Abstract
Free full text
Particulate matter is associated with sputum culture conversion in patients with culture-positive tuberculosis
Abstract
Emerging risk factors for tuberculosis (TB) infection, such as air pollution, play a significant role at both the individual and population levels. However, the association between air pollution and TB remains unclear. The objective of this study was to examine the association between outdoor air pollution and sputum culture conversion in TB patients. In the present study, 389 subjects were recruited from a hospital in Taiwan from 2010 to 2012: 144 controls with non-TB-related pulmonary diseases with negative sputum cultures and 245 culture-positive TB subjects. We observed that a 1 μg/m3 increase in particulate matter of ≤10 μm in aerodynamic diameter (PM10) resulted in 4% higher odds of TB (odds ratio =1.04, 95% confidence interval =1.01–1.08, P<0.05). The chest X-ray grading of TB subjects was correlated to 1 year levels of PM10 (R2=0.94, P<0.05). However, there were no associations of pulmonary cavitation or treatment success rate with PM10. In subjects with TB-positive cultures, annual exposure to ≥50 μg/m3 PM10 was associated with an increase in the time required for sputum culture conversion (hazard ratio =1.28, 95% confidence interval: 1.07–1.84, P<0.05). In conclusion, chronic exposure to ≥50 μg/m3 PM10 may prolong the sputum culture conversion of TB patients with sputum-positive cultures.
Introduction
Tuberculosis (TB) is a communicable disease caused by inhalation of the bacillus Mycobacterium tuberculosis. TB is spread to others when patients with pulmonary TB expel bacteria. In 2013, the World Health Organization (WHO) reported that 9 million people developed TB, and 1.5 million people died from TB.1 In Taiwan, the treatment success rate of new smear-positive cases is 70%.2 More effort still needs to be put into controlling TB and increasing the awareness of other risk factors that can modulate disease treatment.
Air pollutants are thought to influence the development of pulmonary disease by interfering with nonspecific and specific lung defenses.3 Many studies conducted over the past 30 years have shown an association between air pollution and TB.4,5 People who are exposed to particulate matter that is <10 μm in aerodynamic diameter (PM10) generated from biomass burning are more likely to be infected with M. tuberculosis and progress to TB disease.6,7 Smith et al8 observed that outdoor PM10 may be a causative agent for the development of TB. A Taiwan cohort study further showed that exposure to outdoor fine particulate matter was associated with an increased risk of active TB (adjusted hazard ratio =1.39 per 10 μg/m3).5 Jassal et al9 suggested that outdoor fine particulate matter was associated with TB lung pathology and the prevalence of smear-positive TB.9 Occupational exposure to silica particles was identified as a risk factor for TB.10 Additionally, other pollutants, such as sulfur dioxide (SO2), were identified as risk factors for TB infection.11 However, a possible link between air pollution and TB treatment after establishing a diagnosis has not previously been examined.
The reference standard method for diagnosing TB is sputum microscopy to directly observe bacteria. Regarding TB treatment, four standard treatment regimens of first-line TB drugs are used in clinical practice, which achieves success rates of >95%.12 The period for taking different TB drugs is normally between 6 and 9 months. However, a longer period may be required if cavitation, extensive disease, immunosuppression, or a sputum culture that remains positive at 8 weeks occurs in TB patients.13 The objectives of the present study were to investigate the associations of PM10 with 1) the risk of acquiring pulmonary TB; 2) the severity of pulmonary TB disease as graded by a chest X-ray (CXR) appearance; and 3) the rate of sputum culture conversion.
Materials and methods
Study population
A retrospective case–control study of cases reported between January 1, 2010 and December 31, 2012 was conducted. Study subjects were recruited in the chest clinic of Taipei Medical University, Shuang Ho Hospital (New Taipei City, Taiwan). Included in the study were sputum culture-positive patients of both sexes, aged >16 years, who had begun treatment during the period of study. Patients who were smear- and culture-positive were included in the study. TB was diagnosed using sputum culture according to diagnostic standards.13,14 The frequency of sputum culture was the 1st day, and the 2nd, 6th, 9th, and 12th months. A clinical-based control population was recruited from patients who visited the chest clinic and were diagnosed with non-TB pulmonary diseases. Additionally, a sputum culture examination was conducted in control subjects. The sputum culture-negative group served as the control group. Excluded were those for whom the mandatory report was incomplete and for whom missing data could not be retrieved. Cases of TB were assessed at the start and end of TB treatment, taking care to exclude cases ruled out by the attending physician. Personal, clinical, and epidemiological data were obtained from patients’ clinical progress records. Microbiological laboratory data and medical records of patients were reviewed. The TB treatment success rate was defined as having successfully completed treatment, with or without bacteriological evidence of success. This study was approved by the Taipei Medical University Joint Institutional Review Board (Taipei, Taiwan). Records of all patients were anonymized and de-identified before the analysis.
Grading of CXRs
To quantify the severity of chest lesions observed in CXR images of TB patients, a scoring method was applied in the present study.15 Briefly, the presence of nodules, patchy or confluent consolidation, cavitation (size), bronchial lesions or fibrosis, effusion, and lymphadenopathy were recorded. To grade the percentage of the lungs that was affected, a visual estimation of the extent of opacification, cavitation, or other pathology as a percentage of the visible lung was determined. Dense and patchy opacification of a zone was graded. The severity of TB CXRs was scored blindly by two chest physicians (KYL and PHF).
Air pollution exposure
Measurements of air pollutants were obtained from 25 monitoring stations operated by the Taiwan Environmental Protection Administration. The monitoring stations, located throughout northern Taiwan, measured air pollutants and weather data on a daily basis. Daily concentrations of PM10, ozone (O3), nitrogen dioxide (NO2), SO2, and carbon monoxide (CO) were collected to represent exposure to air pollution among the population by indicating the nearest station within 10 km of their residence. If there was more than one station within 10 km of their residence, the participant was also assigned exposure values equal to the weighted average of all monitors in their area of residence, with weights proportional to the inverse of the square of the distance between their residence and the station. All daily air pollution measurements were matched to the date of treatment initiation for study subjects. For the control group, the previous 1 year PM10 data were collected when a subject was recruited for the study. The average environmental data values for the 365 days before the matched date were used to estimate the yearly average of daily levels.
Statistical analysis
The Shapiro–Wilk test was used to test for normality. A logistic regression model was used to assess the risk of air pollutants associated with subjects with TB-positive cultures compared to the risk associated with subjects who had negative cultures. This model produced estimates of the odds ratios and corresponding 95% confidence intervals (CIs). The multivariate Cox proportional-hazards regression model was used to assess the hazard ratio and corresponding 95% CI for sputum culture conversion in subjects with positive sputum cultures relative to PM10 exposure. A linear regression model was used to examine the correlation of quintiles of PM10 with pulmonary cavity, treatment outcomes (success rate), and CXR grading. All statistical analyses for this study were performed using SPSS 15.0 software (SPSS, Chicago, IL, USA). The significance criterion was set to P<0.05.
Results
Demographic characteristics of the study population
Table 1 shows the demographic characteristics of study subjects. Males made up 63% and 71% of patients with TB-negative and -positive cultures, respectively, and average ages of patients in these groups were 56 and 59 years, respectively. Values of the body mass index of all subjects ranged between 22 and 23 kg/m2. Married cases accounted for 87% and 83% of those with TB-negative and -positive cultures, respectively. Percentages of smokers were 12% for culture-negative subjects and 13% for culture-positive subjects. Alcohol consumption rates for the TB-negative and -positive groups were 46% and 27%, respectively. In the study groups, most subjects had less than high school education (46% for TB-negative subjects and 53% for TB-positive subjects), and the majority of subjects were in the middle-income socioeconomic group (95% for culture negative and 83% for culture positive). There were no human immunodeficiency virus coinfections in these subjects. In terms of CXR, 26% of subjects with a positive culture presented with pulmonary cavity lesions, which accounted for 33 in the CXR grading. The number of days for sputum culture conversion from positive to negative was 242 days (range: 32–773 days).
Table 1
Demographic characteristics of subjects with TB-negative (n=144) and TB-positive cultures (n=245) from 2010 to 2012
| Variables | Negative culture subjects (%) | Positive culture subjects (%) |
|---|---|---|
| Sex, no (%) | ||
 Female Female | 53 (37) | 70 (29) |
 Male Male | 91 (63) | 175 (71) |
| Age, years | ||
 Mean ± SD Mean ± SD | 56±20 | 59±21 |
 Range Range | 19–96 | 16–94 |
| BMI, kg/m2 | ||
 Mean ± SD Mean ± SD | 23±4 | 22±4 |
 Range Range | 14–40 | 12–33 |
| Marital, no (%) | ||
 Unmarried Unmarried | 19 (13) | 42 (17) |
 Married Married | 125 (87) | 203 (83) |
| Smoking, no (%) | ||
 Nonsmoker Nonsmoker | 92 (64) | 146 (60) |
 Former smoker Former smoker | 34 (24) | 67 (27) |
 Current smoker Current smoker | 18 (12) | 32 (13) |
| Alcohol consumption, no (%) | ||
 No No | 78 (54) | 179 (73) |
 Yes Yes | 66 (46) | 66 (27) |
| Education, no (%) | ||
 Less than high school Less than high school | 67 (46) | 129 (53) |
 High school or equivalent High school or equivalent | 50 (35) | 77 (31) |
 More than high school More than high school | 27 (19) | 39 (16) |
| Socioeconomic status, no (%) | ||
 High High | 3 (2) | 2 (1) |
 Middle Middle | 137 (95) | 204 (83) |
 Low Low | 4 (3) | 38 (16) |
| HIV infection | ||
 Yes Yes | 0 | 0 |
 No No | 144 (100) | 245 (100) |
| Pulmonary cavity | ||
 Yes Yes | 0 (0) | 63 (26) |
 No No | 144 (100) | 182 (74) |
| CXR grading | ||
 Mean ± SD Mean ± SD | – | 33±29 |
 Range Range | – | 3–140 |
| Sputum culture conversion, days | ||
 Mean ± SD Mean ± SD | – | 242±108 |
 Range Range | – | 32–773 |
| Outcome | ||
 Alive Alive | – | 212 (87) |
 Death Death | – | 33 (13) |
Abbreviations: BMI, body mass index; CXR, chest X-ray; HIV, human immunodeficiency virus; SD, standard deviation; TB, tuberculosis.
Air pollution and TB
Table 2 summarizes the 1 year average data of air pollutants, and the values were 48.7 μg/m3 for PM10, 3.2 ppb for SO2, 21.6 ppb for NO2, 0.6 ppm for CO, and 25.7 ppb for O3. We observed associations of TB risk with increased PM10, after adjusting for age and sex. An increase of 1 μg/m3 of PM10 resulted in a 4% higher odds of TB (odds ratio =1.04, 95% CI: 1.01–1.08, P<0.05). Incident TB was not associated with SO2, NO2, CO, or O3.
Table 2
Levels of 1 year average of air pollutants among study subjects and the estimated ORs (95% CI) for the risk of TB associated with 1-unit increase in 1 year average
| Variables | Concentration Mean ± SD | TB OR (95% CI)a |
|---|---|---|
| PM10, μg/m3 | 48.7±7.0 | 1.04 (1.01–1.08)* |
| SO2, ppb | 3.2±0.8 | 1.24 (0.90–1.69) |
| NO2, ppb | 21.6±3.2 | 1.03 (0.96–1.10) |
| CO, ppm | 0.6±0.1 | 2.32 (0.19–27.9) |
| O3, ppb | 25.7±1.8 | 1.02 (0.89–1.14) |
Notes:
Abbreviations: CI, confidence interval; CO, carbon monoxide; NO2, nitrogen dioxide; O3, ozone; OR, odds ratio; SD, standard deviation; SO2, sulfur dioxide; TB, tuberculosis.
PM10, pulmonary lesions, and treatment outcomes
One-year averages of PM10 for quintiles in relation to pulmonary cavity, treatment success, and CXR grading were examined (Figure 1). We observed a correlation of PM10 with CXR grading (R2=0.94, P<0.05), whereas no correlation observed in PM10 with the pulmonary cavity (R2=0.73, P=0.068) or treatment success (R2=0.14, P=0.52).
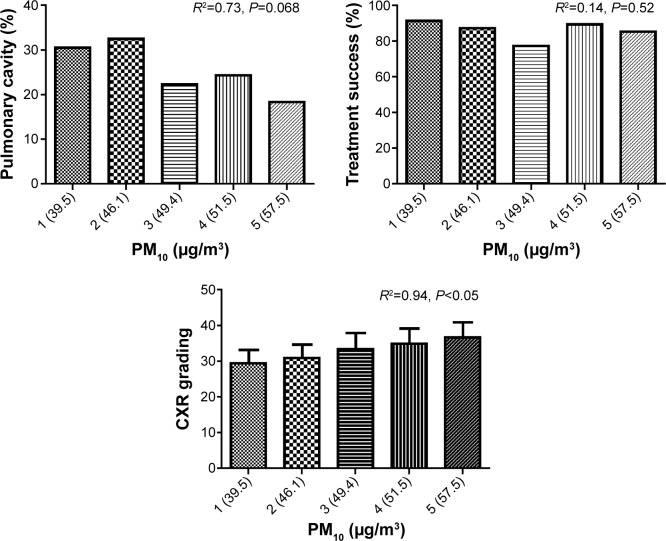
The relationship of pulmonary cavity, treatment success, and CXR grading to 1 year levels of PM10.
Notes: Study subjects were divided equally based on quintiles of PM10. The values in parentheses along the x-axis represent the mean PM10 values for each quintile group. Linear regression was used to examine the correlation between quintiles of PM10 and pulmonary cavity, treatment success and CXR grading. The CXR grading was correlated with 1 year PM10 averages (R2=0.94, P<0.05).
Abbreviation: CXR, chest X-ray.
PM10 and treatment days
The association between different levels of PM10 exposure and the time for sputum culture conversion from positive to negative was investigated, as shown in Table 3. In the present study, the WHO guideline for PM10 of 50 μg/m3 was applied to divide subjects with a positive sputum culture into two groups: <50 and ≥50 μg/m3. We observed an association between exposure to ≥50 μg/m3 PM10 and an increased risk for a longer time for conversion of the sputum culture from positive to negative (hazard ratio =1.28, 95% CI: 1.07–1.84, P<0.05).
Table 3
HR for days of sputum cultures conversion in subjects with TB-positive cultures
| Smoking status | Sputum culture conversion HR (95% CI)a |
|---|---|
| <50 μg/m3 | 1 |
| ≥50 μg/m3 | 1.28 (1.07–1.84)* |
Notes:
Abbreviations: CI, confidence interval; CXR, chest X-ray; HR, hazard ratio; TB, tuberculosis.
Discussion and conclusion
This analysis showed that exposure to PM10, but not other pollutants, was associated with TB that was sputum culture-positive. We found that the severity of pulmonary lesions, based on the score of the CXR grading, increased with increasing levels of PM10. Alteration of the immunity of TB patients by PM10 exposure may be an explanation for our observations, suggesting the possibility that PM10 is a risk factor for regulating sputum culture conversion in TB. We further showed that TB patients exposed to high PM10 levels (≥50 μg/m3) had an elevated risk for prolonged sputum culture conversion from positive to negative.
Examining mucus from the lungs of TB-suspected subjects provides the most accurate diagnosis for active TB. Thirty-six percent of all forms of new TB cases were diagnosed as positive sputum cultures in Taiwan in 2012,16 suggesting that a significant portion of TB patients had sputum-positive cultures. To examine our hypothesis that exposure to air pollution delays sputum culture conversion, TB patients with positive sputum cultures were recruited for the study group. Compared with non-TB patients with negative sputum cultures, we found that a 1 μg/m3 increase in PM10 levels resulted in 4% higher odds of TB patients having positive sputum cultures. These results were consistent with other studies.7,9 Clinically, higher bacterial loads in the sputum are associated with pulmonary lesions in TB patients.17
We investigated the effects of PM10 levels on pulmonary cavity, treatment success rate, and CXR grading. There were no correlations of PM10 levels with pulmonary cavity or the treatment success rate. It is noteworthy that CXR grading was positively associated with PM10 levels. A previous report showed that the time between the appearance of TB symptoms and initiation of anti-TB treatment was related to smoking behavior.18 Our results suggested that pulmonary lesions deteriorated along with exposure to PM10; consequently, the effects of PM10 on the lungs of TB subjects may be associated with TB treatment.
If PM10 causes a faster development of pulmonary lesions, then PM10 may affect the treatment progress of TB patients. TB treatment may be extended for more than the standard 6 months in patients with risk factors for relapse. These risk factors include cavitation, extensive disease, immunosuppression, and a sputum culture that remains positive at 8 weeks.13 The present study referenced the WHO PM10 guideline of 50 μg/m3 as the cutoff point to divide the sputum-positive culture cohort into two groups (<50 and ≥50 μg/m3). Importantly, we found an association between annual exposure to ≥50 μg/m3 PM10 and treatment days for sputum culture conversion. Results showed that clinical exposure to PM10 higher than 50 μg/m3 may prolong the treatment time for sputum culture to convert from positive to negative. This observation was also indicated in other pulmonary diseases. Lung cancer, for example, was linked to cigarette smoke, which may be associated with drug pharmacokinetics and treatment efficacy.19 Based on our findings, we suspect that sputum culture-positive TB patients with PM10 exposure ≥50 μg/m3 may need longer standard treatment periods. However, further research is needed to explore the possible association.
The most crucial cause of the extended course of TB treatment is the dormant state of the M. tuberculosis.20 When M. tuberculosis bacilli reach the alveolar space, dormant and active M. tuberculosis may coexist. Clinically latent infection or active TB depends on the proportions of dormant and active M. tuberculosis. When dormant TB is resuscitated during treatment, the microenvironment changes, the proportion of active TB increases, and an extended period of treatment is necessary. Additionally, the respiratory tract is the system most frequently infected after PM10 exposure, which can result in an increased risk of TB.21 The possible mechanisms for PM10-related infections include an accumulation of chemicals, such as metals, by surface functional groups of PM10.21 Polycyclic aromatic hydrocarbons were also shown to modulate drug-metabolizing enzymes.19
Our study has some limitations. First, factors besides outdoor air pollution could influence the treatment course of TB. Additionally, treatment side effects and multidrug-resistant TB may have affected study outcomes and were not investigated in this study. In addition, indoor air pollution and seasonal effects of air pollution were not determined in the present study, which should be addressed in future work. TB drug medications were not recorded; we could not assess possible interactions between the therapeutic regimens and PM10. In addition, other risk factors for TB, such as alcohol consumption, should be quantified in future work. In conclusion, PM10 was significantly associated with the period required for sputum culture conversion and the severity of pulmonary lesions. Importantly, exposure to PM10 at ≥50 μg/m3 may prolong the TB treatment time. Our findings have important implications for TB treatment strategies, TB control policies, and public health.
Acknowledgments
The authors wish to thank Ms Chiao-Ju Fu and Ching-Ling Li for the technical assistance of this research. This study was funded by the Ministry of Science and Technology of Taiwan (MOST103-2314-B-038-018 and MOST104-2621-M-038-002-MY3).
References
Articles from Therapeutics and Clinical Risk Management are provided here courtesy of Dove Press
Full text links
Read article at publisher's site: https://doi.org/10.2147/tcrm.s92927
Read article for free, from open access legal sources, via Unpaywall:
https://www.dovepress.com/getfile.php?fileID=28546
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.2147/tcrm.s92927
Article citations
Ecological-level factors associated with tuberculosis incidence and mortality: A systematic review and meta-analysis.
PLOS Glob Public Health, 4(10):e0003425, 15 Oct 2024
Cited by: 0 articles | PMID: 39405319 | PMCID: PMC11478872
Long-term exposure to ambient fine particulate matter (PM2.5) and attributable pulmonary tuberculosis notifications in Ningxia Hui Autonomous Region, China: a health impact assessment.
BMJ Open, 14(6):e082312, 04 Jun 2024
Cited by: 0 articles | PMID: 38834325
Association Between Exposure to Ozone (O3) and the Short-Term Effect on Tuberculosis Outpatient Visits: A Time-Series Study in 16 Cities of Anhui Province, China.
J Multidiscip Healthc, 16:2045-2055, 20 Jul 2023
Cited by: 0 articles | PMID: 37496636 | PMCID: PMC10366443
Machine Learning Prediction Model of Tuberculosis Incidence Based on Meteorological Factors and Air Pollutants.
Int J Environ Res Public Health, 20(5):3910, 22 Feb 2023
Cited by: 1 article | PMID: 36900920 | PMCID: PMC10002212
Sputum smear conversion and treatment outcomes among drug-resistant pulmonary tuberculosis patients in eastern Ethiopia: A 9-years data analysis.
Front Med (Lausanne), 9:1007757, 01 Dec 2022
Cited by: 2 articles | PMID: 36530886 | PMCID: PMC9751062
Go to all (13) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effects of particulate air pollution on tuberculosis development in seven major cities of Korea from 2010 to 2016: methodological considerations involving long-term exposure and time lag.
Epidemiol Health, 42:e2020012, 12 Mar 2020
Cited by: 10 articles | PMID: 32164052 | PMCID: PMC7285441
Cigarette smoke is a risk factor for severity and treatment outcome in patients with culture-positive tuberculosis.
Ther Clin Risk Manag, 11:1539-1544, 06 Oct 2015
Cited by: 11 articles | PMID: 26504395 | PMCID: PMC4603723
Correlation of ambient pollution levels and heavily-trafficked roadway proximity on the prevalence of smear-positive tuberculosis.
Public Health, 127(3):268-274, 27 Feb 2013
Cited by: 36 articles | PMID: 23453197
Multicity study of air pollution and mortality in Latin America (the ESCALA study).
Res Rep Health Eff Inst, (171):5-86, 01 Oct 2012
Cited by: 48 articles | PMID: 23311234





