Abstract
Free full text

A comprehensive review of amyotrophic lateral sclerosis
Abstract
Amyotrophic lateral sclerosis (ALS) is a late-onset fatal neurodegenerative disease affecting motor neurons with an incidence of about 1/100,000. Most ALS cases are sporadic, but 5–10% of the cases are familial ALS. Both sporadic and familial ALS (FALS) are associated with degeneration of cortical and spinal motor neurons. The etiology of ALS remains unknown. However, mutations of superoxide dismutase 1 have been known as the most common cause of FALS. In this study, we provide a comprehensive review of ALS. We cover all aspects of the disease including epidemiology, comorbidities, environmental risk factor, molecular mechanism, genetic factors, symptoms, diagnostic, treatment, and even the available supplement and management of ALS. This will provide the reader with an advantage of receiving a broad range of information about the disease.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a fatal motor neuron disorder that is, characterized by progressive loss of the upper and lower motor neurons (LMNs) at the spinal or bulbar level.[251]
ALS was first described in 1869 by French neurologist Jean-Martin Charcot.[213,251,286,323] The disease became well known in the United States when baseball player Lou Gehrig was diagnosed with the disease in 1939.[165,213] ALS is also known as Charcot disease in honor of the first person to describe the disease, Jean-Martin Charcot, and motor neuron disease (MND) as it is one of the five MNDs that affect motor neurons. There are four other known MNDs: Primary lateral sclerosis (PLS), progressive muscular atrophy (PMA), progressive bulbar palsy (PBP), and pseudobulbar palsy.[165,213,251,286]
ALS is categorized in two forms. The most common form is sporadic (90–95%) which has no obvious genetically inherited component. The remaining 5–10% of the cases are familial-type ALS (FALS) due to their associated genetic dominant inheritance factor.[2,107,289,299] The first onset of symptoms is usually between the ages of 50 and 65.[169,170,186,228] The most common symptoms that appear in both types of ALS are muscle weakness, twitching, and cramping, which eventually can lead to the impairment of muscles.[101,323] In the most advanced stages, ALS patients will develop symptoms of dyspnea and dysphagia.[164,238]
Most of the reviews about ALS focus on a specific area of the diseases such as molecular mechanism, treatment, diagnostic, etc. This review will attempt to provide an up-to-date overview of all aspects of ALS. It will first cover the epidemiology and comorbidities of the disease, followed by known environmental risk factors such as smoking, chemical exposure, and radiation.
Improving the understanding of ALS pathogenesis is critical in developing earlier diagnostic methods as well as proposing new effective treatments. Thus, this review will present the most recent studies related to molecular mechanisms, genetics, ALS symptoms, diagnostic examinations, and treatments. Furthermore, due to the fact that there has been only one Food and Drug Administration (FDA) approved drug for ALS treatment, this review will also address nutritional supplements, as well as respiratory and nutritional managements that help alleviating the symptoms. This comprehensive study will inevitably lead to the better understanding of ALS and assist in extending the life expectancy associated with ALS by establishing a basis of knowledge that can be used to improve care.
THE EPIDEMIOLOGY OF AMYOTROPHIC LATERAL SCLEROSIS
During 1990's, the number of reported cases of ALS was between 1.5 and 2.7 per 100,000 in Europe and North America.[170,228,326] Recent studies have shown that disease prevalence has not increased over the past decade, as the incidence rate remains at 2.7/100,000 (95% confidence interval [CI] 2.63–2.91)[146,169,228] and as of 2008, the prevalence of ALS was 0.32/100,000 (95% CI 9.78–10.86).[2,170,228,305] Multiple studies have shown an increased risk in the male to female ratio (male: female =1.5:1),[123,157,170,228,305] although other studies have shown a balance in this ratio.[2,168,326]
The mean age of onset of ALS varies from 50 to 65 years with the median age of onset of 64 years old. Only 5% of the cases have an onset <30 years of age.[169,170,228,286] ALS incidence is most pronounced in people 80 years or older (10.2/100,00 in men; 6.1/100,000 in women). One hypothesis for the increased incidence in older population is related to the variation in care for these patients.[2,93,146]
Although most cases of ALS are sporadic, about 5% of the cases have a family history. The age of onset for FALS is about a decade earlier than for sporadic cases.[2,107,289,299] There is also geographical loci form of ALS where prevalence is 50–100 times higher in certain locations than in any other part of the world. These population include parts of Japan, Guam, Kii Peninsula of Japan, and South West New Guinea.[157,158,159] Although this evidence is not concrete, it is believed that the increased incidence of ALS in these regions is due to environmental factors, specifically a neurotoxic nonprotein amino acid, β–methylamino-L-alanine (BMAA) in the seeds of the cycad Cycas micronesica produced by a symbiotic cyanobacteria in the roots of the cycad that are commonly found in these areas. It is hypothesized that patients in these regions who develop ALS have an inability in preventing BMAA accumulation.[34,159,206] More research is needed in South America to corroborate data for the rest of the continent, yet new studies show that the incidence of sporadic ALS in Uruguay is similar to those found within North America and Europe.[305,311] Thus, it is important to continue epidemiologic studies of ALS in areas where little work has been done to identify vulnerable populations within Africa, Central America, and South America.
A possible relationship between ALS and sports participation has been proposed but not demonstrated. In a cohort study of Italian professional football players, a severe increase in the incidence of ALS was found.[1,5,48] This study, conducted by the National Institute of Environmental Health Science, showed a correlation between head injuries in football players and an increased risk of ALS (odds ratio [OR] =3.2; 95% CI =1.2–8.1). Traumas to other parts of the body were not associated with increased risk.[46,48]
ANALYSIS OF CO-MORBIDITIES OF AMYOTROPHIC LATERAL SCLEROSIS
Retrospective cohort studies have shown that the incidence in certain concomitant diseases and comorbidities is significantly different in ALS-affected population in comparison to the general population. German cohort studies examining comorbidities prior to diagnosis found that while cardiovascular risk factors were the most common comorbidities in ALS patients (31.5% vs. 40%), they still had a significantly higher incidence in the general population than in their affected cohort.[105,127,151] The discrepancy in incidence could suggest that ALS causes a protective mechanism for cardiac disorders or that there are genetic benefits for those predisposed to the disorder. ALS patients had a significantly lower incidence of arterial hypertension (31.5% vs. 47.2%), coronary heart disease (8.6% vs. 9.3%), myocardial infarctions (6.4% vs. 7.0%), diabetes mellitus (7.2% vs. 10.6%), and hypercholesterolemia (17.9% vs. 65.6%).[105,151,325] These results were echoed when results were stratified by various age groups to prevent confounding. With regards to comparing ALS types and their onset time to patient comorbidities, only a difference in hypercholesterolemia incidence was seen when comparing bulbar onset to spinal onset (23.6% vs. 15.7%; P < 0.05).[151] This shows only a slight association with how cardiovascular comorbidities can alter ALS progression.
Of the diseases most likely to be found in ALS patients prior to their diagnosis, a higher incidence in neurological disorders was noted when compared to the general population.[127,151] The increased incidence of neurological diseases in ALS patients may be indicative of similar underlying genetic factors between ALS and other neurological disorders. A history of depression (22.8% vs. 11.6%), dementia (5.8% vs. 1.3%), Parkinson's (1.8% versus 0.1–0.2%), and epilepsy (1.6% versus 0.45–1%) was found more frequently within the study cohort than with the rest of the population.[38,77,151] The results were echoed in other studies that have indicated a greater likelihood of ALS development and progression when examining patients with a high rate of psychological disorders.[189]
Development of depression is one of the most common secondary symptoms associated with ALS. Previous studies have reported a prevalence of depression of 4–56% depending on the assessment measure.[24,95,113,242,287,322] Depression has a negative effect on the quality of life of patients with ALS.[52,156,173] In a recent study, 131 patients with ALS were evaluated to estimate the prevalence of depression.[116] The results showed 29% prevalence of mild depression and 6% prevalence of severe depression.[153] In this study, more than one-third of ALS patients were receiving antidepressant to treat sialorrhea, pseudobulbar affect, and insomnia, which may explain the lower rates of severe depression in ALS patients compared to the prevalence of depression in the general population (10%).[12,153] Physical impairment and duration of the disease did not predict depression in the cohort which suggests that depression is not related to advanced ALS or approaching end of life.[153] ALS-related symptoms, including cramps, stiffness, shortness of breath, swallowing difficulty, insomnia, loss of appetite, increased saliva, uncontrolled laughing or crying, fasciculations, anxiety, fatigue, and pain, did not appear to contribute to depression with the exception of anxiety which is a known symptom of depression.[153] Other studies have also shown that, even in late stages of ALS, major depression is rare.[102,246]
When comparing the survival rates of patients with mental health comorbidities, it was noted that patients suffering from Parkinson's and ALS had a slower progression of their disease and a greater survival time (P = 0.03).[151] It may be possible that the pathological development of Parkinson's disease (PD) could slow down the effects of ALS onset by altering the speed of nervous degeneration and disease progression. It is also possible that the administration of L-DOPA or the decreased levels of endogenous dopamine could offer an antioxidant protective effect in those susceptible to ALS, similar to that seen in multiple studies comparing ALS incidence and Vitamin E consumption.[279,315] Greater evidence will be necessary to confirm correlations and association between these comorbidities and ALS incidence.
AMYOTROPHIC LATERAL SCLEROSIS ENVIRONMENTAL FACTORS
Previous epidemiologic studies have suggested that ALS patients may have been exposed to environmental toxins.[329] Exposures to agriculture chemicals, heavy metals, solvents, electrical magnetic fields, type of diet, dust/fibers/fumes, and physical activity were all examined for association with ALS.[211,329] The following section will discuss more in depth the role that each of these risk factors have with increased ALS incidence.
SMOKING
Cigarette smoke has been found to increase the probability of developing ALS through inflammation, oxidative stress, and neurotoxicity by heavy metals contained in cigarettes.[317,318] Among those that actively smoke, ALS risk is highest when they start smoking at a younger age.[318] It is not, however, associated with duration or intensity of smoking habits. Furthermore, exhaled cigarette smoke contains formaldehyde which is associated with higher mortality rates in ALS patients.[319] It is thought that cigarette smoking is the most consistent nongenetic risk factor for ALS.[318,319] Finally, a beneficial effect of quitting smoking on ALS patient has not been examined so far.
Physical activity
Athletes have higher ALS risk compared to the general population; however, performing passive to robust physical activity has not shown an increased susceptibility of developing ALS.[123] Nevertheless, there are several genes (i.e., ciliary neurotrophic factor, leukemia inhibitory factor, and vascular endothelial growth factor 2) related to exercise that have been recognized as possible ALS risks factors.[47] Moreover, some studies have obtained inconsistent results in regards to high ALS risk among athletes, thus invalidating any association between physical activity and ALS risk.[17] Hence, the physical activity itself is not yet proven to be a cause of ALS. A possible explanation to the high risk of ALS incidence among athletes involves genetic profiles.
Genetic profiles that promote physical fitness but not necessarily muscles strength could hold a proportional correlation between ALS and physical activity.[298] In other words, a genetic profile altered by exogenous factors promoting physical fitness increases ALS susceptibility.[52,298] This idea is supported by findings of a beneficial vascular risk profile in patients and their relatives.[123] They reported a reduced frequency of coronary heart disease premorbid to ALS and an increased risk of ALS alongside physical fitness but not muscle strength, pointing at a mutual element between physical/cardiovascular fitness, and ALS susceptibility.[123,185,298,322]
Chemical exposure and metals
ALS has shown an association with exposure to agricultural chemicals such as pesticides, fertilizers, herbicides, insecticides, and formaldehyde.[319,329] In a prospective study, it was found that people who reported 4 or more years of exposure to pesticides/herbicides might be at an increased risk of acquiring ALS, but no association was found between mortality rate and amount of exposure. This study also found that among individuals with long period of exposure to formaldehyde, the ALS death rate was more than 2 times higher compared to those unexposed one.[319] Also, as formerly mentioned formaldehyde is a byproduct of cigarette smoke, this may account for 10–25% of indoor air formaldehyde exposure.
Among all the heavy metals that might be associated with ALS, lead exposure seems to be studied the most possibly due to the ALS-like symptoms experienced by people exposed to high concentrations of lead.[138] Since then, recent studies have found a correlation between lead exposure and ALS.[191] As such, professions related to lead exposures, such as welding, have demonstrated a significant association with developing ALS with odds ratio (ORs) ranging from 1.9 to 5.7.[133,191,261]
It is thought that lead's role in ALS has to do with its ability to substitute for calcium in intracellular reactions leading to damage the mitochondria, oxidative damage to neurons, and strengthen glutamate's excitotoxicity.[261,271] However, most case–control studies established the lead and ALS association through self-reported occupational lead exposure. When a group of researchers utilized an expert evaluation panel of industrial hygienists to examine self-reported occupational lead exposures, no association was found between ALS and lead exposure.[191] This suggests that recall bias might have interfered with collected data.[133,138] Hence, more studies are needed in order to establish fully this relationship.
Radiation/electromagnetic fields
Laboratory studies have demonstrated that in vitro exposures to extremely lowfrequency electromagnetic waves generate a bigger quantity of cellular reactive oxygen than normal.[269] In vivo, the same exposure produces oxidative stress and disables the antioxidant properties cells might have.[182] This oxidative damage can lead to ALS since oxidative stress has a role in ALS pathogenesis.[14] In fact, studies have observed that electromagnetic fields cause DNA strands to break in brain cells, leading to cell death (apoptosis and necrosis).[236] Such reaction could be the reason for the association between electromagnetic fields and ALS risk.[236] However, none of the current studies found a conclusive connection among electromagnetic field exposure, oxidative stress in neurons, and/or ALS development.[330]
Diet
Previous studies state that consuming high level of glutamate and fat can have adverse effects on ALS patients while Omega 3 fatty acids, Vitamin E, and fiber can have defensive impact.[211,306] According to previous studies, overstimulation of glutamate receptors leads to high intracellular calcium levels, which can initiate selective neuron death similar to ALS mechanism.[130] Glutamate is found in protein-rich foods, tomatoes, mushrooms, milk, and cheese.[216] Normally glutamate does not cross the blood-brain barrier, hence it is not known if dietary glutamate affects neurotransmission.[276] Moreover, there are areas of the brain called circumventricular organs, which are susceptible to plasma glutamate levels.[276] Omega 3 has been known to possess anti-inflammatory characteristics, which in turn would theoretically reduce inflammation caused by neuronal death.[76] Omega 3 in conjunction with Vitamin E has been reported to reduce ALS risks up to 60%.[306] These nutrients appear to act together in a summative way.
A large number of the information regarding environmental factors are based off questionnaires, all of which rely on subjects’ memories, leading to recall bias. Because of this, there may be a lack of information about the frequency and the amount of exposure to environmental factors. Also, this may also lead to the absence of biological markers in order to validate patient claims of exposure or pinpoint the possible action site.
Furthermore, due to ALS prolonged onset, it is difficult to isolate an exact environmental factor. In order to identify or narrow down possible ALS risk factors, a cohort study utilizing mice as a control and experimental group could be appropriate. Starting out with an emphasis on the most sought out factors (smoking, heavy metals, physical activity, diet, radiation, and chemical exposure). In order to track changes accurately, a type of biomarker specific to the possible risk factor could be designed, to theoretically track the progression of the disease.
MOLECULAR MECHANISM
Finding the molecular mechanisms by which motor neurons degenerate in ALS will aid in better understanding the disease's progress. Also, elucidation of molecular mechanisms can yield insight into developing strategies for newer treatments. The molecular basis of ALS is an intriguing issue that warrants in-depth research and investigation.
The most common cause of ALS is a mutation of the gene encoding the antioxidant enzyme superoxide dismutase 1 (SOD1).[63,70,129,233,304] Mutant SOD1 has a structural instability that causes a misfold in the mutated enzyme, which can lead to aggregation in the motor neurons within the central nervous system (CNS).[94] Several hypotheses have been proposed in regards to the mechanism underlying the mode of action of mutant SOD and the subsequent neurodegeneration seen in ALS. The most important proposed hypothesis for the pathogenesis of ALS includes glutamate excitotoxicity structural and functional abnormalities of mitochondria, impaired axonal structure or transport defects, and free radical-mediated oxidative stress.[69,78,94,132,178,207,265,317,331] Even though these mechanisms play a critical role in neurodegeneration, they all are considered as secondary events in the causes behind ALS onset.[312]
Glutamate excitotoxicity
Glutamate is synthesized in the presynaptic terminal. Uptake of glutamate into synaptic vesicles is facilitated by vesicular glutamate transporters.[266] During a normal neurotransmission process, glutamate is released into the synaptic cleft, where it activates postsynaptic receptors. Upon release of the vesicle, glutamate is removed from the synaptic cleft by several glial and neuronal cell transporter proteins, such as excitatory amino acid transporters (EAATs).[283] This continuous release and removal of glutamate maintain a concentration gradient balance and avoid the induction of excitotoxic neuronal damage.[263]
The motor cortex and spinal cord of ALS patients and transgenic SOD1 mouse model were found to have reduced astroglial glutamate transporter EAAT2, which leads to increased extracellular glutamate, over-stimulation of glutamate receptors, and excitotoxic neuronal degeneration.[167,297] Furthermore, this causes an excessive influx of calcium, excessive firing of motor neurons, and initiation of several destructive biochemical processes within the cell, which are all known as important pathophysiological processes in familial and sporadic forms of ALS.[126,313] Glutamate excitotoxicity contributes to the neurodegeneration either through activation of Ca2+-dependent enzymatic pathways by increasing the influx of Na+ and Ca2+ ions or by the generation of free radicals.[88,94,125]
Aberrant EAAT2 messenger RNAs (mRNAs) were found in neuro-pathologically affected areas and cerebrospinal fluid (CSF) of ALS patients. These abnormalities included intron-retention and exon-skipping. According to these findings, aberrant mRNA is the main reason for the decrease in EAAT2 receptors among ALS patient.[120,167]
Structural and functional abnormalities of mitochondria
In addition to glutamate excitotoxicity, mitochondrial dysfunction also plays an important role in the motor neuron degeneration. Mitochondria are membrane bound organelles that have a significant role in vital processes such as intracellular energy production, cellular respiration, calcium homeostasis, and control of apoptosis.[94] Accumulating evidence suggests that abnormalities in mitochondrial morphology and biochemistry contribute to the pathogenesis of ALS. Functional defects and altered mitochondrial morphology such as fragmented network, swelling, and augmented cristae were found in soma and proximal axons of skeletal muscle and spinal motor neurons of ALS patients.[25,53,83,178,180,259,307]
In the spinal cords of ALS patients, mutant SOD1 is deposited on the cytoplasmic face of the outer membrane and matrix of mitochondria.[233,304] The increase of misfolded mutant SOD1 in spinal cord mitochondria is considered as the main reason for mitochondrial dysfunction that leads to abnormal functioning of ATP production, calcium homeostasis, axonal transport of mitochondria, and apoptotic triggering.[25,168]
Mitochondria act as the powerhouse of every cell by converting energy into ATP that is, essential for the metabolism of the cells. Disturbed energy homeostasis and ATP deficits have been reported in the skeletal muscle biopsies of ALS patients. The normal process of electron transport chains is perturbed by the presence of mutant SOD1, causing less production of ATP. Some studies have demonstrated a decreased activity of respiratory chain complexes I and IV that are associated with defective energy metabolism.[234]
In addition to energy homeostasis, another major function of mitochondria in neurons regards buffering cytosolic calcium levels. Thus, unraveling the relationship between aberrant mitochondria, calcium dysregulation, and neuronal death is critical for the understanding of ALS pathogenesis. Calcium is one of the most significant intracellular messengers that play an important role in the regulation of metabolic pathways, neuronal development, and synaptic transmission. Mutant SOD1 has been found to disrupt calcium homeostasis. Several studies have shown that intracellular calcium is misregulated in ALS patients. A lower cytosolic Ca2+ buffering ability has been found as a principal risk factor for motor neuron damage.[7,16,131]
Several studies reported the loss of Ca2+ binding proteins such as calbindin-D28K and parvalbumin in the motor neurons of ALS patients.[6,21,185] These findings are in agreement that with studies showing neurons lost early in the ALS development have low cytosolic Ca2+ buffering capabilities due to the loss of Ca2+ binding proteins. Meanwhile increasing the cytosolic Ca2+ buffering capacity has shown to reduce motor neuron degeneration.[16,132,196,302]
Regulating mitochondrial transport along axons is an essential task for the survival of neurons due to the mitochondria's key role in ATP generation, calcium buffering, and apoptotic signaling. Mitochondria are constantly being transported and docked at the same time in areas with high demand of ATP and calcium homeostasis such as growth cones, nodes of Ranvier, and synaptic terminals.[178,331] Thus, any defects in mitochondrial transport will lead to energy depletion and disruption in Ca2+ buffering, activating synaptic dysfunction and a loss of neurons. Several laboratories have identified disrupted axonal transport of mitochondria in ALS patients.[31,210] The axonal transport alteration impairs the degradation and recycling process of abnormal mitochondria, thus increasing the amount of dysfunctional mitochondria at distal axons.[178,229,233] Moreover, mitochondrial movement can also be suppressed in both anterograde and retrograde directions.[70,178,265]
Increased mitochondrial transport may slow axonal degeneration by delivering healthy mitochondria to axons while removing the damaged one from distal synapses.[264,331] Mitochondria have been an attractive target for ALS therapy development and drugs, such as olexisome, are already in clinical trial for ALS patients.[88]
Finally, mutant SOD1 aggregates may also interfere with components of mitochondrial-dependent apoptotic machinery, such as B-cell lymphoma 2 (Bcl-2), which is a regulator protein that controls cell death.[184,280] Thus, causing the stimulation of premature apoptotic cascade activation is leading to the release of cytochrome C in the presence of Bcl-2, which directly contributes to neuromuscular degeneration and neuronal dysfunction.[25]
Impaired axonal structure and transport defects
Motor neurons are highly polarized cells with long axons that can be more than a meter in length and are thus vulnerable to damage. In addition to transmitting nerve impulses axons also transport organelles, RNA, proteins, lipids, and other cell parts to the axonal compartments. Moving toward the soma is called retrograde and is performed by cytoplasmic dynein molecular motors while moving toward the synaptic structures at the neuromuscular junction is an anterograde transport and is conducted by microtubule-dependent kinesin.[70]
Axonal transport in ALS patients is compromised. Dysregulation of axonal transport and the axonal compartment play a critical role in the pathophysiology of ALS. In several experiment with mutant SOD1 mice, loss of neurotrophic signaling and defective axonal transport were observed early in the disease process.[88,145,324] Both anterograde and retrograde transport were impaired by the presence of mutant SOD1.[22,70]
Several pathways may be responsible for the impaired axonal transport in cases with mutant SOD1. Some of the most important mechanisms involve defective mitochondrial function or energy depletion, disruption of kinesin function by tumor necrosis factor, and excitotoxic damage by glutamate.[69,142,291] Defective axonal transport causes an accumulation of neurofilaments, mitochondria, and autophagosomes in degenerated motor neurons. This leads to further hindrance of axonal transport and eventual motor neuron death.[124]
Free radical-mediated oxidative stress
Reactive oxygen species (ROS) or free radicals form as natural byproducts of the normal metabolism of oxygen.[75] ROS accumulation causes severe damages to cell structures. The term oxidative stress is used to define a disturbance in the balance between the production ROS and cell's antioxidant defenses.[94] Losing the ability to detoxify the harmful reactive intermediates will lead to cell demise.
Increased oxidative damage has been reported in ALS case biopsies and altered redox reactions were among the earliest theories of how mutant SOD1 could cause cytotoxicity.[41]
SOD1 is a major antioxidant protein, thus a mutation in this gene could cause cytotoxicity. Elevation of free radicals and increased oxidative damage were found in CSF, serum, and urine samples of ALS patients.[267,272,277] In addition, oxidative damage to RNA species was found in both mutant SOD1 mouse models, as well as in human CNS biopsies.[44]
GENETICS OF AMYOTROPHIC LATERAL SCLEROSIS
Sporadic ALS accounts for the majority of the cases of ALS, but genetic causes have been known to play a role.[39] FALS occurs due to mutations in specific genetic loci.[73] The inheritance follows a clear Mendelian pattern and is primarily autosomal dominant.[118,146]
The clinical and pathological presentation of FALS and sporadic ALS are similar. Genetic testing can be used to differentiate inherited versus sporadic ALS and also to rule out other diseases that clinically mimic ALS.[33,115,171] Hence, genetic testing enables researchers to categorize and conceptualize the disease and will aid in mapping the various genetic mechanisms of each type of ALS.[231]
Starting with the discovery of mutations in the SOD1 gene, which codes for copper/zinc ion-binding SOD, 18 other genes have been identified in association with FALS.[73,239,308] The additional genes that are known to cause FALS include: TARDBP, encodes TAR DNA-binding protein 43 (TDP-43); FUS, which codes for fusion in sarcoma; ANG, which codes for angiogenin, ribonuclease, and the RNAase A family 5; OPTN, which codes for optineurin; and C9orf72.[23,49,56,100,135,160,179]
SOD1 mutations, which account for 20% of cases of FALS and 5% of SALS, cause cytotoxicity, which still has an unclear pathophysiology.[146,254,260] TARDBP mutations represent 5–10% of FALS mutations.[146] TDP-43 and FUS, which represents 5% of FALS mutations, are part of the process of gene expression and regulation including transcription, RNA splicing, transport, and translation, as well as processing small regulatory RNAs.[146] ANG, responsible for the remaining 1% of FALS, is a gene, coding for an angiogenic factor that responds to hypoxia. OPTN is a gene involved in open-angle glaucoma, where a mutation in this gene eradicates the inhibition of nuclear factor kappa-beta activation, changing the distribution of OPTN in the cytoplasm.[146] Approximately, 50–60% of FALS patients have mutations arising from the 19 genes that have been identified to date.[4] SOD1 and C9orf72 mutations most often cause FALS, but their rates vary across population.[4]
FALS is inherited at a rate of 5–10% for all cases of ALS where family history of the disease is known.[73,146] In the United States, a founder effect has been identified for the A4V mutations in SOD1, whereas in Europe, this mutation is uncommon.[39,255] OPTN mutations occur most often in Japanese population.[39] To date, there has been no evidence for geographic variability in FUS and TARDBP.[39] As soon as more causative genes are identified in FALS, mutation frequencies across different FALS population will be available. The lifetime risk of ALS is 1:450 for women and 1:350 for men.[39] As family size increases, there is a greater likelihood of two family members having SALS.[39]
Close to 50% of FALS cases can be attributed to specific genes, and most are seemingly rare, highly penetrant, de novo mutations within affected families. Genome-wide association studies (GWAS) has allowed for the identification of common variables that are coupled to this disease.[118] Another technique is next-generation sequencing (NGS), otherwise known as massively parallel sequencing, which provides a way to map mutations for single gene diseases.[118,245] Together, GWAS and NGS have helped to identify genetic variables that are seen in parallel with a higher risk for developing ALS.[118] Ascertaining accurate clinical phenotypes is essential for the success of these techniques to avoid false positive results.[96,118]
Family aggregation studies for SALS patients have shown that many people who have common neurodegenerative disorders also have ALS, possibly indicating the presence of a susceptible gene that could be responsible for increasing neurodegeneration in kindreds.[146]
Many GWAS for SALS have resulted in identifying genes that are associated to the ALS disease.[146] Two new susceptible loci, 19p13.3 (UNC13A) and 9p21.2, were identified through collaborative research that combined study pools to elucidate effectively both genes.[146,303] If more research groups work on collaborative efforts, it is highly probably that more molecular pathways and genetic markers could be identified.[146]
AMYOTROPHIC LATERAL SCLEROSIS SYMPTOMS
The different ALS phenotypical expressions are classified mainly as: Limb-onset ALS with a combination of upper motor neuron (UMN) and LMN signs in the limbs; bulbar onset ALS, characterized with speech and swallowing difficulties followed by limb weakening in later stages of the disease; PLS with pure UMN involvement; and finally, PMA with pure LMN involvement.[146,312,323] The main clinical feature in ALS is a combination of UMN and LMN damage involving brainstem and multiple spinal cord innervation regions. Limb-onset ALS is the predominant type with 70% of the cases among patients. Bulbar onset accounts for 25% of the cases, with the final 5% of the cases having initial trunk or respiratory involvement.[146]
ALS patients experience localized muscle weakness that begins distally or proximally in their upper and lower limbs. Usually, the onset symptoms are asymmetric and develop in progressive generalized weakness and wasting of the muscles. The majority of the patients develop bulbar and respiratory symptoms and spasticity, which affects manual dexterity and gait.[101] Pseudobulbar symptoms including emotional lability and excessive yawning have been observed in a substantial number of cases.[323] About 5% of the patients with respiratory weakness usually do not show significant limb or bulbar symptoms.[66] Instead, these patients present type 2 respiratory failure or nocturnal hypoventilation including dyspnea, orthopnea, disturbed sleep, excessive somnolence in daytime, morning headaches, anorexia, decreased concentration, and irritability or mood changes.[238] Muscle atrophy, including muscles of the hands, forearms or shoulders, and proximal thigh or distal foot muscle in lower limbs, is usually discovered early in the development of limb-onset ALS.[312]
Speech disturbances tend to appear before the development of dysphagia for solids and liquids. Symptoms characteristic of limb-onset can develop simultaneously with bulbar symptoms occurring within 1–2 years. Patients with bulbar symptoms suffer from sialorrhea (excessive drooling) due to difficulty of swallowing saliva and minor bilateral lower facial weakness from UMN damage. The generalized weakness of the lower half of the face causes difficulty with lip seal and blowing cheeks.[323] The rest of the cranial nerves remain intact; however, in very rare cases of late stage ALS disease, patients may develop supranuclear gaze palsy that is a neurodegenerative disorder that causes severe balance problem and gaze dysfunction accompanied cognitive dysfunction.[222]
With the progression of ALS, patients develop the distinctive feature of a combination of upper motor and LMN degeneration signs within the same CNS region.[103] This affects the bulbar, cervical, thoracic, and lumbar areas. The main cause of death in ALS is respiratory failure as the result of pulmonary complications.[54] Patients, who undergo tracheostomy-delivered assisted ventilation to prolong their lives, eventually develop a state motor paralysis known as a “totally locked-in state” (TLS), which involves paralysis of all voluntary muscles and varying degrees of oculomotor impairment.[117]
Some uncommon symptoms of ALS include cramps and fasciculations in the absence of muscle weakness, and frontal lobe-type cognitive dysfunction.[323] Other atypical ALS types might also include weight loss, which is an indicative of a poor prognosis.[87] Patients notice the appearance of fasciculations and cramps before weakness and wasting of muscles, which have subtle onset and are exacerbated by cold temperatures. Fasciculations can be observed in various muscle groups while spasticity is observed in the upper limbs with increased tone and a supinator “catch.” In the lower limbs, a patellar “catch” and clonus is seen along with hypertonia.[323]
Weakness, spasticity, and abrupt deep tendon reflexes are usually characteristic of UMN disturbances involving the limbs. LMN features, on the other hand, include fasciculation, wasting of the muscle, and weakness. Spastic dysarthria characterized by slow, labored, and distorted speech is a consequence of bulbar UMN damage.[80] In bulbar onset ALS, the gag reflex is preserved and abrupt. In contrast, the soft palate reflex may be weak. Symptoms that identify bulbar LMN damage include tongue weakening, wasting, and fasciculations along with flaccid dysarthria.[150] Flaccid dysarthria and palatal weakness ultimately produce nasal speech.[80]
In the majority of the cases, tendon reflexes become pathologically abrupt in a symmetrical pattern.[85] Examples include finger jerks in the upper limbs and a positive crossed adductor reflex in the lower limbs. Tendon reflexes might spread beyond the stimulated muscle group in an abnormal way. Hoffmann's sign shows a plantar stimulation of the extensor muscles and a positive sign in upper limbs.[252] In patients presenting a bulbar defect, dysarthria may develop as a consequence of LMN pathology or pseudobulbar palsy from UMN disorder, which leads to dysarthria of speech.[81] In initial stages of the disease, this may only be apparent after ingestion of small amounts of alcohol.[205]
In late stages of ALS, some patients develop flexor spasms or involuntary spams due to excess of activation of the flexor arc in spastic limbs.[219] Patients have reported bladder dysfunction with the urgency of micturition, sensory symptoms, and cognitive symptoms along with multisystem involvement.[26]
Other common symptoms in ALS are fatigue and reduced exercise capacity. As the disease progresses, patients require assistance with basic daily activities.[114] Dysphagia develops with consequent weight loss and malnutrition.[244] In late stages of the disease, patients may develop respiratory complications such as dyspnea, orthopnea, or hypoventilation, which results in hypercapnia and early morning headaches.[164] Progressive weakening of the respiratory muscles develops into respiratory failure, which is often triggered by pneumonia.
The symptoms of ALS can be further divided into primary and secondary symptoms. Primary symptoms include muscle weakness and atrophy, spasticity, speech disturbances, poor management of oral secretions, difficulty swallowing, and respiratory complications that result in death. Secondary symptoms usually accompany primary symptoms, and they can significantly reduce the quality of life of patients, such as pain or difficulty performing daily tasks.[114]
Even though pain has not been associated with ALS, it has been reported in nearly 70% of ALS patients at some point during the course of the disease.[217,223] Pain is classified as acute or chronic depending on duration and presence of abnormalities affecting how nerves transmit electrical impulses to the CNS.[79,198] Pain in ALS is mostly related to musculoskeletal conditions including muscle cramping and spasticity. Acute pain and chronic pain have been linked to ALS. It has been reported that musculoskeletal pain in ALS develops secondary to muscle atrophy and reduced muscle tone. This can arise as a consequence of damage to bones, tendons, ligaments, joints, nerves, and the affected muscle.[114] Muscle wasting in ALS incites collateral axonal sprouting that enhances the surviving units, creating an enlarged plate zone, and a less synchronized motor unit action potential.[258] A progressive dissociation of the mechanical and electrical properties of muscle is observed over time. This alteration in muscle coordination and force generation causes abnormal stress on the ligaments, tendons, and joints.[72,109,230] Continual muscle wasting and injury produce a decrease in strength, coordination, and tone leading to pain development. Contrary to pain onset, which usually occurs in late stages of ALS, cramps and fasciculations are more frequent at initial stages. Even though patients experience fasciculations before the onset of muscle weakness, concern arises after diagnosis.[275]
Spasticity in ALS is usually due to changes in UMN within the motor cortex. Alteration in UMN processing can create the primitive reflex, also known as the Babinski sign, an important sign of neuropathy.[275] Spasticity may not necessarily produce pain, but it can induce painful cramps, alter manual dexterity and cause muscle fatigue. Other consequences of spasticity include involuntary mobilization of stiff joint, muscle contractures, pressure pain, and decubitus ulcers due to immobility and skin breakdown in flexor creases.[26,27,28,257] All of these changes can alter posture, range of motion, ambulation and gait, thus creating new sources of pain.[217]
DIAGNOSING AMYOTROPHIC LATERAL SCLEROSIS
The complexity and heterogeneous nature of ALS makes early and accurate diagnose a continuous challenge.[8] There is an average delay of 13–18 months from the onset of a patient's symptoms to confirmation of the diagnosis.[51,84] The lack of an established biological marker for ALS, the highly variable initial clinical presentations of the disease, and its pathogenic overlap with several neurodegenerative disorders all contributes to the difficulty in diagnosing ALS with acceptable certainty.[65] ALS is primarily a clinically diagnosed disease based on the exclusion of other causes of progressive UMN and LMN dysfunction.[65,115] There are standard criteria and diagnostic tests that help rule out many of the differential diagnosis of ALS. This process includes obtaining a thorough patient history, conducting a thorough examination, appropriate laboratory, electrodiagnostic, and neuroimaging studies, as well as genetic testing.[35,65,115,121,208]
Criteria and requirements for diagnosis
The El Escorial criteria for diagnosing ALS was published in 1994 by the World Federation of Neurology for inclusion standards for patients entering research studies and clinical trials.[3,35] The importance of laboratory exams as diagnostic tools to exclude differential diagnosis was included in a revised criteria and renamed to the Airlie House Criteria in 1998.[35,262,295] These two criteria are used to predict the degree of certainty of diagnosis and are also used as inclusion criteria for clinical trials and research purposes.[115,171,262] The Awaji algorithm was incorporated in 2000 and includes neurophysiological measurements of LMN degeneration while UMN dysfunction remains clinically based.[57,262] The Awaji criteria place equal emphasis on both electromyogram (EMG) and clinical abnormalities.[203] Several follow-up studies have shown that using the Awaji algorithm has successfully increased the ability to detect patients with ALS without increasing the number of false-positives.[57,65,97,195,262] As a result, patients can benefit from treatment and the corresponding results of the clinical trials. These criteria are based on the probability of the disease and do not take into consideration the behavioral and mental variations of ALS patients.[35,115]
A definitive diagnosis of ALS requires evidence of LMN and UMN degeneration, and progression and spread of neurological symptoms or signs within or toward another anatomical region.[115] The electrophysiological, laboratory, and neuroimaging results should not show evidence of other pathological processes that could explain the observed clinical presentation and exclude ALS as a cause.[35]
Variability in clinical presentation
Based on the onset of symptoms, ALS is categorized as either a bulbar or spinal-onset disease, and further phenotypic subclassification is based on the extent of UMN and LMN dysfunction.[115,154] PLS, PMA, and PBP mimic the phenotype of ALS but vary in severity of the disease and prognosis.[115] PLS is defined as an UMN disorder and diagnosed in patients who have only UMN involvement and are classified as sporadic adult onset if the symptoms have been ongoing for more than 4 years.[115,249,274] Spinal signs are typically the first to manifest in PLS and develop into ALS in 77% of patients within 3–4 years.[115] It is especially important to differentiate PLS from ALS because the median survival of patients with PLS is >20 years, for those who do not develop ALS, whereas the average survival after onset of symptoms of ALS is approximately 3–5 years.[115,177,295] PMA involves LMN signs only, and 30% of the patients with PMA develop UMN signs within 18 months and continue to develop ALS.[115,310] PBP initially presents with affected speech and swallowing because of the LMN involvement of cranial nerves IX, X, and XII.[115] LMN syndromes with the segmental distribution of muscle involvement and disease duration of >4 years have an encouraging prognosis.[115,301] Patients with segmental disease phenotypes that were followed in a prospective study did not develop respiratory insufficiency or substantial changes in respiratory muscle strength, functional impairment, or forced vital capacity (FVC).[115,301] Difference between these clinically similar conditions is essential in providing accurate prognostic information to the patient and their family and is crucial for further treatment and management options.[9]
Differential diagnosis
Lack of disease progression, an unusual patient history, or uncommon symptoms should prompt further investigation of the differential diagnosis of ALS [Table 1].[84,115,295]
Table 1
ALS diagnosis: List of differential diagnosis and clinical overlap with ALS
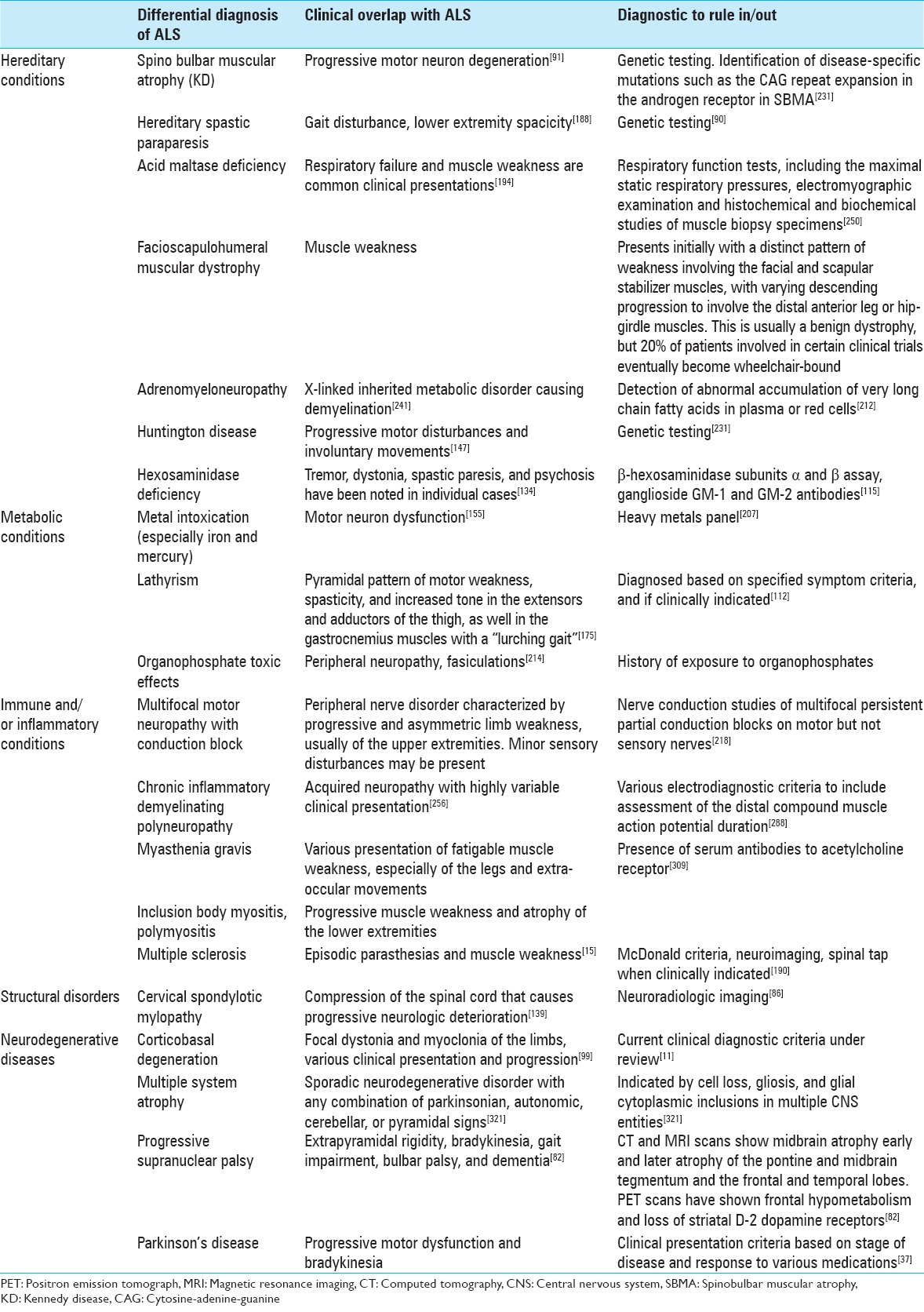
Common misdiagnosis of amyotrophic lateral sclerosis
Conditions that are commonly mistaken for or difficult to differentiate from ALS are multifocal motor neuropathy with conduction block, cervical spondylotic myelopathy, Kennedy disease (KD), and Post-polio syndrome (PPS).[64,115,257] Differentiating multifocal motor neuropathy from ALS is especially important, as patients with this neuropathy may benefit from intravenous immunoglobulin treatment, where ALS patients do not.[42,115] KD, also known as spinobulbar muscular atrophy, is an X-linked disorder associated with an expansion of trinucleotide repeats in the androgen receptor gene.[115,221] Significant features of this rare condition should prompt genetic testing for KD. This includes slow progressive LMN signs in the bulbar region and proximal limbs, absence of sensory nerve action potentials in nerve conduction studies, a family history without any male-to-male inheritance, gynecomastia, and hypogonadism.[148,221,327] Progression of KD is slower than that of typical ALS. Their life expectancy is unaltered, and patients usually do not develop any intellectual impairment.[327]
PPS presents with focal muscle weakness that very slowly progresses to other muscle groups over many years, and does not usually cause death.[257] Patients who present with chronic respiratory muscle weakness should have a thorough evaluation to rule out ALS, as the onset of these symptoms are found in about 3% of ALS patients.[235]
Diagnostic tests
There is no single or absolute test for ALS, but an extensive workup is done to help rule out the various differential diagnosis. Table 2 illustrates a summary of different diagnostic tests for ALS.
Table 2
Diagnostic tests for ALS

Electrodiagnostic tests
Electrodiagnostic studies are a useful diagnostic tool in the investigation of patients who may have ALS. EMG and nerve conduction studies are most sensitive to detecting the disease and can quantify its trademark characteristic of LMN degeneration.[57,65,115] This test can provide a baseline assessment of clinically unaffected areas. Typical EMG abnormalities in patients with ALS are fasciculation (fibrillation) potentials (FPs), and spontaneous denervation discharges, indicative of reinnervation.[35,203] Fibrillation potentials, which are characteristic of positive sharp waves visible on an EMG, may not manifest until one-third of the motor neurons has been lost. Their presence in clinically normal tissue can help facilitate early diagnosis.[295] FPs are also present in benign fasciculation syndrome (BFS), as well as many other conditions and can be highly complex in both ALS and BFS.[65,203] Multifocal distal triggering, axonal conduction variability, and axonal conduction block are factors that lead to variable FP wave shape in ALS and BFS.[203] As ALS progresses, FP discharge rate increases and double same FPs become more prominent, implying that an axonal membrane abnormality has progressed.[203] Electrodiagnostic testing can be limited to confirming ALS in patients with very early signs of the disease due to the range of results produced from those who carry a clinical diagnosis of ALS.[18]
Laboratory studies
Typical labs drawn are erythrocyte sedimentation rate, serum and urine protein electrophoresis, thyroid function tests, serum calcium and phosphate measurements, and CSF analysis.[115] Heavy metal screening is indicated in patients with a potential history of exposure.[115] B-hexaminidase subunits alpha and beta activity should be tested in Ashkenazi Jews because deficiency in this enzyme mimics ALS, but in reality is the rare autosomal recessive genetic disorder, Tay-Sachs.[115]
Neuroimaging
Magnetic resonance imaging (MRI) studies of the brain and spinal cord are the most useful neuroimaging technique in ALS mainly to exclude syndromes that mimic ALS.[115] For example, new chromosome 9p-linked frontotemporal dementia (FTD)-ALS shows a distinct pattern of brain atrophy and neuropathological findings that can help differentiate from classical ALS.[33] Advanced neuroimaging technologies are useful research methods that may help identify specific ALS-associated pathologies in a noninvasive manner, but there are no specific features on an MRI that correlate well with ALS.[115] Neuroimaging is often done to help exclude differential diagnosis rather than confirming the diagnosis of ALS.
TREATMENT
It has been suggested that there are shared environmental and genetic susceptibilities of several different neurological disorders, including PD, FTD, and ALS.[115,171,240] Clinical trials have been conducted giving the same treatment to patients with ALS, PD, and dementia. The assessments of these treatments could influence further diagnostic criteria of ALS.[115,240] Additionally, research has suggested a similarity in the etiology of both Down syndrome and SOD1-related ALS disease, due to their tau hyperphosphorylation.[122] Further understanding of how these mechanisms are connected may play a key role improving treatment and management for patients.
Development of treatments to alleviate ALS symptoms is the foundation for providing proper healthcare to patients. In Table 3, we summarized the current therapeutic agents that have shown promising results in preclinical assessment and some of them have already gone through clinical trials. These compounds were grouped based on the pathophysiological model of the disease.
Table 3
Compounds tested for ALS treatment

Riluzole is currently the only FDA-approved drug treatment identified to have beneficial use in the survival of patients with ALS. Two clinical trials demonstrated evidence of increased survival for the riluzole-treatment group compared with controls.[19,20,162,247] There is some debate on riluzole's precise mechanism, as three mechanisms in decreasing the neuro-toxic effect of glutamate have been recognized. Riluzole is known to trigger presynaptic inhibition and subsequent release of glutamate from cerebrocortical nerve terminals.[316] It inactivates voltage-gated sodium channels and is a noncompetitive NMDA receptor antagonist.[71]
The recommendation dose of riluzole is 50 mg twice daily for patients with definite or probable ALS for duration <5 years, an FVC >60%, and no tracheostomy.[201] Other than riluzole, no other new treatment has been identified that be able to increase the ALS life expectancy.[199] Palliative care can help with the management of ALS symptoms and improving the quality of life. A summary of available palliative care for different symptoms of ALS is provided in Table 4.
Table 4
Palliative care for ALS symptoms

AMYOTROPHIC LATERAL SCLEROSIS MANAGEMENT
Over the past two decades, the management of ALS has changed considerably. Although still incurable, ALS is not untreatable. Emphasis has been made in treatments and interventions that prolong survival.[43,164,204,227,243] While there are no medications that halt or reverse the progressive loss of neurons, importance has been given to management strategies that optimize the quality of life and help maintain the patient's autonomy for as long as possible.[164,270,300,323] Coordinated multidisciplinary care from neurologists, physical therapists, speech therapists, occupational therapists, respiratory therapists, social workers, dietitians, and nursing care managers should be considered for managing patients with ALS to enhance health care delivery, prolong survival, and the quality of life.[201,207,300] Important issues should be discussed with patients and relatives as soon as they are willing to, such as concerns that might arise about the course of the disease, the nutritional and respiratory management during late stages of life, and the patients advanced directives and end-of-life decisions.[270,323]
Multidisciplinary management
In recent years, multidisciplinary ALS treatment facilities have emerged as a result to a shift in the approach of health care delivery to ALS patients. By treating only ALS patients, these multidisciplinary ALS clinics gather great resources and clinical expertise that can facilitate the management and provide optimized care of this progressive disease.[84,293] Although data are limited, some studies, but not all, have suggested that multidisciplinary ALS clinics have improved the quality of life and lengthened survival compared to ALS patients in general neurology clinics.[27,50,84,201,293,300,332]
These multidisciplinary ALS specialized clinics can better assist in managing the complex issues associated with ALS, such as psychosocial problems, nutrition, dysphagia, dysarthria, functional decline, and respiratory symptoms. Both the American Academy of Neurology (AAN) and the European Federation of Neurological Societies recommended that after diagnosis, the patient and caregivers should be referred to a multidisciplinary clinic and receive regular support from a multidisciplinary care team to optimize health care delivery and prolong survival.[84,201]
Respiratory management
The most common cause of death in ALS is due to respiratory failure with or without pneumonia. The presenting symptoms of respiratory muscle weakness, secondary to progressive motor neuron degeneration, result in reduced ventilation.[84,110,323] These symptoms may include dyspnea on exertion or talking, orthopnea, disturbed sleep, excessive daytime somnolence, morning headaches, fatigue, anorexia, depression, poor concentration, vivid nightmares, and nocturia.
Since ALS mortality is mostly caused by respiratory failure, the assessment and management of respiratory function are of great importance. The most common and widely available measure for detecting respiratory decline is the examination of the patient's FVC.[59,92,110] Shorter survival is associated with lower FVC.[62] Another alternative is the Sniff nasal inspiratory pressure test, which acts as a good measure of diaphragmatic strength and had a better predictive value than FVC.[92,176,281] The current guidelines given by the AAN suggest that noninvasive ventilation (NIV) should be considered to treat respiratory insufficiency.[110,176,201] Therapeutic use of NIV is thought to improve survival, slow the decline of FVC, and improve the quality of life in ALS patients.[9,84,110,300]
Nutritional management
Most ALS patients develop dysphagia which leads to malnutrition and weight loss. The consequences of this progressive deterioration include restricting ample nutrition, dehydration, choking, aspiration, and weight loss. As a consequence of the dysphagia present in these patients, the risk of insufficient caloric and fluid intake increases, leading to worsening of weakness and fatigue.[26,51,183,201] Through the use of video fluoroscopic evaluation, it is possible to detect which food consistencies are better handled by the patient. Nutritional management consists initially in altering food consistency, but eventually percutaneous endoscopic gastrostomy (PEG) or similar device may be needed for enteral feeding.[140] Most guidelines recommend that supplementary enteral feeding should be considered in patients whose body weight falls more than 10% of their prediagnostic weight.[84,164] PEG is the standard procedure for enteral feeding and has been found to be helpful in stabilizing weight loss common in ALS.[165,183] However, there is not enough data to refute or support a specific timing of PEG insertion in ALS patients.[140,201,323] Furthermore, there are limited data that correlates prolonged survival with PEG placement and the impact of PEG on the quality of life in ALS patients.[187,201,300] It is suggested that nutritional supplementation using PEG should be done before FVC falls below 50% of predicted values because of the increasing mortality risk of the procedure as respiratory function declines.[164,183,187,201]
DIETARY SUPPLEMENTATION
Vitamin E and Vitamin A
Although the pathophysiologic causes of ALS are not clearly understood, it is hypothesized that free radical stress is a main component of the cell degeneration contributing to ALS progression and onset.[273] Since Vitamin E or α-tocopherol functions as an antioxidant in neural cell membranes, there have been several studies testing its role in ALS.[74,106,197,224,225,227] In a research study by Gurney et al. using transgenic mice, Vitamin E supplements delayed the onset and slowed the course of ALS but did not affect survival rates.[111] When a similar study was applied to humans, Vitamin E intake only slowed the progression of the disease.[74,107,225] Michal Freedman et al. also showed that higher than normal levels of serum Vitamin E was associated with reduced risk of ALS and a small protective effect of Vitamin E supplements is present in patients with lower than normal Vitamin E levels.[197]
In other studies, the efficacy of Vitamin A (beta-carotene) supplementation was investigated among ALS patients. Their results showed that beta-carotene neither has any neuroprotective effect on ALS patients nor helps with slowing down the progression of the disease.[197,209]
Creatine
An investigational study by Klivenyi et al. on transgenic mouse with ALS showed a possible protective effect of dietary creatine supplementation on neurons.[149] Their result presented improved motor performance and extending survival of transgenic mice.[149] A follow-up study by Andreassen et al. explained how creatine intake may improve cellular glutamate transporter, an effect that would prevent a glutamate excitotoxicity, a proposed mechanism of ALS.[10] Clinical trials on human have shown, however, that dietary creatine supplementation did not have an impact on the survival rate of ALS patients or slowing disease progression.[108]
Pu-erh tea extract
A recent research study from Jilin University in China suggests that pu-erh tea extract (PTE) can help in preventing the rapid advancement of ALS in patients. The results of the study suggest that PTE can posttranscriptionally prevent the progression of FET family proteins that are associated with ALS. Also, results from the study suggest that PTE induces FUS/TLS protein degradation via lysosome-dependent pathway. With long-term intake, PTE may prevent protein aggregation and enable cells to maintain function within normal levels of protein. Further studies are required to ascertain the efficacy of PTE on FET in vivo.[329]
SURVIVAL AND PROGNOSIS
ALS is a progressive condition in which more than half of patients diagnosed do not survive within the first 30 months after symptom onset. Only 20% of the patients survive between 5 and 10 years after symptoms onset.[285] Reduced survival to the disease is related to the older age of symptom onset, early respiratory muscle dysfunction, and bulbar onset disease. On the other hand, limb-onset disease, younger age at presentation of the disease and longer diagnostic delay are independent predictors of prolonged survival.[312]
Some ALS subtypes vary according to prognosis. LMN form of ALS, which includes flail-limb variant and PMA, shows a slower progression than other forms of ALS.[285,312] A prognosis of 2–4 years is seen in the pure bulbar palsy phenotype, which usually affects women older than 65 years of age. In this type of ALS, the disease remains localized to the oropharyngeal musculature and UMN features predominate.[285]
CONCLUSION
This study covered a broad range of information about ALS from epidemiology to molecular mechanism and treatment of the disease. Unfortunately, ALS is considered an incurable disease, with an expected life expectancy of 3–5 years after the onset of symptoms.[115] Although there are many antioxidants and supplements that have been proposed as an alternative treatment for ALS, most of them have not been verified in research studies or studies performed lack validity or substantial proof in their methodology.[225] It is important to continue nutritional studies in order to provide better care to ALS patients, as some evidence has shown they may help to alleviate the impact of the disease on their daily lives. For instance, a coherent and in-depth research on alpha-tocopherol and creatine is needed to confirm the known findings on these supplements.
There have been important advancements in the understanding of ALS pathophysiology. Nineteen genes and genetic loci have been found that are associated with ALS.[4] Identifying the molecular pathways underlying ALS will provide the insight to therapeutic approaches. There are currently several clinical trials in place for drugs that are antiapoptotic, anti-aggregation, antioxidant, anti-excitotoxicitory, anti-inflammatory, neuroprotective, and neurotrophic growth factor.[333] Current discoveries of the underlying mechanism of ALS have helped to slow down the progression of the disease. Thus, the future treatments should aim toward preventing neuronal damage, as patients progress from their initial onset.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
Articles from Surgical Neurology International are provided here courtesy of Scientific Scholar
Full text links
Read article at publisher's site: https://doi.org/10.4103/2152-7806.169561
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4653353?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.4103/2152-7806.169561
Article citations
Iron-Status Indicators and HFE Gene Polymorphisms in Individuals with Amyotrophic Lateral Sclerosis: An Umbrella Review of Meta-analyses and Systematic Reviews.
Biol Trace Elem Res, 25 Sep 2024
Cited by: 0 articles | PMID: 39317854
Review
Understanding Amyotrophic Lateral Sclerosis: Pathophysiology, Diagnosis, and Therapeutic Advances.
Int J Mol Sci, 25(18):9966, 15 Sep 2024
Cited by: 0 articles | PMID: 39337454 | PMCID: PMC11432652
Review Free full text in Europe PMC
Downregulation of Lnc-ABCA12-3 modulates UBQLN1 expression and protein homeostasis pathways in amyotrophic lateral sclerosis.
Sci Rep, 14(1):21383, 13 Sep 2024
Cited by: 0 articles | PMID: 39271939 | PMCID: PMC11399266
Takotsubo syndrome in a Sardinian amyotrophic lateral sclerosis cohort.
J Neurol, 10 Sep 2024
Cited by: 0 articles | PMID: 39254699
Advancements in Pharmacological Interventions and Novel Therapeutic Approaches for Amyotrophic Lateral Sclerosis.
Biomedicines, 12(10):2200, 27 Sep 2024
Cited by: 0 articles | PMID: 39457513 | PMCID: PMC11505100
Review Free full text in Europe PMC
Go to all (285) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations.
Ann Neurol, 61(5):427-434, 01 May 2007
Cited by: 626 articles | PMID: 17469116
Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis.
Brain, 131(pt 6):1540-1550, 09 May 2008
Cited by: 278 articles | PMID: 18469020
Delayed disease onset and extended survival in the SOD1G93A rat model of amyotrophic lateral sclerosis after suppression of mutant SOD1 in the motor cortex.
J Neurosci, 34(47):15587-15600, 01 Nov 2014
Cited by: 88 articles | PMID: 25411487 | PMCID: PMC4298650
Genetics of amyotrophic lateral sclerosis.
Rev Neurol (Paris), 173(5):254-262, 25 Apr 2017
Cited by: 29 articles | PMID: 28449881
Review





