Abstract
Free full text

The Kidney: An Organ in the Front Line of Oxidative Stress-Associated Pathologies
Abstract
Both acute kidney injury (AKI) and chronic kidney disease (CKD) are major causes of renal failure in humans and are associated with high incidences of morbidity and mortality rates. AKI and CKD are closely interconnected, and fueled by the obesity and diabetes epidemic, their prevalence is alarmingly increasing to the point that it currently represents a major heath issue worldwide. The kidney is an organ that is particularly sensitive to redox imbalance, resulting in excessive production of reactive oxygen species. Oxidative stress is viewed as a critical pathogenic factor implicated in the initiation, development, and progression of most renal diseases. This Forum discusses the redox-dependent factors and mechanisms accounting for the perturbation of renal function and circulation in the context of the major kidney pathologies linked to hypertension, diabetes, and cancer. Antioxid. Redox Signal. 25, 639–641.
 : renal injury, oxidative stress, reactive oxygen species
: renal injury, oxidative stress, reactive oxygen speciesAcute kidney injury (AKI) and chronic kidney disease (CKD) are the principal causes of renal damages, ultimately leading to organ failure (2, 7). Both disorders are closely intertwined and share similar risk factors, including hypertension, obesity, or diabetes that pertain to these conditions (2, 4, 6–8). The obesity and diabetes epidemics have led to a dramatic increase in the prevalence of CKD and maybe to some extent AKI (4, 6, 8). This interdependence between these different types of kidney diseases and their risk factors are further illustrated by the emerging idea that hypertension and diabetes may increase the risk of kidney cancer (9). The acute and chronic injury as well as the tumorigenic processes responsible for kidney damages and loss of renal function are driven by common mediators and molecular mechanisms, particularly excessive reactive oxygen species (ROS) production (1, 5, 7). These observations are consistent with the fact that the kidney is an organ that is highly sensitive to oxidative stress, due, in part, to its role as an oxygen sensor. This Forum presents compelling evidence of the extreme vulnerability of renal cells in ROS-related kidney diseases.
This Forum of Antioxidants & Redox Signaling delivers a diverse view of old and novel factors affecting ROS production and/or affected by changes in redox status in the context of kidney diseases. The understanding of the molecular mechanisms underlying oxidative stress-mediated kidney injury is a prerequisite for the design of relevant therapeutic approaches to forestall the onset and curb or reverse the progression of renal diseases. This Forum contains four review articles and two original research articles. The articles were chosen to feature topics of intense research in the field, and encompass biochemical, molecular, or physiological studies, as well as discussions of the impact of aberrant ROS generation on renal function and circulation.
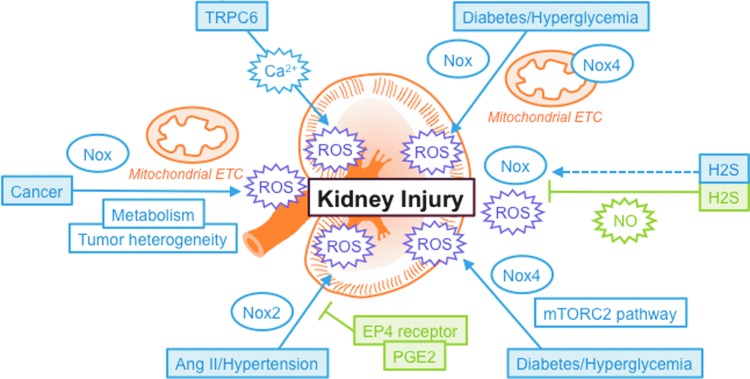
There is emerging evidence that reciprocal interplays between ROS and calcium (Ca2+) signaling are implicated in the control of glomerular and tubulointerstitial cell function. The review by Ma et al. provides comprehensive information on the status of canonical transient receptor potential 6 channel (TRPC6), a member of the Ca2+-conductive channels of the TRPC family, as a novel target of ROS in renal diseases and particularly in the setting of diabetic nephropathy, the complication of diabetes in the kidney. The review stresses the apparent opposing effects of ROS on TRPC6 expression and activity in different kidney cells that appear to be dependent on the cell type and the pathological environment.
Jha et al. provides an in-depth overview of our current understanding of the role of oxidative stress in the pathogenesis of diabetic nephropathy. The authors draw our attention to the importance of NADPH oxidases of the Nox family as mediators of renal cell injury in the diabetic kidney, highlighting how the generation of novel global or cell-specific Nox-deficient mice models was instrumental to help advance the knowledge in the field. The review details which Nox homologues are responsible for diabetes-induced cell injury in the different types of renal cells, including glomerular and tubulointerstitial cells. The role of other sources of ROS in the diabetic kidney, especially the controversial contribution of mitochondrial ROS, is also discussed. This is highly relevant since the general belief that the diabetic state is accompanied by increased ROS production in mitochondria has been recently challenged by the work from Sharma's group, showing a reduction in mitochondrial superoxide in kidney from mice models of type 1 diabetes (3). The present review points out the discrepancies between this report and other in vivo and in vitro studies, demonstrating that homologue Nox4 mediates the increase in mitochondrial superoxide in kidney cortex from diabetic mice and in renal cells exposed to high glucose concentrations. Possible explanations for these opposite results are proposed. Finally, an informed description of the different Nox-specific and Nox-nonspecific inhibitors available and a comparison of their effectiveness as potential therapeutic agents for the treatment of diabetic kidney disease is also presented.
The original article by Eid et al. provides new data demonstrating that mammalian target of rapamycin complex (mTORC)2 signaling cascade contributes to podocyte injury in diabetic nephropathy. The study combines in vitro and in vivo studies and is a continuation of the work of the group that previously documented the implication of mTORC1 pathway in diabetes-mediated podocyte damages. The authors demonstrate that mTORC2 exerts its deleterious actions via Nox4 upregulation and subsequent increase in ROS generation. The causal relationship between mTORC2 and podocyte damage was established in vivo by showing that downregulation of mTORC2 component Rictor with antisense oligonucleotides in OVE26 type 1 diabetic mice preserves podocyte function. The potential for translation to human is high since mTORC2 inhibitors are currently under development in the field of cancer (including renal cell carcinoma) research and could be useful for the treatment of diabetic complications.
The original article by Thibodeau et al. and the review by Feliers et al. both describe factors that can be renoprotective in certain conditions. Thibodeau et al. provide the first evidence that vascular smooth muscle prostaglandin E2 (PGE2) E-prostanoid 4 (EP4) receptor deletion predisposes to renal injury through uncontrolled vasoconstrictive actions of angiotensin II (Ang II) in a hypertension model. The alteration of renal function observed in the absence of PGE2/EP4 receptor signaling may be due to enhanced Nox2 expression and ROS production. The revelation of the beneficial effects of PGE2 and EP4 receptor in Ang II-induced hypertension was made possible by the generation of a novel inducible vascular smooth muscle cell-specific EP4 receptor deletion knockout mice model. In their review, Feliers et al. address the role of hydrogen sulfide (H2S), a gas that has recently received accrued attention, in renal function and pathology. There is a general understanding that H2S can serve as an agent that ameliorates kidney injury in some disease states, including ischemia reperfusion (a form of AKI) and diabetic kidney disease. Nevertheless, the review also points out that the role of H2S is complex and dependent on the context as it may be a mediator kidney injury in some circumstances. The importance of the relationship between H2S and nitric oxide (NO) pathway and its function as a regulator of oxidative stress are also discussed.
Shanmugasundaram and Block examine the key role of oxidative stress in kidney cancers and discuss how intra- and inter-tumor mediators enhance ROS levels and drive tumor heterogeneity. The authors elegantly review the current molecular and biochemical understandings of renal cell carcinoma with emphasis on the role of metabolic syndrome (e.g., effects of diabetes, obesity, and/or hypertension) and tumor environment in tumor heterogeneity, metastasis, and drug resistance. The review makes the compelling argument that the release of ROS by the known major sources of oxidants such as Nox NADPH oxidases or mitochondria in specific subcellular domains and compartments varies over the course of tumor development and actively participates in tumor heterogeneity. These considerations are critical for the design of more specific and effective therapeutic interventions and provide a solid framework for earlier and better diagnosis of renal cell carcinoma.
The scope and context of this Forum are summarized in the accompanying Figure 1. The pathways regulated by ROS or controlling oxidative stress described in this Forum represent potential therapeutic avenues for the treatment of kidney diseases. The exploration of these redox mechanisms is highly relevant since there is an urgent medical need for identification of novel targets for the development of strategies for rational treatment of glomerular and tubulointerstitial injury either at early stages of the disease when it may be possible to prevent initial pathophysiological responses or at more advanced stages to slow down progression of renal disease or even reverse established lesions. These considerations are critical as, in most of the cases, standard care therapies are not fully effective and mainly slow down the disease without being able to reverse it. For instance, in the context of diabetic kidney disease, euglycemia and metabolic control are difficult goals to achieve and blockade of the renin–angiotensin system is often incomplete. It is also becoming clear that therapies targeting multiple molecular redox pathways involved in the pathogenesis of kidney diseases will be more advantageous and effective. Therefore, studies examining the functional relationships between the different redox signaling cascades represent important lines of research with as a primary goal to harness the knowledge of these interactions to intervene and preserve renal function.
Abbreviations Used
| AKI | acute kidney injury |
| Ang II | angiotensin II |
| Ca2+ | calcium |
| CKD | chronic kidney disease |
| EP4 | E-prostanoid 4 |
| H2S | hydrogen sulfide |
| mTORC1 | mammalian target of rapamycin complex 1 |
| mTORC2 | mammalian target of rapamycin complex 2 |
| NO | nitric oxide |
| Nox | NADPH oxidase |
| PGE2 | prostaglandin E2 |
| ROS | reactive oxygen species |
| TRPC6 | transient receptor potential 6 channel |
Acknowledgments
Y.G. is funded by the National Institutes of Health (NIH) Grants UL1 TR001120, RO1 DK 079996, and RO1 DK 33665 as well as an award from the Qatar National Research Fund, National Priorities Research Program (NPRP8-1750-3-360).
References
Articles from Antioxidants & Redox Signaling are provided here courtesy of Mary Ann Liebert, Inc.
Full text links
Read article at publisher's site: https://doi.org/10.1089/ars.2016.6804
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc5069705?pdf=render
Citations & impact
Impact metrics
Article citations
A low-dose pemetrexed-cisplatin combination regimen induces significant nephrotoxicity in mice.
BMC Nephrol, 25(1):370, 21 Oct 2024
Cited by: 0 articles | PMID: 39434019 | PMCID: PMC11494951
Benchmark Dose of Melamine Exposure for a Renal Injury Marker Mediated by Oxidative Stress: Examples in Patients with Urolithiasis and Occupational Workers.
Toxics, 12(8):584, 11 Aug 2024
Cited by: 0 articles | PMID: 39195686 | PMCID: PMC11359403
Multiomics Analyses Identify AKR1A1 as a Biomarker for Diabetic Kidney Disease.
Diabetes, 73(7):1188-1195, 01 Jul 2024
Cited by: 2 articles | PMID: 38394643 | PMCID: PMC11189831
DKK3 promotes oxidative stress injury and fibrosis in HK-2 cells by activating NOX4 via β-catenin/TCF4 signaling.
Mol Cell Biochem, 479(5):1231-1241, 27 Jun 2023
Cited by: 0 articles | PMID: 37368156
Activation of NRF2 Signaling Pathway Delays the Progression of Hyperuricemic Nephropathy by Reducing Oxidative Stress.
Antioxidants (Basel), 12(5):1022, 28 Apr 2023
Cited by: 4 articles | PMID: 37237889 | PMCID: PMC10215166
Go to all (24) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Oxidative stress and autophagy: crucial modulators of kidney injury.
Redox Biol, 4:208-214, 13 Jan 2015
Cited by: 159 articles | PMID: 25613291 | PMCID: PMC4803795
Review Free full text in Europe PMC
Acute Kidney Injury: Tubular Markers and Risk for Chronic Kidney Disease and End-Stage Kidney Failure.
Blood Purif, 41(1-3):144-150, 15 Jan 2016
Cited by: 20 articles | PMID: 26764483
Review
Pyroptosis and Redox Balance in Kidney Diseases.
Antioxid Redox Signal, 35(1):40-60, 17 Mar 2021
Cited by: 19 articles | PMID: 33559516
Review
Mitochondrial dysfunction and the AKI-to-CKD transition.
Am J Physiol Renal Physiol, 319(6):F1105-F1116, 19 Oct 2020
Cited by: 71 articles | PMID: 33073587
Review
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR001120
NIDDK NIH HHS (2)
Grant ID: R01 DK033665
Grant ID: R01 DK079996