Abstract
Background
Treatment with an aromatase inhibitor for 5 years as up-front monotherapy or after tamoxifen therapy is the treatment of choice for hormone-receptor-positive early breast cancer in postmenopausal women. Extending treatment with an aromatase inhibitor to 10 years may further reduce the risk of breast-cancer recurrence.Methods
We conducted a double-blind, placebo-controlled trial to assess the effect of the extended use of letrozole for an additional 5 years. Our primary end point was disease-free survival.Results
We enrolled 1918 women. After a median follow-up of 6.3 years, there were 165 events involving disease recurrence or the occurrence of contralateral breast cancer (67 with letrozole and 98 with placebo) and 200 deaths (100 in each group). The 5-year disease-free survival rate was 95% (95% confidence interval [CI], 93 to 96) with letrozole and 91% (95% CI; 89 to 93) with placebo (hazard ratio for disease recurrence or the occurrence of contralateral breast cancer, 0.66; P=0.01 by a two-sided log-rank test stratified according to nodal status, prior adjuvant chemotherapy, the interval from the last dose of aromatase-inhibitor therapy, and the duration of treatment with tamoxifen). The rate of 5-year overall survival was 93% (95% CI, 92 to 95) with letrozole and 94% (95% CI, 92 to 95) with placebo (hazard ratio, 0.97; P=0.83). The annual incidence rate of contralateral breast cancer in the letrozole group was 0.21% (95% CI, 0.10 to 0.32), and the rate in the placebo group was 0.49% (95% CI, 0.32 to 0.67) (hazard ratio, 0.42; P=0.007). Bone-related toxic effects occurred more frequently among patients receiving letrozole than among those receiving placebo, including a higher incidence of bone pain, bone fractures, and new-onset osteoporosis. No significant differences between letrozole and placebo were observed in scores on most subscales measuring quality of life.Conclusions
The extension of treatment with an adjuvant aromatase inhibitor to 10 years resulted in significantly higher rates of disease-free survival and a lower incidence of contralateral breast cancer than those with placebo, but the rate of overall survival was not higher with the aromatase inhibitor than with placebo. (Funded by the Canadian Cancer Society and others; ClinicalTrials.gov numbers, NCT00003140 and NCT00754845.).Free full text

Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years
Abstract
BACKGROUND
Treatment with an aromatase inhibitor for 5 years as up-front monotherapy or after tamoxifen therapy is the treatment of choice for hormone-receptor–positive early breast cancer in postmenopausal women. Extending treatment with an aromatase inhibitor to 10 years may further reduce the risk of breast-cancer recurrence.
METHODS
We conducted a double-blind, placebo-controlled trial to assess the effect of the extended use of letrozole for an additional 5 years. Our primary end point was disease-free survival.
RESULTS
We enrolled 1918 women. After a median follow-up of 6.3 years, there were 165 events involving disease recurrence or the occurrence of contralateral breast cancer (67 with letrozole and 98 with placebo) and 200 deaths (100 in each group). The 5-year disease-free survival rate was 95% (95% confidence interval [CI], 93 to 96) with letrozole and 91% (95% CI; 89 to 93) with placebo (hazard ratio for disease recurrence or the occurrence of contralateral breast cancer, 0.66; P = 0.01 by a two-sided log-rank test stratified according to nodal status, prior adjuvant chemotherapy, the interval from the last dose of aromatase-inhibitor therapy, and the duration of treatment with tamoxifen). The rate of 5-year overall survival was 93% (95% CI, 92 to 95) with letrozole and 94% (95% CI, 92 to 95) with placebo (hazard ratio, 0.97; P = 0.83). The annual incidence rate of contralateral breast cancer in the letrozole group was 0.21% (95% CI, 0.10 to 0.32), and the rate in the placebo group was 0.49% (95% CI, 0.32 to 0.67) (hazard ratio, 0.42; P = 0.007). Bone-related toxic effects occurred more frequently among patients receiving letrozole than among those receiving placebo, including a higher incidence of bone pain, bone fractures, and new-onset osteoporosis. No significant differences between letrozole and placebo were observed in scores on most subscales measuring quality of life.
CONCLUSIONS
The extension of treatment with an adjuvant aromatase inhibitor to 10 years resulted in significantly higher rates of disease-free survival and a lower incidence of contra-lateral breast cancer than those with placebo, but the rate of overall survival was not higher with the aromatase inhibitor than with placebo. (Funded by the Canadian Cancer Society and others; ClinicalTrials.gov numbers, NCT00003140 and NCT00754845.)
The risk of recurrence of hormone-receptor–positive early breast cancer continues indefinitely.1 Long-term reduction in the risk of recurrence has been achieved with the antiestrogen agent tamoxifen, aromatase inhibitors, or a combination of the two. These treatments are administered in a variety of adjuvant regimens, including tamoxifen for 10 years, tamoxifen for up to 5 years followed by an aromatase inhibitor for 5 years, or an initial aromatase inhibitor for 5 years.2–4 Extrapolating from these results, many patients have chosen to continue taking an aromatase inhibitor for more than 5 years (if they do not have unacceptable side effects), despite the lack of specific data on its value and pending the results of clinical trials. The MA.17R trial was a North American Breast Cancer Group trial that was coordinated by the Canadian Cancer Trials Group. The trial examined the effects of treatment with an aromatase inhibitor for 10 years rather than just 5 years after any duration of prior treatment with tamoxifen.
METHODS
STUDY DESIGN AND PARTICIPANTS
The MA.17R trial was a phase 3, randomized, double-blind, placebo-controlled trial involving postmenopausal women with primary breast cancer who had received 4.5 to 6 years of adjuvant therapy with an aromatase inhibitor, preceded in most patients by treatment with tamoxifen. Within 2 years after completing treatment with the aromatase inhibitor, patients were randomly assigned5 to receive 2.5 mg of letrozole or placebo orally once a day for another 5 years. Participants were stratified according to lymph-node status, prior receipt of adjuvant chemotherapy, the interval between the last dose of aromatase inhibitor and randomization, and the duration of prior receipt of tamoxifen. Women were eligible to participate in the trial if they were disease-free after having completed 4.5 to 6 years of therapy with any aromatase inhibitor. Further eligibility criteria at enrollment included hormone-receptor positivity in the primary tumor (unknown hormone-receptor status was permitted only for patients who had participated in the MA.17 trial), performance status of less than 3 on the Eastern Cooperative Oncology Group scale (a 5-point scale on which higher scores indicate more disease-related disability), and a minimum life expectancy of at least 5 years. Exclusion on the basis of age alone was not permitted.
OVERSIGHT
The institutional review board at each participating institution approved the protocol, which is available with the full text of this article at NEJM.org. All patients provided written informed consent. The trial drug, letrozole, and funding support were provided by Novartis. The Canadian Cancer Trials Group was responsible for the design of the study, the development of the protocol, and the collection and maintenance of the data. All the authors, with assistance from the staff at the central office of the Canadian Cancer Trials Group, contributed to the writing of the manuscript and to the decision to submit the manuscript for publication and vouch for the accuracy and completeness of the data reported and adherence to the protocol. Novartis reviewed the protocol and all amendments before these documents were submitted regulatory agencies and research ethics boards. Novartis did not contribute to the accrual, analysis, or interpretation of the data or to the writing of the manuscript. No one who is not an author contributed to the manuscript.
ASSESSMENTS
Clinical evaluations, which were performed annually, included assessments of new bone fracture and new-onset osteoporosis, routine blood work, mammography, and assessment of toxic effects. Subsequent new diagnoses were reported at follow-up visits. Bone mineral density was measured by means of dual-energy x-ray absorptiometry, and scans were obtained within 12 months after study entry, every 2 years thereafter, and at the completion of treatment. Treatment was discontinued if there was a serious intercurrent illness, unacceptable toxic effects, or disease recurrence or if requested by the patient. Adherence to study procedures was confirmed during all follow-up visits if a participant answered “yes” when asked if she had “been taking the study medication by mouth once per day.” Adverse events were assessed with the use of the National Cancer Institute Common Toxicity Criteria, version 2.0, and quality of life with the use of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) and the Menopause-Specific Quality of Life (MENQOL) questionnaire at baseline and at 12, 24, 36, 48 and 60 months.6,7 Interim safety analyses were reviewed twice yearly by the independent data and safety monitoring committee of the Canadian Cancer Trials Group. The quality-of-life analysis involved between-group comparisons of the change in scores from baseline on the SF-36 physical and mental component summary scores and its eight subscales (physical functioning, role–physical, bodily pain, general health, vitality, social functioning, role–emotional, and mental health) and on the four MENQOL symptom subscales (vasomotor, psychosocial, physical, and sexual). SF-36 scores range from 0 to 100, with higher scores indicating better quality of life, and MENQOL scores range from 1 to 8, with higher scores indicating more bothersome symptoms. The minimum clinically important difference for SF-36 and MENQOL scores is 5 and 0.5, respectively.8,9 (See Section S1 in the Supplementary Appendix, available at NEJM.org, for the methods used to perform the analyses.) Women with at least one assessment were included in the quality-of-life analyses.
TRIAL END POINTS
The primary end point was disease-free survival, which was defined as the time from randomization to recurrence of breast cancer (in the breast or chest wall or at nodal or metastatic sites) or the development of a new primary breast cancer. The occurrence of a second type of cancer or death without breast cancer recurrence were not included as events in the analysis of disease-free survival; data for patients who died without breast cancer recurrence were censored at the date of death. Secondary end points included overall survival, the incidence of contralateral breast cancer, quality of life, and long-term safety.
STATISTICAL ANALYSIS
On the basis of the 43% lower hazard of recurrence found with letrozole as compared with placebo in the preceding MA.17 trial,10 we hypothesized that a 33% lower hazard of recurrence with letrozole as compared with placebo would be seen in the MA.17R trial. We calculated that for the study to have 80% power, at a two-sided 0.05 level, to detect this improvement, 196 events would need to be observed, and we estimated that the target enrollment for that number of events would be 1800 patients. On the basis of the estimate of disease-free survival of 89% at 5 years in the placebo group, it was projected that 196 events would be observed after patients were followed for 4 years. At the 6-year point, by June 4, 2015, only 176 events had been observed and, on the basis of the relatively small loss in the power of the study, a continued decline in the event rate, and limitations in resources, the trial design was amended, with the primary analysis becoming time-based rather than event-based. These changes were approved by the data and safety monitoring committee, the institutional review boards of the participating institutions, and other regulatory bodies. The final database, which was locked on November 13, 2015, had 165 events (cleaned data), providing 80% power to detect a hazard ratio of 0.655 for disease-free survival.
Analyses of pretreatment characteristics and efficacy end points were based on data from all patients who underwent randomization. Analyses of safety and the effects of exposure to the study medication included all participants who received at least one dose of the trial medication. A log-rank test with adjustment for stratification factors performed at the time of randomization was the primary method that we used for the analysis of time-to-event outcomes; binary outcomes were assessed with the use of Fisher’s exact test, and continuous outcomes with the use of the Wilcoxon test. All comparisons between the two groups were made with the use of a two-sided test at an alpha level of 5%, unless otherwise specified. No adjustments were made for multiplicity of inferences for multiple clinical end points.
We report results for the primary outcomes and selected secondary outcomes. Other secondary outcomes are listed in the protocol.
RESULTS
STUDY POPULATION AND DURATION OF TREATMENT
The target enrollment was reached on May 8, 2009, which was 4.5 years after randomization began. A total of 1918 patients were randomly assigned to receive letrozole (959 patients) or placebo (959 patients) (see Fig. S1 in the Supplementary Appendix). Randomization with stratification was used to ensure that the treatment groups were well balanced at baseline (Table 1). The rate of adherence to the study regimen was 62.5% among the patients receiving letrozole and 62.3% among those receiving placebo.
Table 1
Baseline Characteristics.*
| Characteristic | Letrozole (N = 959) | Placebo (N = 959) | Total (N = 1918) |
|---|---|---|---|
| Age — yr† | |||
 Median Median | 65.6 | 64.8 | 65.1 |
 Interquartile range Interquartile range | 60.3–72.0 | 59.6–71.1 | 60.0–71.5 |
| Time from first diagnosis of breast cancer — yr | |||
 Median Median | 10.6 | 10.6 | 10.6 |
 Interquartile range Interquartile range | 7.5–11.5 | 7.8–11.6 | 7.6–11.5 |
| Tumor stage at diagnosis — no. (%)‡ | |||
 T1–T2 T1–T2 | 865 (90.2) | 870 (90.7) | 1735 (90.5) |
 T3–T4 T3–T4 | 87 (9.1) | 79 (8.2) | 166 (8.7) |
 TX TX | 7 (0.7) | 10 (1.0) | 17 (0.9) |
| Nodal stage of disease at diagnosis — no. (%)§ | |||
 N0 N0 | 446 (46.5) | 448 (46.7) | 894 (46.6) |
 N1 N1 | 456 (47.5) | 455 (47.4) | 911 (47.5) |
 N2–N3 N2–N3 | 36 (3.8) | 39 (4.0) | 75 (3.9) |
 NX NX | 21 (2.2) | 17 (1.8) | 38 (2.0) |
| Hormone-receptor status: estrogen, progesterone, or both — no. (%) | |||
 Positive Positive | 945 (98.5) | 950 (99.1) | 1895 (98.8) |
 Negative Negative | 3 (0.3) | 2 (0.2) | 5 (0.3) |
 Unknown or missing Unknown or missing | 11 (1.1) | 7 (0.7) | 18 (0.9) |
| Treatment with tamoxifen — yr | |||
 Median Median | 5.0 | 5.0 | 5.0 |
 Interquartile range Interquartile range | 2.0–5.0 | 2.0–5.0 | 2.0–5.0 |
| Duration of tamoxifen therapy — no. (%) | |||
 0 yr 0 yr | 199 (20.8) | 198 (20.6) | 397 (20.7) |
 >0 to <2 yr >0 to <2 yr | 40 (4.2) | 40 (4.2) | 80 (4.2) |
 2 to <4.5 yr 2 to <4.5 yr | 43 (4.5) | 29 (3.0) | 72 (3.8) |
 4.5 to 6 yr 4.5 to 6 yr | 670 (69.9) | 684 (71.3) | 1354 (70.6) |
 >6 yr >6 yr | 7 (0.7) | 8 (0.8) | 15 (0.8) |
| Duration of previous aromatase-inhibitor therapy¶ | |||
 Median (interquartile range) — yr Median (interquartile range) — yr | 5.0 (5.0–5.1) | 5.0 (5.0–5.1) | 5.0 (5.0–5.1) |
 Distribution — no. (%) Distribution — no. (%) | |||
  <4.5 yr <4.5 yr | 3 (0.3) | 2 (0.2) | 5 (0.3) |
  4.5–6 yr 4.5–6 yr | 949 (99.0) | 950 (99.1) | 1899 (99.0) |
  >6 yr >6 yr | 7 (0.7) | 6 (0.6) | 13 (0.7) |
  Data missing Data missing | 0 | 1 (0.1) | 1 (0.1) |
The median time between the initial diagnosis of breast cancer and randomization was 10.6 years (interquartile range, 7.6 to 11.5). The median duration of prior treatment with tamoxifen was 5 years, with 68.5% of patients having received tamoxifen for 4.5 to 5.5 years and 20.7% having received no tamoxifen. The median duration of prior treatment with an aromatase inhibitor was 5 years, with 95.4% having received 4.5 to 5.5 years of treatment. Almost all patients (99.5%) had been without continuous breaks of longer than 6 months while taking their prior aromatase inhibitor. The median interval between the last dose of aromatase inhibitor and randomization was less than 6 months for 90% of participants, and the median duration of the study regimen was 5 years (mean, 4.3 years). Median follow-up was 75 months (6.3 years).
EFFICACY END POINTS
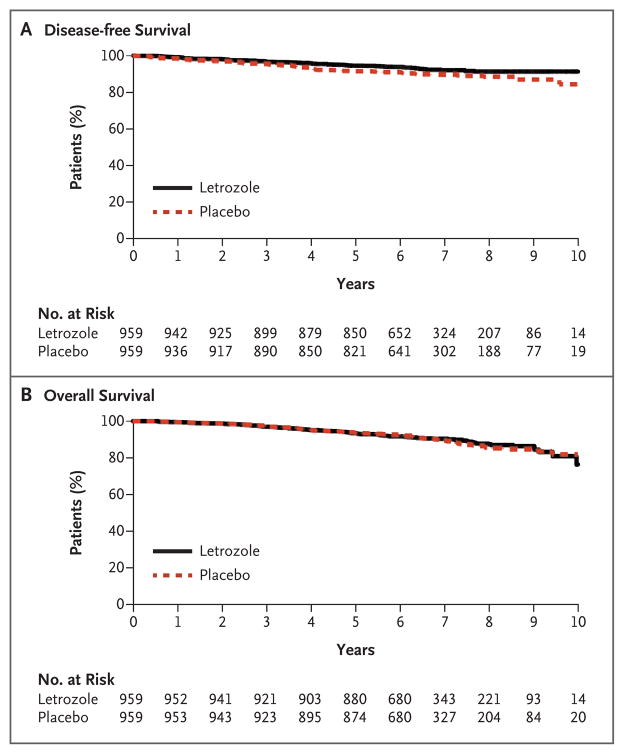
A total of 165 participants had an event involving disease recurrence or the occurrence of contralateral breast cancer (67 in the letrozole group and 98 in the placebo group). Among these patients, 55 in the letrozole group and 68 in the placebo group had recurrent breast cancer, and 13 and 31, respectively, had contralateral breast cancer (Table 2). The Kaplan–Meier curves for disease-free survival are shown in Figure 1A. The rate of 5-year disease-free survival was 95% (95% confidence interval [CI], 93 to 96) in the letrozole group and 91% (95% CI, 89 to 93) in the placebo group. The hazard ratio involving disease recurrence or the occurrence of contralateral breast cancer with letrozole versus placebo was 0.66 (95% CI, 0.48 to 0.91; P = 0.01).
Table 2
Recurrence of Breast Cancer or Occurrence of Contralateral Breast Cancer.
| Variable | Letrozole (N = 959) | Placebo (N = 959) |
|---|---|---|
| number (percent) | ||
| Patients with a recurrence of the primary cancer or with contralateral breast cancer | 67 (7.0) | 98 (10.2) |
|
| ||
| Recurrence*† | 55 (5.7) | 68 (7.1) |
|
| ||
 Local breast Local breast | 8 (0.8) | 10 (1.0) |
|
| ||
 Local chest wall Local chest wall | 6 (0.6) | 7 (0.7) |
|
| ||
 Regional Regional | 5 (0.5) | 13 (1.4) |
|
| ||
 Distant Distant | 42 (4.4) | 53 (5.5) |
|
| ||
  Ascites Ascites | 1 (0.1) | 4 (0.4) |
|
| ||
  Bone Bone | 28 (2.9) | 37 (3.9) |
|
| ||
  Brain Brain | 3 (0.3) | 2 (0.2) |
|
| ||
  Liver Liver | 11 (1.1) | 12 (1.3) |
|
| ||
  Lung Lung | 14 (1.5) | 14 (1.5) |
|
| ||
  Bone marrow Bone marrow | 1 (0.1) | 2 (0.2) |
|
| ||
  Omentum Omentum | 1 (0.1) | 0 |
|
| ||
  Peritoneum Peritoneum | 1 (0.1) | 0 |
|
| ||
  Pleural effusion Pleural effusion | 5 (0.5) | 4 (0.4) |
|
| ||
  Pleura Pleura | 1 (0.1) | 2 (0.2) |
|
| ||
  Other Other | 8 (0.8) | 12 (1.3) |
|
| ||
| Contralateral breast cancer† | 13 (1.4) | 31 (3.2) |
A prespecified sensitivity analysis of disease-free survival that included all deaths from breast cancer as events yielded the same results as the primary analysis because all patients who died of breast cancer also had recurrence before or at the time of their death. A post hoc sensitivity analysis of disease-free survival that included all deaths as events showed a rate of 5-year disease-free survival of 90% (95% CI, 88 to 92) with letrozole versus 88% (95% CI, 86 to 90) with placebo. The hazard ratio for disease recurrence, the occurrence of contralateral breast cancer, or death with letrozole as compared with placebo was 0.80 (95% CI, 0.63 to 1.01; P = 0.06); in a multivariate analysis that was adjusted for stratification factors and the duration of aromatase-inhibitor therapy received before the trial, the hazard ratio was 0.79 (95% CI, 0.63 to 1.00; P=0.05).
The effect of letrozole on disease-free survival was also explored in subgroups defined according to each stratification factor and according to the duration of prior treatment with an aromatase inhibitor; all the subgroups were prespecified in the analysis plan. The superior effect of letrozole was observed in all subgroups, and no significant interactions were observed, indicating a homogeneity of treatment effect across all subgroups (Fig. S2 in the Supplementary Appendix).
A total of 200 participants had died by the time of data cutoff (100 in each study group). The major causes of death in the letrozole and placebo groups were breast cancer (31 and 34 deaths, respectively), other primary cancers (26 and 25), and cardiovascular events (14 and 11). The Kaplan–Meier curves for overall survival are shown in Figure 1B. The rate of 5-year overall survival was 93% (95% CI, 92 to 95) in the letrozole group and 94% (95% CI, 92 to 95) in the placebo group, with a hazard ratio for death of 0.97 (95% CI, 0.73 to 1.28; P = 0.83). No significant difference in overall survival between letrozole and placebo was found in any of the prespecified subgroups.
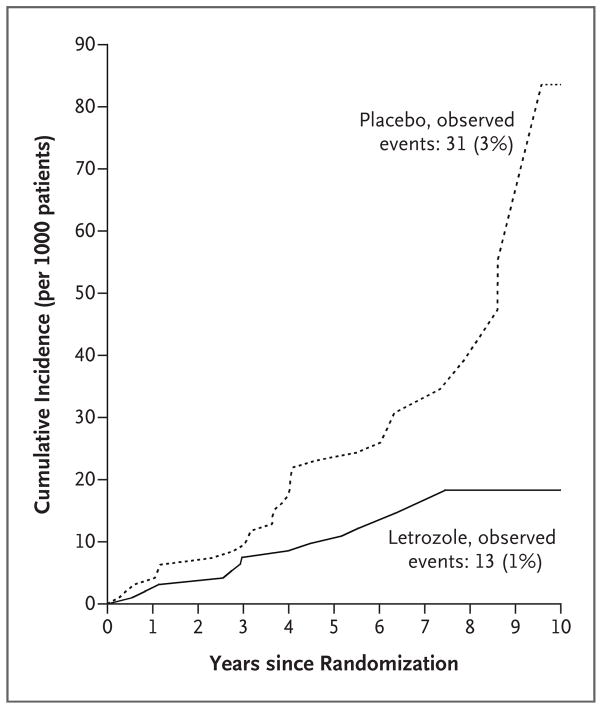
The annual incidence rate of contralateral breast cancer was 0.21% (95% CI, 0.10 to 0.32) in the letrozole group and 0.49% (95% CI, 0.32 to 0.67) in the placebo group (P = 0.007), with a hazard ratio of 0.42 (95% CI, 0.22 to 0.81). The cumulative incidence plot for the time to the development of contralateral breast cancer is shown in Figure 2.
SAFETY
The incidence of most toxic effects was similar in the two groups, with the exception of bone-related toxic effects, which were more common in the letrozole group (Table 3). Few women discontinued treatment because of toxic effects (5.4% in the letrozole group vs. 3.7% in the placebo group).
Table 3
Adverse Events.*
| Event | Letrozole (N = 959) | Placebo (N = 954) | P Value |
|---|---|---|---|
| number (percent) | |||
| Toxic effect during receipt of trial regimen
| |||
| Edema | 158 (16) | 136 (14) | 0.19 |
|
| |||
| Hypertension | 157 (16) | 145 (15) | 0.48 |
|
| |||
| Hot flashes | 360 (38) | 354 (37) | 0.84 |
|
| |||
| Fatigue | 346 (36) | 355 (37) | 0.61 |
|
| |||
| Constipation | 117 (12) | 140 (15) | 0.10 |
|
| |||
| Diarrhea | 105 (11) | 81 (8) | 0.07 |
|
| |||
| Arthritis | 317 (33) | 288 (30) | 0.18 |
|
| |||
| Hypercholesterolemia | 203 (21) | 184 (19) | 0.31 |
|
| |||
| Dizziness | 145 (15) | 139 (15) | 0.74 |
|
| |||
| Headache | 151 (16) | 138 (14) | 0.43 |
|
| |||
| Insomnia | 269 (28) | 243 (25) | 0.20 |
|
| |||
| Arthralgia | 513 (53) | 475 (50) | 0.10 |
|
| |||
| Myalgia | 268 (28) | 240 (25) | 0.31 |
|
| |||
| Bone pain | 174 (18) | 133 (14) | 0.01 |
|
| |||
| Dyspnea | 148 (15) | 165 (17) | 0.27 |
|
| |||
| Vaginal dryness | 102 (11) | 96 (10) | 0.68 |
|
| |||
| Elevated alkaline phosphatase level — no./total no. (%)† | 111/928 (12) | 78/916 (9) | 0.01 |
|
| |||
| Elevated aspartate aminotransferase level — no./total no. (%)† | 133/928 (14) | 131/915 (14) | 0.92 |
|
| |||
| Elevated alanine aminotransferase level — no./total no. (%)† | 97/909 (11) | 128/894 (14) | 0.02 |
|
| |||
| Bone fracture‡ | 133 (14) | 88 (9) | 0.001 |
|
| |||
 Spine Spine | 17 (2) | 9 (1) | 0.12 |
|
| |||
 Wrist Wrist | 27 (3) | 16 (2) | 0.09 |
|
| |||
 Pelvis Pelvis | 1 (<1) | 7 (1) | 0.08 |
|
| |||
 Hip Hip | 7 (1) | 6 (1) | 0.79 |
|
| |||
 Femur Femur | 9 (1) | 4 (<1) | 0.17 |
|
| |||
 Tibia Tibia | 5 (1) | 4 (<1) | 0.74 |
|
| |||
 Ankle Ankle | 19 (2) | 11 (1) | 0.14 |
|
| |||
 Other Other | 68 (7) | 48 (5) | 0.06 |
|
| |||
| New-onset osteoporosis | 109 (11) | 54 (6) | <0.001 |
|
| |||
| Cardiovascular event | 116 (12) | 98 (10) | 0.21 |
|
| |||
| Toxic effect after discontinuation of trial regimen
| |||
| Hot flashes | 25 (3) | 17 (2) | 0.22 |
|
| |||
| Arthralgia | 22 (2) | 24 (3) | 0.75 |
|
| |||
| Hypertension | 8 (1) | 4 (<1) | 0.25 |
|
| |||
| Superventricular arrhythmia | 6 (1) | 3 (<1) | 0.32 |
|
| |||
| Bone fracture‡ | 54 (6) | 57 (6) | 0.75 |
|
| |||
 Spine Spine | 13 (1) | 9 (1) | 0.40 |
|
| |||
 Wrist Wrist | 4 (<1) | 4 (<1) | 1 |
|
| |||
 Pelvis Pelvis | 3 (<1) | 4 (<1) | 0.70 |
|
| |||
 Hip Hip | 5 (1) | 0 | 0.07 |
|
| |||
 Femur Femur | 2 (<1) | 7 (1) | 0.09 |
|
| |||
 Tibia Tibia | 3 (<1) | 2 (<1) | 0.65 |
|
| |||
 Ankle Ankle | 3 (<1) | 6 (1) | 0.31 |
|
| |||
 Other Other | 32 (3) | 31 (3.2) | 0.92 |
|
| |||
| New-onset osteoporosis | 20 (2) | 11 (1.2) | 0.11 |
Whereas patients receiving letrozole had a mean loss of bone mineral density in the total hip (mean loss, −3.2%) and an increase of 1.4% in the lumbar spine at the time that treatment was discontinued, there was an increase in bone mineral density in both the hip and the spine in patients receiving placebo (mean gain in the hip, 22.4%, and in the spine, 4.5%). The between-group difference in the mean change in bone mineral density was significant, favoring placebo (P<0.001). A significantly greater number of participants receiving letrozole than those receiving placebo had a T score at the lumbar spine that was less than −2.5 at any time after baseline (10% vs. 7%, P = 0.03). A similar percentage of patients in the two groups used bone-protecting medications during the trial, including calcium supplements (86.1% in both groups), vitamin D supplements (84.5% in both groups), a selective estrogen-receptor modulator (0.3% in both groups), and bisphosphonates (46.2% in the letrozole group and 46.6% in the placebo group). Among 133 patients receiving letrozole who had a fracture during the trial period, 56% were taking bisphosphonates, 90% were taking a calcium supplement, and 86% were taking a vitamin D supplement; among 88 patients receiving placebo who had a fracture during protocol therapy, 55% were taking bisphosphonates, 86% a calcium supplement, and 88% a vitamin D supplement.
QUALITY OF LIFE
In both groups, more than 85% of the participants completed the quality-of-life assessment at each time point. No significant between-group differences were observed in the SF-36 summary scores or in the majority of the subscale scores. Overall, the reduction in scores (indicating worse quality of life) was greater among women in the letrozole group than among those in the placebo group in the role–physical subscale (between-group difference in the change in score, 3.2; P = 0.009). The interaction between group assignment and time was significant for the bodily pain (P = 0.03) and the role–emotional (P = 0.03) subscales, indicating a change in between-group differences over time. Specifically, bodily pain was greater with letrozole than with placebo at 12, 24, and 36 months but lower at months 48 and 60. However, when single time points were compared, the between-group difference was of only borderline significance at 12 months (P = 0.07). Regarding the role–emotional sub-scale, women receiving letrozole had better scores than those receiving placebo at months 12, 36, and 60, but their scores were worse than the scores for those receiving placebo at months 24 and 48. Comparison at single time points showed a significant difference only at 60 months, in favor of letrozole (change in score, −3.1 with letrozole vs. −8.6 with placebo; P = 0.01). No significant between-group differences were observed on any of the four MENQOL symptom subscales.
DISCUSSION
The MA.17R trial explored the effect of extending adjuvant treatment with an aromatase inhibitor beyond 5 years in women with early breast cancer. We showed that treatment with an aromatase inhibitor for an additional 5 years after initial treatment for 4.5 to 6 years was beneficial in preventing disease recurrence, independent of nodal status, prior adjuvant chemotherapy, time since the last dose of aromatase inhibitor, and duration of prior therapy with tamoxifen or an aromatase inhibitor. The risk of disease recurrence and contralateral breast cancer was significantly lower (by 34%) among women who continued aromatase inhibitor for 10 years than among women who received placebo after the initial 5 years of aromatase-inhibitor therapy. No overall difference in survival was noted at a median follow-up of 6.3 years. The significant benefit in disease-free survival includes not only a numerically larger reduction in events of local, regional, and distant recurrence but also an apparently greater proportional reduction in events of contralateral breast cancer, which may partly explain the absence thus far of an observed overall survival benefit.
For patients receiving up-front treatment with tamoxifen, the extension of tamoxifen2,3 or an aromatase inhibitor to 10 years has been shown to be beneficial in reducing the ongoing risk of recurrence.10,11 However, most postmenopausal patients with hormone-receptor–positive early breast cancer now receive 5 years of treatment with an aromatase inhibitor as up-front therapy. For this overwhelming majority of patients with breast cancer, it was previously unclear whether the extension of treatment with an aromatase inhibitor beyond 5 years would be beneficial.
As patients in the placebo group stopped taking the aromatase inhibitor and quickly reverted to normal postmenopausal estrogen levels, they had an improvement in bone health, as would be expected when aromatase-inhibitor therapy is stopped.12 In addition, a significantly greater percentage of the women taking letrozole than those taking placebo had both new-onset osteoporosis and more clinical fractures — either major osteoporotic fractures (i.e., fragility fractures at the typical osteoporotic sites of the proximal femur [hip], thoracic or lumbar vertebrae [spine], distal forearm [wrist], or proximal humerus [shoulder]) or other fragility fractures (i.e., fragility fractures at any other skeletal site). The increases in bone mineral density that were observed among patients receiving placebo were anticipated. Only a minority of the fractures in both groups were located in the hip, spine, pelvis, or femur, and no significant change in physical health was recorded in either group, perhaps because most of the women in both groups took bone-protecting supplements or medications during the study. Overall, the low incidence of reported toxic effects is probably due to self-selection on the part of study participants who had had few unacceptable side effects through the first 5 years of aromatase-inhibitor therapy and were thus willing to undergo another 5 years of treatment. It is also reassuring that there were no significant between-group differences in the outcomes related to most measures of quality of life. The significant differences in favor of placebo were observed in only one subscale of the SF-36 — role function related to physical health — but the difference (3.2 points) was less than the minimum clinically important difference (5 points on a 100-point scale).8
The validity of the adherence data may be limited because we used a measure that relies on self-report. The rates of adherence observed were at the lower end of the range reported in other studies of early adjuvant endocrine therapy on the basis of longitudinal claims data13,14 or combinations of prescription records and physician recall.15
Our study shows that it is safe and beneficial for postmenopausal patients with hormone-receptor–positive breast cancer to take an aromatase inhibitor as adjuvant therapy for 5 years after initial treatment. Although most post-menopausal patients now receive an aromatase inhibitor as up-front therapy, only 21% of the patients in the MA.17R trial had not been previously treated with tamoxifen. Although the hazard ratios for disease progression and contralateral breast cancer were similar among patients with different durations of prior tamoxifen exposure and no interaction effects between study regimen and duration of prior tamoxifen exposure were observed in our study, it is likely that the benefits of treatment, toxic effects, and quality of life differ among these groups. It is conceivable, for example, that since the baseline hazard of recurrence declines over time, the absolute benefit of extended endocrine therapy is higher in the first few years after diagnosis and declines over time (in the presence of similar hazard ratios, as shown). Consequently, women who are discontinuing treatment after having received an aromatase inhibitor for 5 years without prior treatment with tamoxifen might be the ones who realize the most benefit. Ultimately, the decision of whether a patient should receive prolonged therapy with an aromatase inhibitor will depend largely on the extent of its effect on her in terms of toxic effects and quality of life, the extent to which bone mineral density is maintained, as indicated by sequential scans, and the patient’s individual risk of disease recurrence.
Acknowledgments
Supported by grants from the Canadian Cancer Society Research Institute (021039 and 015469), the National Cancer Institute (CA180888, CA189953, CA180828, CA13612, CA37981, CA077202, CA180863, CA67753, CA189805 [to Dr. Sturtz], CA16116, CA180802 [to Dr. Wolff], CA16116, and CA180802 [to Dr. Robert]), the Canadian Cancer Trials Group (CA077202 and CA180863), the ECOG-ACRIN Cancer Research Group (CA180820 and CA21115), and Novartis Pharmaceuticals. Dr. Goss was funded in part by the Avon Foundation.
Dr. Pritchard reports receiving fees for serving on advisory boards from AstraZeneca, Pfizer, Roche, Amgen, Novartis, GlaxoSmithKline and Eisai, consulting fees from Pfizer and Novartis, and lecture fees from Novartis; Dr. Muss, serving as an uncompensated consultant and advisor to Pfizer and Harbor-Path and serving on the board of directors of HarborPath; Dr. Gralow, serving on data safety and monitoring committees for Novartis and Roche-Genentech and on a steering committee for Roche-Genentech; Dr. Whelan, receiving fees for serving on an advisory board from Genomic Health and fees for testing reagents from NanoString; Dr. Winer, receiving grant support through his institution from Novartis; Dr. Hudis, receiving consulting fees and fees for serving on advisory boards from Novartis, Pfizer, and AstraZeneca; and Dr. Stopeck, receiving consulting fees from Amgen, Genentech, and BioMarin and honoraria from Amgen and serving on a data safety and monitoring committee for Pfizer and a steering committee for Sandoz. No other potential conflict of interest relevant to this article was reported.
We thank Jessica St. Louis for her administrative support in the preparation and submission of an earlier version of this manuscript.
APPENDIX
The authors’ full names and academic degrees are as follows: Paul E. Goss, M.D., Ph.D., James N. Ingle, M.D., Kathleen I. Pritchard, M.D., Nicholas J. Robert, M.D., Hyman Muss, M.D., Julie Gralow, M.D., Karen Gelmon, M.D., Tim Whelan, B.M., B.Ch., Kathrin Strasser-Weippl, M.D., Sheldon Rubin, M.D., Keren Sturtz, M.D., Antonio C. Wolff, M.D., Eric Winer, M.D., Clifford Hudis, M.D., Alison Stopeck, M.D., J. Thaddeus Beck, M.D., Judith S. Kaur, M.D., Kate Whelan, M.Sc., Dongsheng Tu, Ph.D., and Wendy R. Parulekar, M.D.
The authors’ affiliations are as follows: the Massachusetts General Hospital Cancer Center, Avon International Breast Cancer Research Program (P.E.G.), Harvard Medical School (P.E.G., E.W.), and Dana–Farber Cancer Institute (E.W.), Boston; the Department of Oncology, Mayo Clinic, Rochester, MN (J.N.I., J.S.K.); Sunnybrook Odette Cancer Centre, Toronto (K.I.P.), British Columbia Cancer Agency, Vancouver (K.G.), Canadian Cancer Trials Group, Queen’s University, Kingston, ON (K.W., D.T., W.R.P.), Department of Oncology, McMaster University, Hamilton, ON (T.W.), and Dalhousie University Faculty of Medicine, Moncton Hospital, Moncton, NB (S.R.) — all in Canada; Virginia Cancer Specialists–US Oncology Network, Fairfax (N.J.R.); University of North Carolina–Lineberger Comprehensive Cancer Center, Chapel Hill (H.M.); University of Washington School of Medicine, Seattle (J.G.); Center of Oncology and Hematology, Wilheminen Hospital, Vienna (K.S.-W.); Colorado Cancer Research Program, Denver (K.S.); Johns Hopkins Kimmel Cancer Center, Baltimore (A.C.W.); Memorial Sloan Kettering Cancer Center, New York (C.H.); University of Arizona, Tucson (A.S.); and Highlands Oncology Group, Fayetteville, AR (J.T.B.).
Footnotes
References
Full text links
Read article at publisher's site: https://doi.org/10.1056/nejmoa1604700
Read article for free, from open access legal sources, via Unpaywall:
https://www.nejm.org/doi/pdf/10.1056/NEJMoa1604700?articleTools=true
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1056/nejmoa1604700
Article citations
Patient-reported outcomes, and perceptions and knowledge about recurrence in women with hormone receptor-positive breast cancer.
Breast Cancer Res Treat, 21 Oct 2024
Cited by: 0 articles | PMID: 39432162
Impact of extended endocrine therapy for patients with risk factors for late recurrence in estrogen receptor-positive, human epidermal growth factor receptor 2-negative breast cancer after 5 years of endocrine therapy.
Breast Cancer Res Treat, 17 Oct 2024
Cited by: 0 articles | PMID: 39417907
Adjuvant endocrine therapy and risk of contralateral breast cancer: a systematic review and meta-analysis of observational studies.
Cancer Causes Control, 09 Oct 2024
Cited by: 0 articles | PMID: 39382775
Review
Updates in Systemic Treatment of Hormone Receptor-Positive Early-Stage Breast Cancer.
Curr Treat Options Oncol, 25(10):1323-1334, 03 Oct 2024
Cited by: 0 articles | PMID: 39361142
Review
Clinical Treatment Score Post-5 Years (CTS5) and Late Recurrence Risk in Hormone Receptor-Positive, HER2-Positive Breast Cancer.
J Natl Compr Canc Netw, 22(7):463-468, 26 Aug 2024
Cited by: 0 articles | PMID: 39191270 | PMCID: PMC11473094
Go to all (298) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT00003140
- (1 citation) ClinicalTrials.gov - NCT00754845
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer.
N Engl J Med, 361(8):766-776, 01 Aug 2009
Cited by: 273 articles | PMID: 19692688 | PMCID: PMC2921823
Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial.
Lancet Oncol, 20(1):88-99, 30 Nov 2018
Cited by: 63 articles | PMID: 30509771 | PMCID: PMC6691732
Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a.
J Natl Cancer Inst, 99(24):1845-1853, 11 Dec 2007
Cited by: 153 articles | PMID: 18073378
Reducing the risk for breast cancer recurrence after completion of tamoxifen treatment in postmenopausal women.
Clin Ther, 29(8):1535-1547, 01 Aug 2007
Cited by: 8 articles | PMID: 17919537
Review
Funding
Funders who supported this work.
NCI NIH HHS (29)
Grant ID: CA37981
Grant ID: N01 CA013612
Grant ID: U10 CA067753
Grant ID: CA077202
Grant ID: CA180802
Grant ID: CA180820
Grant ID: CA189805
Grant ID: CA21115
Grant ID: CA67753
Grant ID: U10 CA180791
Grant ID: U10 CA180802
Grant ID: CA180888
Grant ID: U10 CA013612
Grant ID: U10 CA037981
Grant ID: U10 CA180820
Grant ID: U10 CA180863
Grant ID: UG1 CA189805
Grant ID: CA189953
Grant ID: U10 CA016116
Grant ID: UG1 CA189953
Grant ID: CA180828
Grant ID: P30 CA008748
Grant ID: U10 CA021115
Grant ID: U10 CA077202
Grant ID: U10 CA180828
Grant ID: U10 CA180838
Grant ID: CA16116
Grant ID: CA180863
Grant ID: U10 CA180888