Abstract
Free full text

Long-Term Effects of In Utero Antiretroviral Exposure: Systolic and Diastolic Function in HIV-Exposed Uninfected Youth
Abstract
The aim of this study was to evaluate the association of in utero exposure to highly active antiretroviral therapy (HAART) with left ventricular (LV) function and structure in HIV-exposed uninfected (HEU) children. A prospective, multisite cohort study in HEU children was conducted by the Pediatric HIV/AIDS Cohort Study (PHACS). Echocardiographic measures of LV systolic and diastolic function and cardiac structure were obtained from HEU subjects aged ≥6 years enrolled in the PHACS Surveillance Monitoring of ART Toxicities study. Echocardiographic Z-scores were calculated using normative data from an established reference cohort. We used adjusted linear regression models to compare Z-scores for echocardiographic measures from HEU children exposed in utero to HAART with those exposed to non-HAART, adjusting for demographic and maternal health characteristics. One hundred seventy-four HEU subjects with echocardiograms and maternal ARV information were included (mean age 10.9 years; 48% male, 56% black non-Hispanic). Among 156 HEU youth with any ARV exposure, we observed no differences in Z-scores for LV systolic function measures between youth exposed in utero to HAART (39%) and HAART-unexposed youth in either unadjusted or adjusted models. In adjusted models, those exposed to HAART had significantly lower mitral late diastolic inflow velocities (adjusted mean Z-score =
= 0.00 vs. 0.52, p
0.00 vs. 0.52, p =
= .04) and significantly higher adjusted mean LV mass-to-volume ratio Z-scores (adjusted mean Z-score
.04) and significantly higher adjusted mean LV mass-to-volume ratio Z-scores (adjusted mean Z-score =
= 0.47 vs. 0.11, p
0.47 vs. 0.11, p =
= .03) than HAART-unexposed youth. Uninfected children with perinatal exposure to HAART had no difference in LV systolic function. However, small but significant differences in LV diastolic function and cardiac structure were observed, suggesting that continued monitoring for cardiac outcomes is warranted in this population.
.03) than HAART-unexposed youth. Uninfected children with perinatal exposure to HAART had no difference in LV systolic function. However, small but significant differences in LV diastolic function and cardiac structure were observed, suggesting that continued monitoring for cardiac outcomes is warranted in this population.
Introduction
The effects of HIV and antiretroviral (ARV) exposure on the cardiovascular system of uninfected children born to mothers infected with HIV are not completely understood. Based on current recommendations for treatment of HIV-infected women during pregnancy, most HIV-exposed uninfected (HEU) children are now exposed in utero to highly active antiretroviral therapy (HAART) during a critical period of development of the cardiovascular system. Past studies have been somewhat inconsistent in identification of negative or positive cardiac effects of in utero ARV exposure. An early study of in utero zidovudine monotherapy exposure in 382 HEU infants born before 1995 found no adverse cardiac effects,1 but another study observed an association of in utero exposure to two-drug nucleoside reverse transcriptase inhibitor therapy with mitochondrial dysfunction, which has the potential for negative cardiac effects.2
The National Heart, Lung, and Blood Institute-funded Cardiovascular Status of HAART in HIV-Exposed Infants and Children (CHAART 1) study of HEU children found that in utero exposure to ARVs was associated with decreased septal thickness and left ventricular (LV) mass, as well as increased LV contractility at the age of 2 years.3 More recently, a cross-sectional evaluation of 30 HEU children (mean age 8 years) by Cade et al.4 found reduced diastolic function and LV mass index when exposed to ARVs in utero compared with those born to HIV-negative mothers without ARV exposure.
With the exception of the later study, most evaluations of cardiac function in HEU children have focused on infants and very young children. However, as combination regimens become more widely used, potential long-term cardiac effects of in utero exposure warrant monitoring. Lipshultz et al.5 evaluated cardiac function and structure in 189 HEU children (mean age =
= 11.0 years) and found significantly higher LV fractional shortening compared with perinatally HIV-infected (PHIV) children as well as significant differences in mean Z-scores for LV structural measures. However, that study focused on comparison of echocardiographic measures for PHIV versus HEU, and no evaluation of associations with maternal ARV exposure was conducted.
11.0 years) and found significantly higher LV fractional shortening compared with perinatally HIV-infected (PHIV) children as well as significant differences in mean Z-scores for LV structural measures. However, that study focused on comparison of echocardiographic measures for PHIV versus HEU, and no evaluation of associations with maternal ARV exposure was conducted.
The use of HAART among pregnant women with HIV infection has increased dramatically over the last decade at the same time that changes in the natural history of the epidemic in women have resulted in a much lower percentage of illicit drug use during pregnancy.6,7 Thus, comparison of cardiac measures by HAART exposure requires careful consideration of a number of potential confounding measures. As children transition to adolescence, the home environment may exert a stronger influence on child health outcomes.
We conducted an evaluation of cardiac function and structure in a group of older HEU youth with information on maternal ARV exposure based on echocardiograms conducted as part of our Pediatric HIV/AIDS Cohort Study (PHACS), with adjustment for a number of child, maternal, and household characteristics. Based on the prior studies noted above, we hypothesized that youth exposed in utero to HAART would have decreased LV diastolic function and LV mass compared with those not exposed to HAART.
Materials and Methods
Study population
The PHACS network has two ongoing studies of HIV-affected youth: the Adolescent Master Protocol (AMP) is a prospective cohort study conducted at 14 U.S. sites designed to evaluate development of children and adolescents born to mothers with HIV infection, including both PHIV and HEU youth. The study enrolled children aged 7–16 years between March 2007 and November 2009. The PHACS Surveillance Monitoring for ART Toxicities (SMARTT) study is an ongoing prospective cohort study of potential adverse outcomes of maternal ARV use on HEU infants and children conducted at 22 U.S. sites. This study enrolls HIV-infected mothers and their infants from birth to age of 12 years. HEU children aged seven or older may coenroll in both the AMP and SMARTT studies, allowing evaluation of maternal exposure on long-term outcomes in HEU adolescents.
The current analysis leveraged this opportunity by evaluating cardiac function measured in the AMP study in relation to in utero ARV exposure, measured primarily in the SMARTT study. Both study protocols were approved by the institutional review board of each participating site and the Harvard T. H. Chan School of Public Health. Written informed consent was obtained from the parent or legal guardian and assent was obtained from older participants as per local institutional review board rules.
At each annual AMP and SMARTT study visit, information about participants and their families was gathered through clinical interviews and chart reviews. Current health status was ascertained through physical and laboratory evaluations. For SMARTT, detailed information was collected through medical chart abstraction on maternal ARV use during pregnancy, and start and stop dates for all ARVs were used to code trimester of exposure to each agent. The AMP study also collected such maternal ARV information when available, but it was not required to be available as per eligibility criteria. HAART regimens were defined as containing three or more ARVs, including two or more drug classes.
Data on pregnancy and infant outcomes were abstracted from hospital medical records and from prior studies.6 Data on self-reported substance use during pregnancy, including smoking, alcohol, and illicit drug use (e.g., marijuana, cocaine, opiates), were collected by interview. The latest maternal CD4+ lymphocyte counts and percentages and HIV RNA viral load levels available before labor and delivery were abstracted from medical records, as well as the earliest corresponding measures during pregnancy if available.
This analysis includes 174 children ≥6 years of age at the time of an echocardiogram performed predominantly as part of the AMP study (98%) and who had information available on maternal ARV use during pregnancy. Participants with cardiac congenital defects were excluded.
Echocardiographic assessment of cardiac structure and function
The echocardiogram was performed by PHACS-trained site staff, including LV M-mode, which was recorded under two-dimensional control. To improve reliability, all echocardiograms were centrally remeasured at the echocardiographic core laboratory at Boston Children's Hospital. The following structural and functional parameters were measured from M-mode recordings: LV end-diastolic and end-systolic diameters, end-diastolic interventricular septal, and LV posterior wall thicknesses, LV fractional shortening, and LV velocity of circumferential fiber shortening. The 5/6 × area × length algorithm was used to calculate LV end-diastolic and end-systolic LV volumes, LV mass, and the LV ejection fraction.
Mitral inflow velocities recorded using pulsed-wave Doppler with the sample volume placed at the tips of mitral leaflets from the apical four-chamber view were used to measure the following diastolic parameters: peak early transmitral flow velocity (E), peak late transmitral flow velocity (A), deceleration time of the E wave (DT), and the E/A ratio. Pulsed tissue Doppler (TDI) samples were recorded from the apical four-chamber view, with the sample volume placed within the basilar aspects of the myocardium in the LV lateral wall and interventricular septum. The TDI images were used to measure the peak early diastolic tissue velocities (E′) in the interventricular septum and lateral wall and the E/E′ ratios were calculated as LV diastolic function indices.
Echocardiographic Z-scores were calculated using normative data from an established reference cohort of healthy children from Boston Children's Hospital, with Z-scores calculated relative to age and body surface area, as previously described.8 This reference population was not known to be HIV infected or ARV exposed and was not matched to the study cohort for race, ethnicity, or socioeconomic status. Echocardiograms were discontinued from the study protocol in 2010 when preset targets were reached.
Statistical methods
Our primary analysis compared echocardiographic parameter Z-scores between HEU youth exposed in utero to HAART compared with HAART-unexposed youth (excluding those not exposed to any ART, n =
= 18). We compared demographic and anthropomorphic characteristics between these two groups using Fisher's exact test for binary measures, chi-square tests for categorical variables, ANOVA for body measurement Z-scores, and Kruskal–Wallis tests for other continuous measures.
18). We compared demographic and anthropomorphic characteristics between these two groups using Fisher's exact test for binary measures, chi-square tests for categorical variables, ANOVA for body measurement Z-scores, and Kruskal–Wallis tests for other continuous measures.
The distributions of Z-scores were compared between HAART-exposed and HAART-unexposed youth using general linear regression models, both unadjusted and adjusted for age, sex, race, ethnicity, BMI Z-score, and systolic blood pressure Z-score. Models for diastolic parameters were also adjusted for heart rate. Similar analyses were conducted to compare youth with versus without in utero exposure to ARVs during the first trimester, given this critical period in fetal cardiac development.
Several sensitivity analyses were conducted. First, we compared HEU youth exposed to zidovudine monotherapy, to regimens containing two or more ARV drugs, both unadjusted and adjusted for the covariates noted above (age, sex, race/ethnicity, BMI Z-score, systolic blood pressure Z-score, and for diastolic parameters, heart rate). Second, multivariable adjusted models comparing HAART-exposed with HAART-unexposed youth and comparing monotherapy to 2+ ARV drugs were repeated, adding those unexposed to any ARV (n =
= 18) in the lesser-exposed group. General linear regression models were fit to the subset of youth with information available on substance use (~72%) and maternal health measures such as CD4% and HIV viral load during pregnancy (52%–74%). Each measure of maternal health and substance use was separately evaluated for association with echocardiographic Z-scores with adjustment for the aforementioned covariates. Comparisons of HAART-exposed vs. HAART-unexposed youth were then repeated among these subsets, controlling for measures for which p
18) in the lesser-exposed group. General linear regression models were fit to the subset of youth with information available on substance use (~72%) and maternal health measures such as CD4% and HIV viral load during pregnancy (52%–74%). Each measure of maternal health and substance use was separately evaluated for association with echocardiographic Z-scores with adjustment for the aforementioned covariates. Comparisons of HAART-exposed vs. HAART-unexposed youth were then repeated among these subsets, controlling for measures for which p <
< .10.
.10.
Last of all, we attempted to account more appropriately for the inter-relationships among echocardiographic parameters by fitting multivariate models, assessing the association of in utero ARV exposure with the echocardiographic Z-scores as a vector of outcomes grouped by type of measurement (LV systolic function, diastolic function, and structure). All analyses were conducted using SAS (Version 9.2) and were based on data submitted as of January 2013.
Results
Participant characteristics
Of the 678 children in the PHACS AMP Study, 227 were HEU. Of these 227, 192 (85%) had echocardiograms available for analysis (119 coenrolled in SMARTT) and 177 had detailed information on maternal ARV use during pregnancy. Three children were excluded due to cardiac congenital anomalies [one with ventricular septal defect, one with mitral valve prolapse, and the last with multiple cardiac defects (atrial septal defect, patent ductus arteriosus, ventricular septal defect) associated with trisomy 21]. Previous analyses indicated that the AMP children with echocardiograms were demographically similar to those without echocardiograms.5
Background characteristics of the 174 HEU youth included in the analysis are summarized in Table 1. Just under half (48%) were male and 56% were black non-Hispanic; 67 (39%) were exposed in utero to HAART, 58 (33%) to zidovudine monotherapy, 31 (18%) to other non-HAART ARV, and 18 (10%) were unexposed to any ARV. During the first trimester, 31% (54/174) were exposed to ARV: 25 to HAART, 14 to other non-HAART ARV, and 15 to zidovudine monotherapy. Their mean age at the time of the echocardiogram was 10.9 years overall, but HAART-exposed youth were significantly younger than those unexposed to HAART (9.2 years. vs. 12.0 years.), reflecting the increasing use of HAART during pregnancy over time. Other characteristics were generally similar between HAART-exposed and -unexposed youth, except that HAART-exposed youth had a lower prevalence of maternal alcohol use during pregnancy.
Table 1.
Characteristics of HIV-Exposed Uninfected Children Born to HIV-Infected Mothers and Exposed to HAART In Utero, by Mother's Treatment Status, Among Subset with Completed Echocardiogram at Age Six or Older and Available Maternal ARV Information
| HAART exposure in utero | |||||
|---|---|---|---|---|---|
| Characteristic | Not exposed to any ARVs (n = = 18) 18) | Exposed to non-HAART ARV (n = = 89) 89) | HAART exposed (n = = 67) 67) | Total (n = = 174) 174) | pa |
| Age at time of echo (years), Mean (SD) | 11.6 (2.8) | 12.0 (2.6) | 9.2 (1.4) | 10.9 (2.6) | <.001 |
| Male, n (%) | 9 (50) | 41 (46) | 34 (51) | 84 (48) | .86 |
| Race/ethnicity, n (%) | |||||
 White non-Hispanic White non-Hispanic | 2 (11) | 3 (3) | 5 (7) | 10 (6) | .42 |
 Black non-Hispanic Black non-Hispanic | 11 (61) | 48 (54) | 38 (57) | 97 (56) | |
 Hispanic Hispanic | 5 (28) | 32 (36) | 23 (34) | 60 (34) | |
 Other Other | 0 (0) | 6 (7) | 1 (1) | 7 (4) | |
| Gestational age <37 weeks, n (%) | 6 (38) | 18 (20) | 11 (17) | 35 (21) | .17 |
| Body measurement Z-scores, mean (SD) | |||||
 Height Height | −0.25 (1.14) | 0.33 (1.12) | 0.22 (1.04) | 0.22 (1.10) | .13 |
 Weight Weight | 0.34 (1.03) | 0.83 (1.41) | 0.69 (1.39) | 0.73 (1.37) | .37 |
 Body mass index Body mass index | 0.60 (0.90) | 0.77 (1.30) | 0.72 (1.37) | 0.73 (1.29) | .87 |
 Body surface area Body surface area | 0.00 (1.02) | 0.82 (1.84) | 0.30 (1.57) | 0.53 (1.69) | .059 |
| Blood pressure (BP) Z-scores, mean (SD) | |||||
 Systolic BP Systolic BP | −0.36 (1.10) | −0.25 (1.04) | −0.45 (0.96) | −0.34 (1.01) | .49 |
 Diastolic BP Diastolic BP | 0.11 (0.88) | 0.46 (0.86) | 0.31 (0.80) | 0.37 (0.84) | .21 |
 Mean BP Mean BP | −0.51 (0.99) | −0.42 (0.90) | −0.53 (0.89) | −0.47 (0.90) | .78 |
| AV heart rate (b/min), Median (IQR) | 75 (70, 81) | 76 (64, 84) | 81 (72, 87) | 78 (68, 85) | .13 |
Maternal health measures before CD4 < < 350 cells/mm3 delivery, n (%) 350 cells/mm3 delivery, n (%) | |||||
 CD4 CD4 < < 350 cells/mm3 350 cells/mm3 | 2 (40) | 19 (30) | 21 (35) | 42 (32) | .71 |
 HIV-1 RNA VL >400 HIV-1 RNA VL >400 cpm cpm | 2 (100) | 15 (48) | 17 (30) | 34 (38) | .039 |
| Maternal substance use during pregnancy, n (%) | |||||
 Tobacco use Tobacco use | 3 (33) | 14 (23) | 10 (18) | 27 (22) | .49 |
 Alcohol use Alcohol use | 4 (44) | 10 (17) | 6 (11) | 20 (16) | .05 |
 Illicit drug use Illicit drug use | 2 (22) | 5 (8) | 3 (5) | 10 (8) | .23 |
 =
= 45), maternal VL (N
45), maternal VL (N =
= 84), and maternal substance use (N
84), and maternal substance use (N =
= 49).
49).ARV, antiretroviral; AV, aortic valve; HAART, highly active antiretroviral therapy; SD, standard deviation; VL, viral load.
Echocardiographic parameters by in utero HAART exposure
Mean Z-scores for the 174 youth were generally close to zero or within half a standard deviation of the normative reference group from Boston Children's Hospital, both overall and within each of the ARV-unexposed, HAART-exposed, and non-HAART ARV-exposed groups (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid). After excluding the 18 youth unexposed to any ARV, there were no significant differences in Z-scores comparing HAART-exposed with HAART-unexposed youth for the three LV systolic function measures in either unadjusted or adjusted models (Table 2).
Table 2.
Results of Linear Regression Models Comparing HIV-Exposed Uninfected Children Born to HIV-Infected Mothers and Exposed or Unexposed In Utero to HAART
| Unadjusted Z-score mean (SE) | AdjustedaZ-score mean (SE) | |||||
|---|---|---|---|---|---|---|
| Echocardiographic Z-score | Exposed to non-HAART ARV (n = = 89) 89) | HAART exposed (n = = 67) 67) | P | Exposed to non-HAART ARV (n = = 89) 89) | HAART exposed (n = = 67) 67) | P |
| Functional systolic parameters | ||||||
 Contractility Contractility | 0.30 (0.12) | 0.38 (0.13) | .67 | 0.35 (0.12) | 0.32 (0.14) | .87 |
 Fractional shortening Fractional shortening | 0.34 (0.10) | 0.43 (0.12) | .57 | 0.34 (0.11) | 0.43 (0.13) | .62 |
 Ejection fraction Ejection fraction | 0.15 (0.09) | 0.20 (0.11) | .75 | 0.19 (0.10) | 0.15 (0.12) | .81 |
| Functional diastolic parameters | ||||||
 Peak early velocity Peak early velocity | 0.34 (0.10) | 0.39 (0.12) | .79 | 0.37 (0.11) | 0.35 (0.13) | .91 |
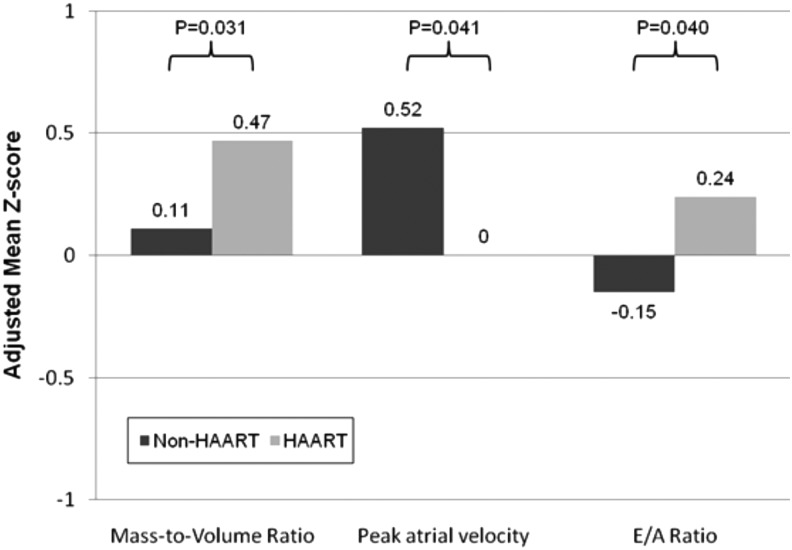
 Peak atrial velocity Peak atrial velocity | 0.46 (0.15) | 0.08 (0.16) | .091 | 0.52 (0.15) | 0.00 (0.17) | .040 |
 Peak early/atrial velocity (E/A ratio) Peak early/atrial velocity (E/A ratio) | −0.13 (0.11) | 0.20 (0.12) | .041 | −0.15 (0.11) | 0.24 (0.13) | .041 |
 Peak early tissue (E′) Peak early tissue (E′) | 0.45 (0.17) | 0.38 (0.20) | .80 | 0.51 (0.18) | 0.28 (0.21) | .46 |
 Peak early inflow/early tissue (E/E′) Peak early inflow/early tissue (E/E′) | 0.04 (0.16) | 0.11 (0.18) | .77 | 0.03 (0.16) | 0.13 (0.19) | .71 |
 Peak early deceleration time Peak early deceleration time | −0.40 (0.11) | −0.26 (0.12) | .40 | −0.41 (0.12) | −0.25 (0.14) | .44 |
| Structural parameters | ||||||
 LV mass LV mass | 0.03 (0.09) | 0.15 (0.11) | .39 | 0.02 (0.10) | 0.18 (0.11) | .32 |
 ED LV volume ED LV volume | −0.17 (0.11) | −0.18 (0.12) | .95 | −0.11 (0.11) | −0.26 (0.12) | .40 |
 ES LV volume ES LV volume | −0.33 (0.11) | −0.10 (0.12) | .16 | −0.20 (0.10) | −0.26 (0.11) | .73 |
 Mass-to-volume ratio Mass-to-volume ratio | 0.25 (0.10) | 0.28 (0.12) | .89 | 0.11 (0.10) | 0.47 (0.12) | .031 |
Results of linear regression modeling, excluding those unexposed to any ARVs during pregnancy (N =
= 18). Each row represents a separate linear regression model comparing youth exposed in utero to HAART with those unexposed to HAART.
18). Each row represents a separate linear regression model comparing youth exposed in utero to HAART with those unexposed to HAART.
BMI, body mass index; D, diastolic; ED, end-diastolic; ES, end-systolic; LV, left ventricular; S, systolic; SBP, systolic blood pressure.
In adjusted models for LV diastolic function parameters, HAART-exposed youth had significantly lower atrial velocity Z scores (0.00 vs. 0.52, p =
= .04) and higher E/A ratio Z-scores (0.24 vs. −0.15, p
.04) and higher E/A ratio Z-scores (0.24 vs. −0.15, p =
= .04) than HAART-unexposed youth (Fig. 1). Those exposed to HAART also had significantly higher adjusted mean mass-to-volume ratio Z-scores than HAART-unexposed youth.
.04) than HAART-unexposed youth (Fig. 1). Those exposed to HAART also had significantly higher adjusted mean mass-to-volume ratio Z-scores than HAART-unexposed youth.
Results were generally similar when comparing HEU youth exposed in utero to zidovudine monotherapy vs. those exposed to two or more ARV drugs in combination; there remained no significant difference in mean Z-scores for LV systolic function parameters, but for LV diastolic function measures, peak early deceleration time Z-score differed significantly between exposure groups (0.10 for zidovudine monotherapy vs. −0.59 for combination ARVs, p =
= .016). A higher adjusted mean LV mass-to-volume ratio Z-score was also observed for combination regimens versus zidovudine monotherapy (0.46 vs. −0.08; see Supplementary Table S2).
.016). A higher adjusted mean LV mass-to-volume ratio Z-score was also observed for combination regimens versus zidovudine monotherapy (0.46 vs. −0.08; see Supplementary Table S2).
Comparisons of mean Z-scores for echocardiographic parameters by first trimester ARV exposure are summarized in Table 3. There were no significant differences in mean Z-scores in either unadjusted or adjusted models for any of the LV systolic or diastolic function parameters.
Table 3.
Results of Linear Regression Models Comparing HIV-Exposed Uninfected Children Born to HIV-Infected Mothers and Exposed or Unexposed In Utero to Any Antiretroviral Therapy During the First Trimester of Pregnancy
| Unadjusted Z-score mean (SE) | AdjustedaZ-score Mean (SE) | |||||
|---|---|---|---|---|---|---|
| Echocardiographic Z-score | Unexposed in first trimester (n = = 102) 102) | Exposed in first trimester (n = = 54) 54) | P | Unexposed in first trimester (n = = 102) 102) | Exposed in first trimester (n = = 54) 54) | P |
| Functional systolic parameters | ||||||
 Contractility Contractility | 0.28 (0.11) | 0.44 (0.15) | .42 | 0.29 (0.10) | 0.43 (0.15) | .43 |
 Fractional shortening Fractional shortening | 0.32 (0.09) | 0.48 (0.13) | .31 | 0.32 (0.09) | 0.50 (0.13) | .26 |
 Ejection fraction Ejection fraction | 0.18 (0.09) | 0.15 (0.12) | .81 | 0.19 (0.09) | 0.15 (0.13) | .81 |
| Functional diastolic parameters | ||||||
 Peak early velocity Peak early velocity | 0.47 (0.10) | 0.16 (0.13) | .063 | 0.45 (0.09) | 0.20 (0.13) | .13 |
 Peak atrial velocity Peak atrial velocity | 0.38 (0.14) | 0.13 (0.18) | .27 | 0.34 (0.13) | 0.21 (0.18) | .57 |
 Peak early/atrial velocity (E/A ratio) Peak early/atrial velocity (E/A ratio) | 0.02 (0.10) | 0.02 (0.14) | .98 | 0.04 (0.10) | −0.02 (0.14) | .73 |
 Peak early tissue (E′) Peak early tissue (E′) | 0.48 (0.16) | 0.31 (0.22) | .53 | 0.47 (0.16) | 0.30 (0.22) | .55 |
 Peak early inflow/early tissue (E/E′) Peak early inflow/early tissue (E/E′) | 0.13 (0.15) | −0.05 (0.20) | .47 | 0.12 (0.14) | −0.01 (0.20) | .61 |
 Peak early deceleration time Peak early deceleration time | −0.37 (0.10) | −0.27 (0.14) | .59 | −0.37 (0.10) | −0.29 (0.14) | .69 |
| Structural parameters | ||||||
 LV mass LV mass | 0.05 (0.09) | 0.15 (0.12) | .52 | 0.05 (0.08) | 0.15 (0.12) | .50 |
 ED LV volume ED LV volume | −0.28 (0.10) | 0.04 (0.14) | .063 | −0.26 (0.09) | −0.01 (0.13) | .094 |
 ES LV volume ES LV volume | −0.37 (0.10) | 0.04 (0.14) | .018 | −0.31 (0.08) | −0.07 (0.12) | .096 |
 Mass-to-volume ratio Mass-to-volume ratio | 0.37 (0.09) | 0.05 (0.13) | .046 | 0.33 (0.09) | 0.13 (0.12) | .17 |
Results of linear regression modeling, excluding those unexposed to any ARVs during pregnancy (N =
= 18). Each row represents a separate linear regression model comparing youth exposed to any ARV during the first trimester of pregnancy with those unexposed to ARVs during the first trimester
18). Each row represents a separate linear regression model comparing youth exposed to any ARV during the first trimester of pregnancy with those unexposed to ARVs during the first trimester
Sensitivity analyses
In the subset of ARV-exposed youth with information on maternal substance use during pregnancy (n =
= 116 of 156, 74%), we observed few associations with echocardiographic parameters. Illicit drug use was associated with a lower mean LV mass (adjusted mean Z
116 of 156, 74%), we observed few associations with echocardiographic parameters. Illicit drug use was associated with a lower mean LV mass (adjusted mean Z =
= −0.46 vs. 0.14, p
−0.46 vs. 0.14, p =
= .029). When the models presented in Tables 2 and and33 were repeated within this subset with further adjustment for illicit drug use, there were essentially no changes to results. Low maternal CD4 cell count (<350 cells/mm3) before delivery was associated with significantly higher LV mass (adjusted mean Z
.029). When the models presented in Tables 2 and and33 were repeated within this subset with further adjustment for illicit drug use, there were essentially no changes to results. Low maternal CD4 cell count (<350 cells/mm3) before delivery was associated with significantly higher LV mass (adjusted mean Z =
= 0.42 vs. −0.04, p
0.42 vs. −0.04, p =
= .004), higher LV volume (adjusted mean Z
.004), higher LV volume (adjusted mean Z =
= 0.18 vs. −0.30, p
0.18 vs. −0.30, p =
= .004), and higher LV contractility (adjusted mean Z
.004), and higher LV contractility (adjusted mean Z =
= 0.61 vs. −0.18, p
0.61 vs. −0.18, p =
= .033). However, the high percentage of youth missing this information precluded comparisons between HAART-exposed and HAART-unexposed subgroups adjusting for maternal health measures.
.033). However, the high percentage of youth missing this information precluded comparisons between HAART-exposed and HAART-unexposed subgroups adjusting for maternal health measures.
In our three sets of multivariate models, fit using sets of LV systolic functional, diastolic functional, and structural Z-scores as vectors of outcomes, we observed no association between in utero HAART exposure and any of these outcomes.
Discussion
We found that HIV-uninfected children born to HIV-infected women who were exposed to HAART in utero had significant differences in LV chamber structure compared with children not exposed to HAART, but LV systolic function was not different between the HAART-exposed and -unexposed cohorts. Specifically, there was a higher LV mass-to-volume ratio in those exposed to HAART in association with LV diastolic function differences consisting of a lower A-wave velocity and a higher E/A ratio.
Abnormalities of LV structure and function associated with antiretroviral therapy have been previously reported,2,9 but the impact of HAART on the myocardium remains controversial. Lipshultz et al.3 reported that in HIV-negative children of HIV-positive mothers, aged 0–2 years, ART exposure was associated with reduced LV mass, interventricular septal thickness, and short axis LV dimension in conjunction with increased LV fractional shortening and contractility. However, the long-term cardiac effects of fetal exposure to ART remain uncertain.
Recently, Cade et al.4 reported a comparison between 30 HIV-negative children (mean age =
= 8
8 ±
± 2 years) born to HIV-positive women with prenatal exposure to ART vs. 30 age-matched HIV-negative children born to HIV-negative women. In their substantially smaller and somewhat younger cohort than in this study, the ART-exposed group had no differences in systolic function, but did have a lower LV mass index and lower early diastolic mitral annular velocity. These findings suggest the possibility of late myocardial effects of in utero ART exposure. These findings are somewhat difficult to interpret because the method used for adjusting LV mass for body size through indexing for body surface area is of questionable validity,10 and notably, nonindexed LV mass was not significantly different between these groups who were in fact matched for age and BSA.
2 years) born to HIV-positive women with prenatal exposure to ART vs. 30 age-matched HIV-negative children born to HIV-negative women. In their substantially smaller and somewhat younger cohort than in this study, the ART-exposed group had no differences in systolic function, but did have a lower LV mass index and lower early diastolic mitral annular velocity. These findings suggest the possibility of late myocardial effects of in utero ART exposure. These findings are somewhat difficult to interpret because the method used for adjusting LV mass for body size through indexing for body surface area is of questionable validity,10 and notably, nonindexed LV mass was not significantly different between these groups who were in fact matched for age and BSA.
The two significant findings in our study of higher LV mass-to-volume ratio in conjunction with differences in LV diastolic behavior (lower late mitral inflow velocity that results in a higher E/A) in the ART-exposed cohort may be causally related since a higher LV mass-to-volume ratio is typically associated with lower ventricular compliance. Alterations in LV diastolic function can be secondary to changes in diastolic relaxation, compliance, or both and are not intrinsically related to systolic properties such as LV ejection fraction. Abnormalities of ventricular compliance and relaxation can be demonstrated by characteristic changes in mitral inflow, tissue Doppler velocity, and pulmonary venous Doppler flow patterns.11
The association between LV hypertrophy and LV diastolic dysfunction has been extensively described in LV hypertrophy models.12 In HIV-infected adults, Grandi13 showed in 60 asymptomatic patients that chronic HAART was associated with an increased LV mass-to-volume ratio and preclinical LV diastolic dysfunction, independent of blood pressure values, findings that parallel the LV remodeling pattern and LV diastolic function differences found in this study.
The fact that the alteration in LV diastolic function in our study cohort may be related to altered LV mass-to-volume ratio is important insofar as it calls into question whether this therapy has a direct adverse effect on diastolic myocardial properties, particularly since no differences were seen in E′ values, which are thought to be a more direct measure of myocardial properties. Although the potential implications of these minor variations from normal are unknown, seemingly mild abnormalities in LV structure have been associated with adverse late outcomes in other situations. For example, LV mass values that were slightly but insignificantly different from normal early after completion of therapy were associated with progressive decline over the ensuing 10 years.14
Because of the observational design of our study, there are some clear limitations. We were unable to fully control for maternal disease severity given the lack of information on maternal CD4 count and viral load for a large percentage of participants, which could have resulted in residual confounding. The two subgroups compared in our primary analyses defined by HAART exposure had some differences in age distributions, although we accounted for such differences through the use of echocardiographic Z-scores calculated relative to age and body size, as well as adjustment for age in our analyses. Despite these limitations, our study is the largest evaluation of long-term cardiac functioning in youth and adolescents who were exposed perinatally to HAART or other ARV regimens. Our consistent standards for echocardiogram measurement and ability to adjust for a number of potential demographic and maternal confounders are additional strengths of our study.
Altered LV systolic function in children with HIV infection is well described predominantly in studies that precede the introduction of ART.15 Lipshultz et al.16 found LV dysfunction and increased LV wall thickness as risk factors for mortality in HIV-infected children (median age 2.1 years). Cunha et al.17 evaluated 93 infected children (average age 3.07 years) exposed and nonexposed to HAART and reported a positive association between the absence of combined ART and LV systolic dysfunction. In the current cohort, there were no significant differences in LV systolic function associated with HAART exposure (Table 2). Preserved LV systolic function associated with impaired LV diastolic function secondary to abnormal LV stiffness and impaired relaxation is a well-known cause of heart failure in adults18 that has to date remained relatively recalcitrant to therapy.19
Conclusions
Taken together, our findings point toward the conclusion that prenatal ART exposure is associated with mild direct cardiac effects that are independent of other factors commonly seen in conjunction with the in utero environment of HIV-infected women. Whether the increase in the LV mass-to-volume ratio and LV diastolic dysfunction with preserved LV systolic function is of long-term importance requires longer follow-up of these vulnerable children. Further studies will be required to confirm this finding and the outcome in adult age and its clinical relevance.
Supplementary Material
Acknowledgments
The authors thank the children and families for their participation in PHACS and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with cofunding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart, Lung, and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T. H. Chan School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) and the Tulane University School of Medicine (HD052104, 3U01HD052104-06S1). Data management services were provided by Frontier Science and Technology Research Foundation, and regulatory services and logistical support were provided by Westat, Inc. The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with cofunding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart, Lung, and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T. H. Chan School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) and the Tulane University School of Medicine (HD052104, 3U01HD052104-06S1). NIH representatives were part of the study team and therefore the sponsor was involved in study design, coordination, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclaimer
The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the U.S. National Institutes of Health or the U.S. Department of Health and Human Services.
References
Articles from AIDS Research and Human Retroviruses are provided here courtesy of Mary Ann Liebert, Inc.
Full text links
Read article at publisher's site: https://doi.org/10.1089/aid.2015.0281
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4931731?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/170591713
Article citations
Letter to the Editor: Cardiac Dysfunction Among Youth With Perinatal HIV Acquisition and Exposure.
J Acquir Immune Defic Syndr, 95(3):e2-e4, 01 Mar 2024
Cited by: 0 articles | PMID: 38408218
Cardio-Metabolic Health of Offspring Exposed in Utero to Human Immuno-Deficiency Virus and Anti-Retroviral Treatment: A Systematic Review.
Biology (Basel), 13(1):32, 06 Jan 2024
Cited by: 0 articles | PMID: 38248463 | PMCID: PMC10813696
Review Free full text in Europe PMC
Direct and indirect cardiovascular and cardiometabolic sequelae of the combined anti-retroviral therapy on people living with HIV.
Front Physiol, 14:1118653, 27 Mar 2023
Cited by: 3 articles | PMID: 37078025 | PMCID: PMC10107050
Review Free full text in Europe PMC
In-utero HIV exposure and cardiometabolic health among children 5-8 years: findings from a prospective birth cohort in South Africa.
AIDS, 37(1):173-182, 19 Oct 2022
Cited by: 2 articles | PMID: 36476456 | PMCID: PMC9751971
Mitochondrial DNA Instability Is Common in HIV-Exposed Uninfected Newborns.
J Clin Med, 10(11):2399, 28 May 2021
Cited by: 1 article | PMID: 34071681 | PMCID: PMC8197798
Go to all (10) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cardiac effects of in-utero exposure to antiretroviral therapy in HIV-uninfected children born to HIV-infected mothers.
AIDS, 29(1):91-100, 01 Jan 2015
Cited by: 21 articles | PMID: 25562493 | PMCID: PMC4287991
Left ventricular diastolic dysfunction in HIV-uninfected infants exposed in utero to antiretroviral therapy.
AIDS, 34(4):529-537, 01 Mar 2020
Cited by: 7 articles | PMID: 31764073 | PMCID: PMC8806162
Cardiac Effects of Highly Active Antiretroviral Therapy in Perinatally HIV-Infected Children: The CHAART-2 Study.
J Am Coll Cardiol, 70(18):2240-2247, 01 Oct 2017
Cited by: 14 articles | PMID: 29073951 | PMCID: PMC5687306
Health and survival of HIV perinatally exposed but uninfected children born to HIV-infected mothers.
Curr Opin HIV AIDS, 11(5):465-476, 01 Sep 2016
Cited by: 28 articles | PMID: 27716731
Review
Funding
Funders who supported this work.
NICHD NIH HHS (2)
Grant ID: U01 HD052102
Grant ID: U01 HD052104
 1
1