Abstract
Free full text

Predictors of Weight-Loss Response with Glucagon-Like Peptide-1 Receptor Agonist Treatment among Adolescents with Severe Obesity
Abstract
Background
In two previous, separate clinical trials, we demonstrated significant reductions in BMI with exenatide in adolescents with severe obesity. In the present study, we pooled data from these near identical trials to evaluate factors that may predict BMI reduction at 3 months.
Methods
Data from 32 patients (mean age 14.3±2.2 years; 69% female; mean BMI 39.8±5.8 kg/m2) were included. Exenatide treatment consisted of 5 mcg twice daily for 1 month, followed by an increase to 10 mcg twice daily for 2 additional months. Predictor variables included baseline BMI, BMI percent change at 1 month, incidence of nausea/vomiting, and baseline appetite and satiety measures. Treatment effects of percent change in BMI from baseline were estimated within predictor subgroups using generalized estimating equations with exchangeable working correlation and robust variance estimation for confidence intervals and P-values to account for paired observations.
Results
The pooled data treatment effect on absolute BMI at 3 months was −3.42% [95% CI: −5.41%, −1.42%] compared to placebo. Within treated participants, appetite at baseline (treatment effect in high [−4.28%] vs. low [1.02%], p=0.028) and sex (treatment effect in female [−4.78%] vs. male [0.76%], p=0.007) were significant predictors of change in BMI at 3 months. Baseline BMI, BMI percent change at 1 month, age, incidence of nausea, vomiting, or other gastrointestinal symptoms, and satiety scores did not predict 3 month responses.
Conclusions
Sex and measures of appetite may serve as useful predictors of glucagon-like peptide-1 receptor agonist treatment response among adolescents with severe obesity.
Introduction
Mirroring the rise in obesity rates observed in adults, the prevalence of obesity among children has increased dramatically over the past 30 years. Perhaps more troubling is the rise in rates of youth with severe obesity (1), defined as having a body mass index (BMI) ≥1.2 times the 95th percentile or BMI ≥35 kg/m2 (2). Health problems stemming from obesity such as insulin resistance and type 2 diabetes, hypertension, dyslipidemia, and early markers of cardiovascular disease have emerged among this population with severe obesity compared to those with lesser forms of obesity (2). Moreover, since obesity in childhood tracks into adulthood (3), these children with severe obesity are at risk for future obesity-related health problems as adults.
The alarming increase in severe obesity has created a need for different approaches to combat this disease. Although lifestyle modification remains a cornerstone treatment for individuals with obesity, this approach is often ineffective and does not result in the amount of weight loss needed to improve co-morbidities and reduce the risk for other chronic diseases in adolescents with severe obesity (4;5). Weight loss surgery is emerging as an effective treatment approach in this patient population, yet availability and appropriate studies in this population remain low (6–8). Medical approaches using pharmacotherapy provide a third option and an opportunity to correct maladaptive physiologic and hormonal changes associated with severe obesity.
Previously, we performed two clinical trials investigating the impact of exenatide, a glucagon-like peptide-1 (GLP-1) receptor agonist, on weight loss among adolescents with severe obesity (9;10). Treatment with exenatide in these studies resulted in a 3–5% reduction in BMI, compared to either a control- or placebo-group. Although the mean BMI reductions in these trials were promising, and paralleled treatment effects for other pharmacologic interventions for obesity in the pediatric population (11–13), there was some variability in the magnitude of weight loss response. This prompted the question of whether individual characteristics might predict responsiveness and perhaps even a greater BMI reduction. Therefore, the primary aim for this study was to identify predictors of weight loss responsiveness to exenatide in adolescents with severe obesity.
Materials and Methods
Study Design and Eligibility Criteria
For this study we pooled data from two independent clinical trials conducted by our research group, with nearly identical study design (9;10) for which change in BMI from baseline with 3 months of GLP-1 receptor agonist treatment was the primary endpoint. Briefly, the first study was a 6-month, randomized, open-label, crossover, clinical trial of exenatide consisting of two, 3-month phases: 1) a control phase of lifestyle modification and 2) a drug phase of lifestyle modification plus exenatide (9). Participants were equally randomized to phase-order (i.e., starting with control or drug therapy) then crossed-over to the other treatment. The second study was a 3-month, randomized, double-blind, placebo-controlled, multicenter clinical trial of exenatide followed by a three-month open-label extension during which time active medication was offered to all patients (10). Data collected while participants were on placebo for the first three months contributed to results for the placebo group whereas data from the three month period on the open-label extension of exenatide contributed to results for the exenatide group.
The combined age range for inclusion in the studies was 8–19 years old, and both trials selectively enrolled participants with severe obesity (BMI ≥1.2 times the 95th percentile or BMI-SDS>2.3). Importantly, for this analysis, only participants aged 12–19 years were included since this group also completed the Child Eating Behavior Questionnaire (CEBQ), which was used to assess some of the predictive factors whereas the 8–11 year participants did not. Recruiting centers included the University of Minnesota Masonic Children’s Hospital Weight Management Clinic (Minneapolis, MN), the McNeely Pediatric Diabetes Center and Endocrinology Clinic at Children’s Hospitals and Clinics of Minnesota (St. Paul, MN), and the International Diabetes Center at Park Nicollet (St. Louis Park, MN). Exclusion criteria included the following: prior diagnosis of diabetes mellitus (type 1 or 2), use of medications associated with weight loss/gain within 3 months of screening, history of bariatric surgery, initiation of any new prescription drug within 30-days of screening, current pregnancy/plans to become pregnant, history of pancreatitis, and obesity-associated genetic disorders.
Lifestyle modification therapy was provided to all participants in both studies and consisted of dietary- and physical activity counseling. The protocols for both studies were approved by the University of Minnesota and the Children’s Hospitals and Clinics of Minnesota institutional review boards. Consent and assent were obtained from parents and participants, respectively. An investigational new drug exemption was obtained for each study from the FDA prior to study initiation. Both trials were registered on the clinicaltrials.gov website (NCT00886626; NCT01237197).
Exenatide and Placebo Dosing, Administration, and Tracking
In both trials, exenatide was initiated at a dose of 5 mcg, subcutaneously, twice per day. After 1 month, the exenatide dose was increased to 10 mcg, twice per day, for the remaining 2 months of the respective treatment phases. In the second trial, which was placebo-controlled, placebo delivery devices were identical in appearance to the active-medication devices. Compliance for both studies was assessed by medication logs and inspection of medication/placebo devices.
Quantification of Appetite and Satiety
Patient self-reported appetite and satiety were quantified using two subscales of the CEBQ, a validated tool in pediatric obesity research (14–17). These measurements were available only from the first study (n = 22) and only the first period was used to avoid mis-classification from perceptions at the beginning of the cross-over period being influenced by the treatment over the first period. Response categories for all items within each subscale were evaluated using a 5-point Likert scale ranging from “never” to “always.” Participant responses were then transfigured to an integer score where 1 is “never” and 5 is “always.” The mean total scores for appetite (or “Food Responsiveness” from the CEBQ) and satiety (or “Satiety Responsiveness” from the CEBQ) subscales were then calculated for each participant and classified as high or low based upon a cut-point of 2.5.
Statistical Analysis
Characteristics were tabulated for the overall group of patients and also according to the treatment groups. Five baseline covariates (starting BMI [<35, 35–40, >40 kg/m2], sex, age, appetite at baseline, satiety at baseline) and three additional covariates assessed at 1 month after initiating treatment (presence of nausea within 30 days, frequency of gastrointestinal symptoms, BMI % change at 1 month) were chosen a priori as primary predictors. Treatment effects were estimated using generalized estimating equations with exchangeable correlation structure to account for the correlated nature of paired observations. Robust variance estimation was used for confidence intervals and P-values. All analyses were conducted using R v2.15.2.
Results
A total of 32 participants from both studies were included. Baseline characteristics, as well as appetite and satiety from the CEBQ, are shown in table 1.
Table 1
(values presented are mean (SD) or N (%) as indicated).
| Covariate | All* (N=32) | Placebo (N=20) | Exenatide (N=29) |
|---|---|---|---|
| Male | 10 (31.2%) | 5 (25.0%) | 10 (34.5%) |
| Age (yrs.) | 14.3 (2.19) | 13.9 (2.29) | 14.5 (2.01) |
| Race | |||
 Black Black | 3 (9.4%) | 2 (10.0%) | 3 (10.3%) |
 White White | 25 (78.1%) | 15 (75.0%) | 22 (75.9%) |
 Other Other | 4 (12.5%) | 3 (15.0%) | 4 (13.8%) |
| Tanner Stage | |||
 2 2 | 1 (3.1%) | 1 (5.0%) | 0 (0.0%) |
 3 3 | 3 (9.4%) | 3 (15.0%) | 3 (10.3%) |
 4 4 | 6 (18.8%) | 2 (10.0%) | 6 (20.7%) |
 5 5 | 21 (65.6%) | 12 (60.0%) | 20 (69.0%) |
| BMI (kg/m2) | 39.8 (5.8) | 38.9 (6.54) | 40.2 (5.71) |
| Experienced Nausea or Vomiting | 13 (40.6%) | 3 (15.0%) | 13 (44.8%) |
Baseline Covariates as Predictors
As a group, exenatide-treated participants had a significant decrease in BMI compared to placebo (Figure) that was not dependent on starting BMI category. The difference between treated and placebo participants appeared to be in part related to sex, as among females, there was a mean decrease in BMI compared to placebo (p<0.001) whereas within the male group, no significant difference was observed between treatment and placebo groups (Figure). Participants reporting high measures of appetite at baseline responded significantly better on exenatide compared to placebo (−4.28% vs. 1.02%, p = 0.028). The covariates of high or low satiety at baseline did not predict response at 3 months. In addition, no meaningful associations were identified between any baseline predictor and metabolic function (fasting glucose and insulin values) at baseline (or 1 and 3 month time points).
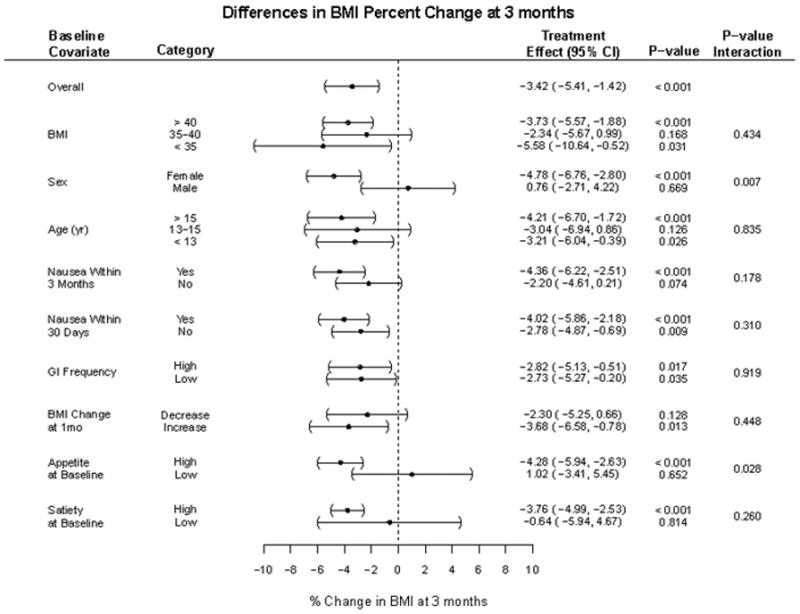
Categories within each covariate were assessed for their association with change in BMI%. Treatment effects describe relative BMI% change among exenatide treated vs. placebo participants within that category. Statistical differences between treated and placebo are displayed under p-value. Statistical differences between category groups in that covariate are noted under p-value interaction.
One and Three Month Covariates as Predictors
Overall, there were no significant predictors of response after 1 month of therapy. All exenatide-treated participants, regardless of whether they had a BMI decrease at 1-month, had a similar degree of BMI reduction at 3 months. All participants treated with exenatide had a similar BMI reduction regardless of whether they had symptoms of nausea at 30 days or not. Participants with any episode of nausea that occurred over the three month treatment period had a greater treatment effect (−4.36% [95% CI: −6.22 to −2.51]), compared to those that did not (−2.20% [95% CI: −4.61 to 0.21]), albeit not statistically significant (p = 0.18). Episodes of nausea were infrequent or intermittent overall, and no participants dropped out of the study due to this side effect. Participants that reported a high frequency of any gastrointestinal complaints did not have a significantly different treatment effect compared to those who had no such complaints (−2.82% vs. −2.73%, respectively).
Discussion
In this hypothesis-generating study, we have identified potential factors that may be associated with a better weight loss response to GLP-1 receptor agonist treatment among adolescent patients with severe obesity: female sex and self-reported, high baseline appetite. Individualizing medical therapies is a promising direction for the treatment of many chronic medical disorders. Treatment of obesity in children (and adults) requires a close examination of all potential factors that may have contributed to the disease. Genetic factors, environmental influences, behavioral components, and physiologic alterations (e.g. leptin in appropriate patients) are all areas that should be explored when attempting to construct a therapeutic plan for an individual patient. Although all cases of obesity result from a relative excess of caloric energy compared to caloric expenditure, the reasons for this “imbalance” can be different among individuals. Therefore, a tailored treatment approach targeting factors specific to the individual patient, rather than prescribing a “one size fits all” therapy, is a promising strategy worthy of further investigation. Tailored treatment is particularly relevant in the field of pediatric obesity medicine since the goal should be to use the fewest number of medications as possible to achieve clinically-meaningful weight loss, especially considering that most patients may need to remain on the treatment(s) for extended periods of time, perhaps for life.
The proposed mechanisms for weight loss with GLP-1 receptor agonists are thought to be through alterations in hypothalamic centers for appetite and satiety combined with a slowing of gastric motility that induces a greater sense of fullness (18–20). It is therefore not completely unexpected that individuals with higher baseline levels of appetite responded better than those who did not. Although the physiologic alterations associated with changes in levels of appetite were not measured in these clinical trials, this baseline predictor nonetheless supports this principle mechanism of action.
Of equal interest is the dichotomy of responses between females and males. Other weight loss pharmacologic interventions have demonstrated a similar difference (21), implying a sex-specific effect from different pharmacologic interventions. One could hypothesize that differences in response may be related to interaction with other hormones known to be different between sexes such as leptin. Leptin levels continue to increase during puberty in girls whereas they remain relatively constant in boys (22; 23). Moreover, GLP-1 agonists appear to have an additive effect on the anorexogenic response to leptin (24). Thus, this mechanism of action could be hypothesized to account for the sex specific differences observed. Importantly, some male participants did respond with modest weight loss though as a group, it was no different from placebo/control and was significantly less compared to the females. Thus, factors related to sex appear to impart some degree of effect, but clearly other factors are also involved that may influence individual responses within both sexes. Future investigations would need to clarify this hypothesis.
GLP-1 receptor agonists have both peripheral and central actions, at least in part, through vagal nerve mediated pathways to induce known gastrointestinal effects (25;26). In our analysis, nausea appeared to be associated with a larger weight loss treatment effect compared to those who did not experience nausea; however, the small number of participants in this analysis limited our precision of this difference as evidenced by the lack of statistical significance. This suggests that the known gastrointestinal side effect profile is perhaps important to weight loss, but other central mechanisms are probably involved. Although not reaching the level of statistical significance, results indicated that individuals reporting higher levels of satiety at baseline had a greater mean reduction in BMI. This result was unexpected considering the satiety-enhancing mechanism of action associated with GLP-1 receptor agonist treatment and warrants further investigation in future studies.
The primary strengths of the current study include the near- identical design of the two clinical trials from which data were pooled and the use of a standardized and validated measure of appetite and satiety (CEBQ). Limitations of the study include the relatively small sample size, short duration of treatment, the fact that the analysis was secondary and post-hoc in nature, and that the population enrolled were predominantly white with a greater proportion of female participants. As such, results should be viewed as hypothesis-generating. These results will need to be confirmed in subsequent studies.
In conclusion, treatment of adolescents with severe obesity with exenatide results in weight loss, but with some individual variability in response. Our analysis suggests that female sex and higher self-reported appetite are the strongest baseline predictors of a better weight loss response to exenatide. Importantly, these are characteristics that can be measured easily in the clinical setting and therefore be potentially clinically useful. The results of the current study should be tested in a larger and longer clinical trial designed to evaluate predictors of weight loss responsiveness to GLP-1 receptor agonist treatment and to determine whether other characteristics or additive combinations may further define the most responsive populations.
Table 2
CEBQ Based Measures of Appetite and Satiety
| All | Placebo | Exenatide | |
|---|---|---|---|
| CEBQ Baseline Appetite | (n=22) | (n=10) | (n=12) |
 Rarely responsive to food Rarely responsive to food | 6 (27.3%) | 3 (30.0%) | 3 (25.0%) |
 Sometimes responsive to food Sometimes responsive to food | 11 (50.0%) | 4 (40.0%) | 7 (58.3%) |
 Often responsive to food Often responsive to food | 3 (13.6%) | 2 (30.0%) | 1 (8.3%) |
 Always responsive to food Always responsive to food | 2 (9.1%) | 1 (10.0%) | 1 (8.3%) |
| CEBQ Baseline Satiety | |||
 Never full Never full | 1 (4.5%) | 0 (0.0%) | 1 (8.3%) |
 Rarely full Rarely full | 11 (50.0%) | 3 (30.0%) | 8 (66.7%) |
 Sometimes full Sometimes full | 9 (40.9%) | 6 (60.0%) | 3 (25.0%) |
 Often full Often full | 1 (4.5%) | 1 (10.0%) | 0 (0.0%) |
Acknowledgments
Funding for this study was provided by the Minnesota Obesity Center (NIH Grant P30DK050456 NORC), a Community Health Collaborative Grant from the University of Minnesota Clinical and Translational Science Institute, and from Award Number UL1TR000114 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences, or the National Institutes of Health. Study drug and placebo were generously provided by Amylin Pharmaceuticals, Inc. BN, CF, and AK were responsible for study design and BN, CK, MJA and EB carried out study visits. BN, CF, KR, BC, and AK performed data analysis and interpretation. BN and AK performed associated literature review. All authors were involved in writing and editing the paper and had final approval of the submitted version. The authors would like to thank Angela Fitch, M.D., and Betsy Schwartz, M.D., for assistance in recruitment, and Andrea Metzig for assistance in study coordination.
Footnotes
Conflicts of Interest/Disclosures:
Dr. Kelly has received research funding from Amylin/Eli Lilly, served on a pediatric obesity advisory board (clinical trial design) for Novo Nordisk Pharmaceuticals and Takeda pharmaceuticals but did not receive personal or professional income for his services. Dr. Abuzzahab reports participation in two industry sponsored trials investigating GLP-1 analogs (H8O-MC-GWBQ, NN2211-3659), outside the submitted work. None of the other authors have relevant disclosures.
References
Full text links
Read article at publisher's site: https://doi.org/10.1111/cob.12128
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4721217?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/cob.12128
Article citations
Evaluating appetite/satiety hormones and eating behaviours as predictors of weight loss maintenance with GLP-1RA therapy in adolescents with severe obesity.
Pediatr Obes, 19(5):e13105, 09 Feb 2024
Cited by: 0 articles | PMID: 38339799
Evaluating potential predictors of weight loss response to liraglutide in adolescents with obesity: A post hoc analysis of the randomized, placebo-controlled SCALE Teens trial.
Pediatr Obes, 18(9):e13061, 01 Jun 2023
Cited by: 3 articles | PMID: 37264767 | PMCID: PMC10926323
Circulating levels of proglucagon-derived peptides are differentially regulated by the glucagon-like peptide-1 agonist liraglutide and the centrally acting naltrexone/bupropion and can predict future weight loss and metabolic improvements: A 6-month long interventional study.
Diabetes Obes Metab, 25(9):2561-2574, 29 May 2023
Cited by: 2 articles | PMID: 37246799 | PMCID: PMC10524619
Usefulness of circulating EPAC1 as biomarkers of therapeutic response to GLP-1 receptor agonists.
Acta Diabetol, 59(11):1437-1442, 04 Aug 2022
Cited by: 0 articles | PMID: 35925404
Vicious cycle between severity of childhood obesity and pandemic: Potential impact of metformin.
Obes Med, 33:100433, 13 Jun 2022
Cited by: 3 articles | PMID: 35720680 | PMCID: PMC9190200
Go to all (20) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT01237197
- (1 citation) ClinicalTrials.gov - NCT00886626
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial.
JAMA Pediatr, 167(4):355-360, 01 Apr 2013
Cited by: 80 articles | PMID: 23380890 | PMCID: PMC4010226
Evaluating appetite/satiety hormones and eating behaviours as predictors of weight loss maintenance with GLP-1RA therapy in adolescents with severe obesity.
Pediatr Obes, 19(5):e13105, 09 Feb 2024
Cited by: 0 articles | PMID: 38339799
Experiences with Glucagon-Like Peptide-1 Receptor Agonist in Children with Acquired Hypothalamic Obesity.
Obes Facts, 13(4):361-370, 11 Aug 2020
Cited by: 4 articles | PMID: 32781455 | PMCID: PMC7590745
Funding
Funders who supported this work.
Minnesota Obesity Center (1)
Grant ID: P30DK050456 NORC
NCATS NIH HHS (2)
Grant ID: UL1TR000114
Grant ID: UL1 TR000114
NCRR NIH HHS (1)
Grant ID: UL1 RR033183
NIDDK NIH HHS (3)
Grant ID: P30 DK050456
Grant ID: P30DK050456
Grant ID: T32 DK007161
National Center for Advancing Translational Sciences (1)
Grant ID: UL1TR000114





