phuka J.C., Gladstone M., Maleta K., Thakwalakwa C., Cheung Y.B., Briend A., et al (2012) Developmental outcomes among 18‐month‐old Malawians after a year of complementary feeding with lipid‐based nutrient supplements or corn‐soy flour. Maternal & Child Nutrition 8, 239–248. [Europe PMC free article] [Abstract] [Google Scholar]
- Phuka J.C., Maleta K., Thakwalakwa C., Cheung Y.B., Briend A., Manary M.J., et al (2009) Postintervention growth of Malawian children who received 12‐mo dietary complementation with a lipid‐based nutrient supplement or maize‐soy flour. American Journal of Clinical Nutrition 89, 382–390. [Abstract] [Google Scholar]
- Pollitt E. (2000) A developmental view of the undernourished child: background and purpose of the study in Pangalengan, Indonesia. European Journal of Clinical Nutrition 54 (Suppl 2), S2–S10. [Abstract] [Google Scholar]
- Pollitt E., Saco‐Pollitt C., Jahari A., Husaini M.A. & Huang J. (2000) Effects of an energy and micronutrient supplement on mental development and behavior under natural conditions in undernourished children in Indonesia. European Journal of Clinical Nutrition 54(Suppl 2), S80–S90. [Abstract] [Google Scholar]
- Prado E.L. & Dewey K.G. (2014) Nutrition and brain development in early life. Nutrition Reviews 72, 267–284. [Abstract] [Google Scholar]
- Qawasmi A., Landeros‐Weisenberger A. & Bloch M.H. (2013) Meta‐analysis of LCPUFA supplementation of infant formula and visual acuity. Pediatrics 131, e262–e272. [Europe PMC free article] [Abstract] [Google Scholar]
- Qawasmi A., Landeros‐Weisenberger A., Leckman J.F. & Bloch M.H. (2012) Meta‐analysis of long‐chain polyunsaturated fatty acid supplementation of formula and infant cognition. Pediatrics 129, 1141–1149. [Europe PMC free article] [Abstract] [Google Scholar]
- Raichle M.E. (2010) Two views of brain function. Trends in Cognitive Sciences 14, 180–190. [Abstract] [Google Scholar]
-
Ramakrishnan U., Nguyen P. & Martorell R. (2009) Effects of micronutrients on growth of children under 5
 y of age: meta‐analyses of single and multiple nutrient interventions. American Journal of Clinical Nutrition
89, 191–203. [Abstract] [Google Scholar]
y of age: meta‐analyses of single and multiple nutrient interventions. American Journal of Clinical Nutrition
89, 191–203. [Abstract] [Google Scholar] -
Rosado J.L., Lopez P., Garcia O.P., Alatorre J. & Alvarado C. (2011) Effectiveness of the nutritional supplement used in the Mexican Oportunidades programme on growth, anaemia, morbidity and cognitive development in children aged 12–24
 months. Public Health Nutrition
14, 931–937. [Abstract] [Google Scholar]
months. Public Health Nutrition
14, 931–937. [Abstract] [Google Scholar] - Schmidt M.K., Muslimatun S., West C.E., Schultink W. & Hautvast J.G. (2004) Mental and psychomotor development in Indonesian infants of mothers supplemented with vitamin A in addition to iron during pregnancy. British Journal of Nutrition 91, 279–286. [Abstract] [Google Scholar]
- Servili C., Medhin G., Hanlon C., Tomlinson M., Worku B., Baheretibeb Y., et al (2010) Maternal common mental disorders and infant development in Ethiopia: the P‐MaMiE Birth Cohort. BMC Public Health 10, 693. [Europe PMC free article] [Abstract] [Google Scholar]
- Siegel E.H., Kordas K., Stoltzfus R.J., Katz J., Khatry S.K., LeClerq S.C., et al (2011) Inconsistent effects of iron‐folic acid and/or zinc supplementation on the cognitive development of infants. Journal of Health, Population, and Nutrition 29, 593–604. [Europe PMC free article] [Abstract] [Google Scholar]
- Singla D.R., Shafique S., Zlotkin S.H. & Aboud F.E. (2014) A 22-Element Micronutrient Powder Benefits Language but Not Cognition in Bangladeshi Full-Term Low-Birth-Weight Children. The Journal of Nutrition 144, 1803–1810. [Abstract] [Google Scholar]
- Smithers L.G., Gibson R.A., McPhee A. & Makrides M. (2008) Effect of long‐chain polyunsaturated fatty acid supplementation of preterm infants on disease risk and neurodevelopment: a systematic review of randomized controlled trials. American Journal of Clinical Nutrition 87, 912–920 [Abstract] [Google Scholar]
- Stein A.D., Wang M., DiGirolamo A., Grajeda R., Ramakrishnan U., Ramirez‐Zea M., et al (2008) Nutritional supplementation in early childhood, schooling, and intellectual functioning in adulthood: a prospective study in Guatemala. Archives of Pediatrics and Adolescent Medicine 162, 612–618. [Europe PMC free article] [Abstract] [Google Scholar]
- Sudfeld C.R., McCoy D.C., Danaei G., Fink G., Ezzati M., Andrews K.G., et al (2015) Linear growth and child development in low‐ and middle‐income countries: a meta‐analysis. Pediatrics 135, e1266–e1275. [Abstract] [Google Scholar]
- Surkan P.J., Siegel E.H., Patel S.A., Katz J., Khatry S.K., Stoltzfus R.J., et al (2013) Effects of zinc and iron supplementation fail to improve motor and language milestone scores of infants and toddlers. Nutrition 29, 542–548. [Europe PMC free article] [Abstract] [Google Scholar]
-
Taneja S., Bhandari N., Bahl R. & Bhan M.K. (2005) Impact of zinc supplementation on mental and psychomotor scores of children aged 12 to 18
 months: a randomized, double‐blind trial. Journal of Pediatrics
146, 506–511. [Abstract] [Google Scholar]
months: a randomized, double‐blind trial. Journal of Pediatrics
146, 506–511. [Abstract] [Google Scholar] - The Chilenje Infant Growth, Nutrition and Infection (CIGNIS) Study Team (2010)Micronutrient fortification to improve growth and health of maternally HIV-unexposed and exposed Zambian infants: a randomised controlled trial. PLoS One 5. [Europe PMC free article] [Abstract]
- Thilly C.H. (1981) Psychomotor development in regions with endemic goiter In: Fetal Brain Disorders: Recent Approaches to the Problem of Mental Deficiency (eds Hetzel B.S. & Smith R.M.). Elsevier/North‐Holland Biomedical Press: New York, NY, USA. [Google Scholar]
- Thilly C.H., Lagasse R., Roger G., Bourdoux P., Ermans A.M. (1980a) Impaired fetal and postanal development and high perinatal death in a severe iodine deficient area. In: Thyroid Research VIII: Proceedings of the Eighth International Thyroid Congress, Sydney, Australia, 3–8 February, 1980. (ed J.R. Stockigt, Nagataki, S.). Pergamon, Oxford, NY, USA.
- Thilly C.H., Roger G., Lagasse R., Tshibangu D., Vanderpas J.B., Berquist, H. , et al (1980b) Fetomaternal relationship, fetal hypothyroidism, and psychomotor retardation In: Role of Cassava in the Etiology of Endemic Goitre and Cretinism (eds Ermans A.M., Mbulamoko N.M., Delange F. & Ahluwalia R.). International Development Research Centre: Ottawa, Canada. [Google Scholar]
- Tofail F., Kabir I., Hamadani J.D., Chowdhury F., Yesmin S., Mehreen F., et al (2006) Supplementation of fish‐oil and soy‐oil during pregnancy and psychomotor development of infants. Journal of Health, Population, and Nutrition 24, 48–56. [Abstract] [Google Scholar]
- Tofail F., Persson L.A., El Arifeen S., Hamadani J.D., Mehrin F., Ridout D., et al (2008) Effects of prenatal food and micronutrient supplementation on infant development: a randomized trial from the Maternal and Infant Nutrition Interventions, Matlab (MINIMat) study. American Journal of Clinical Nutrition 87, 704–711. [Abstract] [Google Scholar]
- Walker S.P., Chang S.M., Vera‐Hernandez M. & Grantham‐McGregor S. (2011) Early childhood stimulation benefits adult competence and reduces violent behavior. Pediatrics 127, 849–857. [Abstract] [Google Scholar]
- Werker J.F. & Tees R.C. (2005) Speech perception as a window for understanding plasticity and commitment in language systems of the brain. Developmental Psychobiology 46, 233–251. [Abstract] [Google Scholar]
- Yousafzai A.K., Rasheed M.A., Rizvi A., Armstrong R. & Bhutta Z.A. (2014) Effect of integrated responsive stimulation and nutrition interventions in the Lady Health Worker programme in Pakistan on child development, growth, and health outcomes: a cluster‐randomised factorial effectiveness trial. The Lancet. [Abstract] [Google Scholar]
Articles from Maternal & Child Nutrition are provided here courtesy of Wiley
Full text links
Read article at publisher's site: https://doi.org/10.1111/mcn.12229
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6866072?pdf=render
Citations & impact
Impact metrics
Article citations
Developing a context-relevant psychosocial stimulation intervention to promote cognitive development of children with severe acute malnutrition in Mwanza, Tanzania.
PLoS One, 19(5):e0285240, 09 May 2024
Cited by: 0 articles | PMID: 38722956 | PMCID: PMC11081340
Oral nutritional supplementation with dietary counseling improves linear catch-up growth and health outcomes in children with or at risk of undernutrition: a randomized controlled trial.
Front Nutr, 11:1341963, 10 Jul 2024
Cited by: 1 article | PMID: 39050140 | PMCID: PMC11266289
Engaging Fathers for Effective Child Nutrition and Development in Tanzania (EFFECTS): study protocol for a five-arm, cluster-randomized trial.
Trials, 25(1):188, 14 Mar 2024
Cited by: 0 articles | PMID: 38486278 | PMCID: PMC10938806
Attitude towards healthy nutrition and mental toughness: a study of taekwondo athletes.
PeerJ, 12:e17174, 29 Mar 2024
Cited by: 0 articles | PMID: 38563010 | PMCID: PMC10984172
Developmental and Nutritional Changes in Children with Severe Acute Malnutrition Provided with n-3 Fatty Acids Improved Ready-to-Use Therapeutic Food and Psychosocial Support: A Pilot Study in Tanzania.
Nutrients, 16(5):692, 28 Feb 2024
Cited by: 2 articles | PMID: 38474820 | PMCID: PMC10934689
Go to all (40) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association of Early Interventions With Birth Outcomes and Child Linear Growth in Low-Income and Middle-Income Countries: Bayesian Network Meta-analyses of Randomized Clinical Trials.
JAMA Netw Open, 2(7):e197871, 03 Jul 2019
Cited by: 16 articles | PMID: 31348509 | PMCID: PMC6661710
Nutrition and maternal, neonatal, and child health.
Semin Perinatol, 39(5):361-372, 10 Jul 2015
Cited by: 100 articles | PMID: 26166560
Review
Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review).
Evid Based Child Health, 8(1):112-201, 01 Jan 2013
Cited by: 99 articles | PMID: 23878126
Review
Effects of Preventive Nutrition Interventions among Adolescents on Health and Nutritional Status in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis.
Nutrients, 12(1):E49, 23 Dec 2019
Cited by: 10 articles | PMID: 31878019 | PMCID: PMC7019616
Review Free full text in Europe PMC
 1
and
1
and