Abstract
Free full text

Argentinian pistachio oil and flour: a potential novel approach of pistachio nut utilization
Abstract
In order to searching a potential novel approach to pistachio utilization, the chemical and nutritional quality of oil and flour from natural, roasted, and salted roasted pistachios from Argentinian cultivars were evaluated. The pistachio oil has high contents of oleic and linoleic acid (53.5 - 55.3, 29 - 31.4 relative abundance, respectively), tocopherols (896 - 916 μg/g oil), carotenoids (48 - 56 μg/g oil) and chlorophylls (41 - 70 μg/g oil), being a good source for commercial edible oil production. The processing conditions did not affect significantly the fatty acid and minor composition of pistachio oil samples. The content of total phenolic (TP) and flavonoids (FL) was not significantly modified by the roasting process, whereas free radical scavenging (DPPH radical) and antioxidant power decreased in a 20% approximately. Furthermore, salted roasted pistachio flour (SRPF) showed a significant decrease in TP and FL content in comparison to others samples. The phenolic profile of pistachio flours evaluated by LC-ESI-QTOF-MS. The major compounds identified were (+)-catechin (38 - 65.6 μg/g PF d.w.), gallic acid (23 - 36 μg/g PF d.w.) and cyanidin-3-O-galactoside (21 - 23 μg/g PF d.w.). The treatments effects on the phenolics constituents of pistachio flour. Roasting caused a significant reduction of some phenolics, gallic acid and (+)- catechin, and increased others, naringenin and luteolin. Salting and roasting of pistachio increased garlic acid and naringenin content.
Abstract
In order to searching a potential novel approach to pistachio utilization, the chemical and nutritional quality of oil and flour from natural, roasted, and salted roasted pistachios from Argentinian cultivars were evaluated. The pistachio oil has high contents of oleic and linoleic acid (53.5–55.3, 29–31.4 relative abundance, respectively), tocopherols (896–916 μg/g oil), carotenoids (48–56 μg/g oil) and chlorophylls (41–70 μg/g oil), being a good source for commercial edible oil production. The processing conditions did not affect significantly the fatty acid distribution and minor components of pistachio oil samples. The roasting process not diminish total phenolic (TP) and flavonoids (FL) content significantly compared to natural pistachio flour (NPF), even so reduced the DPPH antioxidant capacity (approximately 20 %) in the roasted pistachio flour (RPF). Furthermore, salted roasted pistachio flour (SRPF) showed a slight and significant decrease on TP and FL content in relation to the others samples. The phenolic profile of pistachio flours were evaluated by LC-ESI-QTOF-MS. The major compounds were (+)-catechin (38–65.6 μg/g PF d.w.), gallic acid (23–36 μg/g PF d.w.) and cyanidin-3-O-galactoside (21–23 μg/g PF d.w.). The treatments have different effects on the phenolics constituents of pistachio flour. Roasting caused a significant reduction of some phenolics, gallic acid and (+)-catechin, and increased others, naringenin and luteolin. Otherwise, salting and roasting of pistachio increased levels of gallic acid and naringenin. These results suggest that Argentinian pistachio oil and flour could be considered as ingredients into applications that enhance human health.
Introduction
Nowadays, consumers’ tendency on choosing food is more associated to health and wellness. This situation can clearly be seen on market with the supply of products distinguished by their content on polyunsaturated fatty acids, antioxidants, dietary fibre, and other components that usual consumers are learning to recognize as a healthy contribution. The Dietary Guidelines for Americans recommend that consuming nuts (almonds, walnuts, pistachios, pecans, and peanuts) as a part of daily diet provides beneficial effect on human health (John and Shahidi 2010).
The pistachio tree (Pistacia vera L.) is a member of the Anacardiaceae family, which is a species native from Central and Western Asia but currently distributed throughout the Mediterranean basin (Gentile et al. 2007). Its fruit is a drupe with a large, central located single seed. The seed or kernel is the edible portion of the fruit. Habitually, it is consumed as a snack (natural, roasted or salted-roasted) otherwise in other edible products. The pistachio nuts are excellent sources of nutrients, unsaturated fatty acids, proteins, minerals and fibre, as well as natural bioactive and health-promoting components (tocopherols, carotenoids, chlorophylls, and flavonoides, among others) (Fabani et al. 2013; Gentile et al. 2007; Kornsteiner et al. 2006; US Department of Agriculture 2015).
The popularity of pistachio nuts has grown significantly in recent years, given rise to an incipient agro-industry in Argentina, being the Province of San Juan the main producer. This has been motivated investigation on chemical and nutritional composition (Fabani et al. 2013) and perception of pistachio quality by consumers of Argentinian pistachios (Penci et al. 2013).
Due to their high lipid content (about 50–60 %), pistachio nuts have been used to obtain edible oil. Pistachio oils are produced in small quantities compared to oils obtained from traditional oilseeds; generating a niche market taking account more consumers are now demanding tastier and healthier alternatives, other than traditional oil products. Moreover, pistachio oil production has been proposed as an interesting alternative in order to add value to the increasing pistachio nut production. In addition, the pressing process of pistachio oil extraction generates a defatted cake (pistachio flour), which is typically wasted. This product probably retains nutrients and bioactive compounds present in pistachio nut which may be used as a natural source of phytochemicals for dietary supplements, to prepare food for celiac people (gluten-free foods), to use in bakery foods and it may improve the flavour and nutritional value in preparing gourmet meals (Goli et al. 2005). Besides, in the last decades, there is an increasing tendency towards the addition of natural antioxidants to replace synthetic antioxidants with the aim of increasing the shelf life of food products and to reduce nutritional properties losses by inhibiting and delaying oxidation (Rajaei et al. 2010).
The pistachio nut production has given rise to an incipient agro-industry in Argentina, and it has motivated research on chemical and nutritional prospects of native genetic resources. Fabani et al. (2013) have reported the chemical profile, mineral content, as well as, antioxidant activities of pistachio nuts from Argentinian cultivars. Moreover, Penci et al. (2013) have evaluated the changes in the perception of pistachio quality by consumers through sensory and instrumental analyses. However, until now, the chemical composition and nutritional quality of pistachio oil and flour from Argentinian cultivars have been not yet reported.
Thus, the main goal of this study was to evaluated the chemical composition and nutritional quality of pistachio oil and flour and the influence of roasted and salted–roasted processes during Argentinian pistachio oil manufacture in order to searching a potential novel approach to Argentinian pistachio nut utilization.
Materials and methods
Chemicals
Ultra-pure water (<5 μg/L TOC) was obtained from a water purification system Arium 126 61,316-RO, plus an Arium 611 UV unit (Sartorius, Germany). Methanol (HPLC grade) and formic acid (puriss. p.a. for mass spectrometry) were obtained from J. T. Baker (State of México, México) and Fluka (Steinheim, Germany), respectively. Commercial Folin–Ciocalteu (FC) reagent, HNO3 (63 %) and HCl (37 %) were purchased from Merck Química Argentina (Buenos Aires, Argentina), 2,2-Diphenyl-1-picrylhydrazyl (DPPH), trichloroacetic acid (TCA) and commercial standards (+)-catechin, (−)-epicatechin, gallic acid, isoquercitrin, malvidin-O-glucoside, myricetin and quercetin were from Sigma-Aldrich (Buenos Aires, Argentina), while apigenin and naringenin were obtained from Extrasynthese (Genay, France). All other chemicals were of analytical grade.
Samples
Pistachio samples (Pistachia vera L. cv Kerman) were provided by Pisté S.R.L., an industrial factory located in Carpintería, Pocito district, from the province of San Juan, Argentina (2014 crop year). Samples were obtained at both riverbanks of San Juan River (lat. 31° S, long. 69° W, altitude 650–750 m.a.s.l.).
Sample treatment
Pistachio seeds dried at 40 °C (4 h) up to 3 % moisture content in a grain drier (Mega S.A., Argentina), were named natural pistachios (NP). A sub-sample of these NP were roasted (RP) using a rotating bakery oven (Argental, Argentina) at 120 °C during 90 min. Salted and roasted pistachios (SRP) were prepared by immersion of NP in a brine solution (NaCl 10 % w/v) during 1 min, then slurred and toasted (Penci et al. 2013). All samples were stored under vacuum, in individual bags (500 g each) at 5 °C until theirs use.
Pistachio seed composition
Samples of NP, RP and SRP were analysed according to standards AACC (AACC 2003) and American Oil Chemist’s Society (AOCS) (AOCS 2009) for total oil content, fatty acid profile, total protein, total carbohydrates and ash.
Screw press extraction
To optimize oil extraction by screw-press operations, pistachio seeds were conditioned to adjust moisture content to 10 % (w/w) level. Moisture conditioning was achieved by instant water sprinkling according to Singh and Bargale (2000). The water sprinkled samples were then packed in air-tight metal containers and stored for about 48 h for equilibration. The containers were shaken at regular intervals to distribute moisture uniformly throughout the sample. Extractions were carried out in a single step, with a Komet screw-press (Model CA 59 G, IBG Monforts, Monchengladbach, Germany) at a pilot plant scale (5 mm restriction die, 20 rpm screw speed). The screw press was firstly run for 15 min without material, but by heating via, an electrical resistance-heating ring attached around the press barrel, to raise the screw-press barrel temperature to the desired temperature (25 °C). Running temperature was checked with a digital thermometer inserted into the restriction die. After each run, all press devices were cleaned and dried (Martínez et al. 2008).
Oil yield
The oil yield was calculated considering the initial oil content in the incoming material, and the residual oil content in the cake. Oil yield was expressed as g extracted oil per 100 g of total oil present in the incoming material (g/100 g oil).
Fines amount in oil
Press-extracted oils were centrifuged at 11,000 x g during 30 min. The solid sediment was recovered and its content was calculated as g per 100 g of the total extracted oil (Martínez et al. 2008).
Oil analysis
Free fatty acid content (AV), peroxide value (PV), and specific extinction coefficients (K232 and K270) were determined according to standard methods of AOCS (AOCS 2009). The fatty acid profiles were analysed by gas chromatography (GC) according to Martínez et al. (2006). Carotenoids and chlorophylls were measured according to Minguez-Mosquera et al. (1991). The antiradical activity (AA) was analyzed by means of spectrophotometric determinations (Shimadzu Corporation, Kyoto, Japan MultiSpec-1501, equipped with a holder for multiple cells and temperature control) according to Martínez and Maestri (2008).
Pistachio flours
From each treatment (NP, RP, SRP), three independent samples of pistachio flours were collected immediately after pressing pistachio seeds. Flour samples were lyophilized and stored under vacuum in individual bags (50 g each) at 5 °C until theirs further use. Samples were named as follow: natural pistachio flour (NPF), roasted pistachio flour (RPF) and salted roasted pistachio flour (SRPF).
NPF, RPF and SRPF samples were homogenized, weighted (200 mg) and extracted by sonication (40 kHz, 30 min, 25 °C, ultrasound bath model TB02TACA, TESTLAB S.R.L, Buenos Aires, Argentina) using acidified methanol (0.1 % HCl, v/v) (MeOH-H+), according to Fabani et al. (2013). The homogenates was then centrifuged at 10,000 x g during 10 min using a Biofuge® 28RS Heraeus Sepatech Centrifuge (Heraeus Instruments, Hanau, Germany). The supernatant was separated, filtered (0.45 μm) and used for further analyses.
Determination of total phenolic (TP) and flavonoids (FT) content
The total phenolic (TP) and flavonoids (FT) content of acidified methanolic extracts (NPF, RPF and SRPF) were determined according Folin-Ciocalteu method and a colorimetric method with AlCl3 respectively. TP were determined by linear regression from a calibration plot constructed using gallic acid (0–250 μg/mL), and expressed as mg of gallic acid equivalents (GAE) per 100 g of pistachio flour (PF) on a dry weight (d.w.) (mg GAE/100 g PF d.w.). The values of FT were expressed as mg of quercetin equivalents (QE) per 100 g of pistachio flour (PF) on a d.w. basis (mg QE/100 PF g d.w.). For both, the values from triplicates were reported as mean ± SD.
Identification and quantification of phenolic compounds by HPLC-ESI-MS/MS
The phenolic profile was performed on an Agilent Series 1200 LC System (Agilent, Santa Clara, CA, USA) coupled in tandem to a PDA detector (Agilent Series 1200) and a MicrQTOF Q II (Bruker Daltonics, Billerica, MA, USA) high resolution mass spectrometer (MS and MS/MS) equipped with an ESI source. The HPLC system was equipped with a binary gradient pump, solvent degasser, and autosampler (Agilent Series 1200 L).
HPLC analyses were performed on a thermostatized (40 °C) Luna C18 250 × 4.6 mm (5 μm) column (Phenomenex, Torrance, CA, USA), at 0.4 mL/min flow rate, using 0.5 % (v/v) formic acid-water (solvent A) and 0.5 % (v/v) formic acid-methanol (solvent B). HPLC runs were performed using the following gradient: starting with 20 % B, changing to 50 % B along 3 min, kept for 5 min, followed by a second ramp to 80 % B during 5 min, maintained for 17 min, returning to 20 % B in 1 min, remaining at this last condition for 10 min before the next run. The injection volume was 40 μL. ESI-MS and MS/MS detection was performed in successive runs using both negative and positive ionization mode, with mass acquisition between 100 and 1500 Da. Nitrogen was used as drying and nebulizer gas (7 L/min and 3.5 Bar, respectively), and 180 °C for drying temperature. For MS/MS experiments fragmentation was achieved by using the auto MS2 option of the equipment. UV-Vis analyses were carried out in the range between 200 and 700 nm (PDA).
The identification of pistachios flours constituents was achieved by comparison of the spectral properties (UV, ESI-MS and MS/MS) of eluted compounds with those of reference samples, when available, and comparison with literature data. The standards gallic acid, naringenin, apigenin, quercetin, isoquercitrin, (+)-catechin, (−)-epicatechin and myricetin, were prepared at a stock concentration of 1000 mg/L. Calibration standard samples were prepared by appropriate dilutions with methanol from the stock solutions and filtered on Millipore filters (0.45 μm) before used. MS analysis was used for compounds quantification with the specific calibration plot. When reference compounds were not available, the calibration plots from structurally related compounds were used. The compounds concentrations were measured in triplicate, reporting the mean value and the standard deviation in each case.
Antioxidant activity
Free radical scavenger activity on DPPH
Free radical scavenging effects were assessed by the fade of a methanolic solution of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) according to the procedure described by Tapia et al. (2004). Extracts were assayed at concentrations 3.13, 6.25, 12.5, 25 and 50 mg/mL. Scavenging activities were evaluated at 517 nm in a Multiskan FC microplate photometer (Thermo Scientific, USA). Quercetin was used as a reference compound. The extract concentration providing 50 % of radicals scavenging activity (EC50) was calculated by plotting the inhibition percentage at A517 against the extract concentration. Results were extrapolated from the plot by linear regression. Analyses were performed in triplicate; and values were reported as mean ± SD.
Ferric-reducing antioxidant power assay (FRAP)
FRAP assay, measures the reducing capability of the samples, evaluating the conversion of a Fe3+/ferricyanide complex to Fe2+. The iron-reducing power of the samples was tested using the assay reported by Oyaizu (1986). The absorbance was read at 700 nm in a Multiskan FC microplate photometer (Thermo Scientific, USA). Quercetin was used as a reference compound. Analyses were performed in triplicate; values were reported as mean ± SD.
Statistical analysis
Results were analysed by one-way ANOVA and significant differences between mean values were determined by Duncan’s test (P < 0.05) using the software InfoStat (2014). Pearson’s correlation analysis was used to determine correlation coefficients and their statistical significance.
Results and discussion
Natural, roasted and salted roasted pistachio kernels: oil composition and chemical quality
Oil is extracted by mechanical pressing for oil-bearing seeds and nuts (Martínez et al. 2013). This process avoids contact with hydrocarbon solutions; so, the resulting oil is free from further refining processes. In this work, oil yields from screw-pressing of natural pistachios (NP), roasted pistachios (RP) and salted roasted pistachios (SRP) samples were 65 ± 3, 51.9 ± 0.4 and 35 ± 3 % lipid content (DB) and the solids content were 7.30 ± 0.3, 7.6 ± 0.1 and 9.8 ± 0.3 % in the raw oil collected, respectively. The lowest value was obtained in NP sample. Roasted and salting processes affected negatively the oil extraction yield (Fig. (Fig.1).1). Roasted and salting conditions produced pistachios that, during pressing, were more brittle, less plasticity and cohesive than natural pistachio samples. Proximate composition of pistachio kernel samples (NP, RP and SRP) is shown in Table Table1.1. All samples had lower moisture contents than those reported by Arena et al. (2007) (4 to 10 %). A lower moisture content is an important parameter in food safety, e.g., prevents both fungal grow and free fatty acid content increase (Martínez et al. 2008). A significant decrease of moisture content in pistachio kernels upon roasting at 120 °C has been also reported by Nikzadeh and Sedaghat (2008). Moreover, it was observed that salting procedure significantly increased moisture content. This could be related to that salted and roasted pistachios (SRP) were prepared by immersion of NP in a brine solution (NaCl 10 % w/v) during 1 min, then slurred and toasted (Penci et al. 2013). In the present study, both RP and SRP treatments had significantly lesser moisture content than NP samples (Table (Table11).
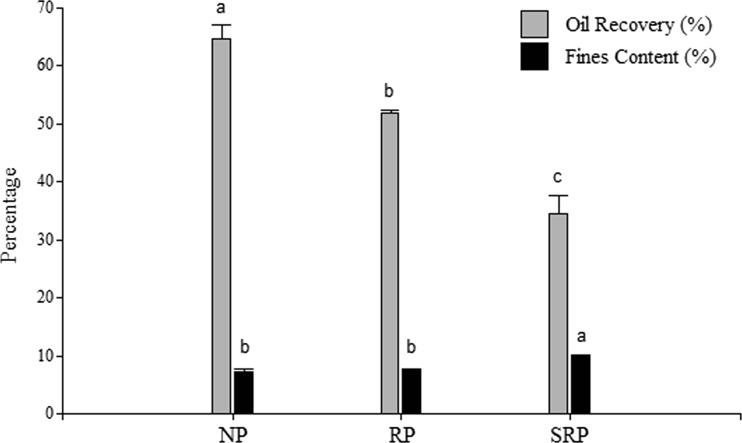
Percentage of oil recovery and solid content in raw oil extracted from NP, RP and SRP. Results are expressed as mean ± SD (standard deviation). Different letters indicate significant difference among treatments, Duncan (P < 0.05)
Table 1
Natural, roasted and salted-roasted pistachios nut composition
| Parameter | NP | RP | SRP |
|---|---|---|---|
| Moisture content (WB) % | 3.3 a ± 0.1 | 1.3 c ± 0.2 | 2.1 b ± 0.1 |
| Lipid content (DB) % | 49.9 a ± 0.1 | 50 a ± 2 | 50.6 a ± 0.1 |
| Protein content (DB) % | 21.6 b ± 0.3 | 22.2 b ± 0.7 | 25.2 a ± 0.7 |
| Ash content (DB) % | 3.0 b ± 0.1 | 3.0 b ± 0.1 | 4.8 a ± 0.1 |
| Carbohydrate content (DB) %* | 22.3 a ± 0.4 | 24 a ± 2 | 17.4 b ± 0.8 |
| Fatty acid distribution (relative abundance) | |||
 Palmitic acid (16:0) Palmitic acid (16:0) | 12.2 a ± 0.4 | 12.9 a ± 0.2 | 12.3 a ± 0.2 |
 Palmitoleic acid (16:1) Palmitoleic acid (16:1) | 1.31 a ± 0.01 | 1.4 a ± 0.1 | 1.3 a ± 0.1 |
 Stearic acid (18:0) Stearic acid (18:0) | 1.05 a ± 0.04 | 1.0 a ± 0.1 | 1.03 a ± 0.02 |
 Oleic acid (18:1) Oleic acid (18:1) | 55.3 a ± 0.5 | 55 a ± 2 | 53.5 a ± 0.6 |
 Linoleic acid (18:2) Linoleic acid (18:2) | 29.6 a ± 0.1 | 29 a ± 3 | 31.4 a ± 0.3 |
 Linolenic acid (18:3) Linolenic acid (18:3) | 0.51 a ± 0.01 | 0.56 a ± 0.01 | 0.51 a ± 0.01 |
| Minor components | |||
 Tocopherol content (μg/g oil) Tocopherol content (μg/g oil) | |||
 α Tocopherol α Tocopherol | 33 a ± 4 | 31 a ± 3 | 31 a ± 31 |
 γ Tocopherol γ Tocopherol | 804 a ± 4 | 826 a ± 23.9 | 815 a ± 18.1 |
 σ Tocopherol σ Tocopherol | 60 a ± 11 | 59 a ± 10 | 50 a ± 10 |
 Total tocopherol content Total tocopherol content | 898 a ± 11 | 916 a ± 30 | 896 a ± 24 |
 Carotenoids (μg/g oil) Carotenoids (μg/g oil) | 48 a ± 7 | 58 a ± 1 | 56.0 a ± 0.4 |
 Chlorophylls (μg/g oil) Chlorophylls (μg/g oil) | 41 c ± 2 | 60 b ± 4 | 70 a ± 2 |
amean ± standard deviation (n = 3). Mean values in each row followed by different superscript letters present significant differences, Duncan (P < 0.05) among natural, roasted and salted roasted pistachios. * by difference
Oil, protein and carbohydrate contents varied between 49.9–50.6, 21.6–25.2, and 17.4–24 %, respectively. These values agree with those ones reported by other authors (Arena et al. 2007; Tsantili et al. 2010). Oil content did not vary significantly among NP, RP and SRP samples, whereas protein content was higher in samples from the SRP treatment. The increment in protein content could be attributed to that salted SRP were prepared by immersion of NP in a brine solution. In this way, during moisture conditioning, the proteins of integument (the outer skin) could be partly solubilized due salt effect, resulting in increased levels of free proteins on the SRP. Consequently, there is an enhancement on the protein content of SRP nuts.
The content of each fatty acid (FA) followed the same order of abundance (oleic > linoleic > palmitic > palmitoleic > stearic > linolenic) in all samples. Overall, the FA composition is in agreement with those ones from pistachio oils originating from Spain (Arranz et al. 2008) and Iran (Tsantili et al. 2010). The processing conditions employed to obtain NP, RP and SRP samples did not affect significantly the FA concentrations and minor components of pistachio oil (Table (Table11).
Regarding tocopherols, it has been reported that pistachios have higher contents as compared with other nuts (peanut, hazelnut and almond) (Shakerardekani 2015). The tocopherol content of NP, RP and SRP is shown in Table Table1.1. These samples have a high performance on total tocopherol content (898 ± 11, 916 ± 30, 896 ± 24 μg/g oil, NP, RP and SRP respectively), and it is higher than those informed by Arranz et al. (2008). Even though, there were not significant differences in tocopherol content among NP, RP and SRP oil, suggesting the chemical stability of these compounds under roasting and salting conditions. Heat pre-treatment was reported in order to cause no change (Chiou and Tsai 1989), increase (Kim et al. 2002) or decrease (Anjum et al. 2006) in the different seeds and nuts tocopherol contents. Those different results imply that roasting may affect tocopherol distribution in different ways depending on the seed variety or the type and intensity of heat pre-treatment. The tocopherol isomers identified in pistachio kernels were α-, γ- and σ-, being γ-Tocopherol the predominant form of tocopherol in pistachio nut oils.
Pigment contents in NP, RP and SRP oils samples were markedly higher than those observed in other nut oils such as walnut and almond oils (Martínez et al. 2013). Values for carotenoids and chlorophylls varied between 48 and 56, and 41–70 μg/g oil, respectively (Table (Table1).1). Thermal processing induces degradation of chlorophylls into pheophytins (Aparicio-Ruiz et al. 2010). Under the assay conditions used in this study (spectrophotometric determination at 670 nm) pheophytins show moderate absorbance. Thus, enhanced absorbance values found in RP and RSP samples may be the result of chlorophyll degradation which is often accompanied by the presence of its various derivatives, such as pheophytins. Data about these minor components are in agreement with those ones from Liu et al. (2014) who showed that γ-tocopherol, chlorophylls and lutein are the main bioactive compounds in whole pistachios from California.
Acidity (AV), peroxide (PV) and UV extinction coefficients (K270 and K232) values are shown in Table Table2.2. The lowest AV and PV values were obtained for NP oil samples. Roasting and salting processes increased significantly these oil quality parameters. These results are in agreement with those reported by Penci et al. (2013) indicating that processes involving heating may increase hydrolytic and oxidative degradation. The AV obtained for all oil samples were lightly lower than those reported by Álvarez-Ortí et al. (2012) for virgin pistachio oils extracted with hydraulic or screw press. K270 and K232 also showed the lowest values in NP oil samples and the highest ones in SRP oil samples.
Table 2
Natural, roasted and salted roasted pistachio oils chemical quality
| Parameter | NP a | RP a | RSP a |
|---|---|---|---|
| Acid value (% oleic acid) | 0.155 b ± 0.009 | 0.184 a ± 0.001 | 0.181 a ± 0.006 |
| Peroxide value (meq O2/Kg oil) | 1.55 b ± 0.05 | 2.1 b ± 0.3 | 4.3 a ± 0.2 |
| K232 | 1.65 b ± 0.05 | 1.68 b ± 0.07 | 1.88 a ± 0.04 |
| K270 | 0.147 b ± 0.002 | 0.158 a ± 0.001 | 0.158 a ± 0.002 |
| EC50 (g oil/g DPPH·) | 381 a ± 4 | 357 b ± 6 | 357 b ± 6 |
| EC50 (mg oil/mL DPPH·) | 15.1 ± 0.2 | 14.2 + 0.6 | 14.2 ± 0.6 |
amean ± standard deviation (n = 3). Mean values in each row followed by different superscript letters present significant differences, Duncan (P < 0.05) among natural, roasted and salted roasted pistachios
NP, RP and SRP oils samples presented antiradical capacity values comparable to those from pistachio oils from Spain (median, EC50 378 ± 32 g oil/g DPPH) and also to those from extra virgin olive oils (439.7 ± 8.4 g oil/g DPPH) (Arranz et al. 2008) (Table (Table2).2). The antiradical capacity (EC50) correlated significantly and inversely with the total chlorophyll content (r = −0.91, p ≤ 0.01).
Total phenolic (TP), flavonoid (FT) content and antioxidant activity in pistachio flour
The acidified methanolic extraction yields from pistachio defatted flour were studied. Significant differences (P < 0.05) among samples of different heat treatments were observed. The results showed that SRPF with 28.5 ± 0.9 % was the best in the extraction, followed by RPF (20.0 ± 0.2 %) and NPF (18.6 ± 0.9 %), respectively.
Folin-Ciocalteau assay was used as rapid methods to evaluate total phenolic (TF) compounds in the pistachio flours samples (Fig. Fig.22). The TP concentration of acidified methanolic extracts was similar between NPF (11.4 ± 0.2 mg GAE/g PF d.w.) and RPF (11.1 ± 0.6 mg GAE/g PF d.w.). However, a slight and significant decrease was observed in SRPF (10.4 ± 0.6 mg GAE/g PF d.w.) (Fig. (Fig.2).2). The results obtained were lower when compared with pistachio green hull extract from Fandoghi and Ahmadaghaei variety, 32.8 and 28 mg/g sample, respectively (Goli et al. 2005; Rajaei et al. 2010). Concerning flavonoids (FL) content, pistachio flours varied from 0.48 ± 0.05 to 0.7 ± 0.1 mg QE/g PF d.w. (Fig. (Fig.2).2). The SRPF presented the minor FT content, which was significantly different in relation to the others pistachio flours analyzed.
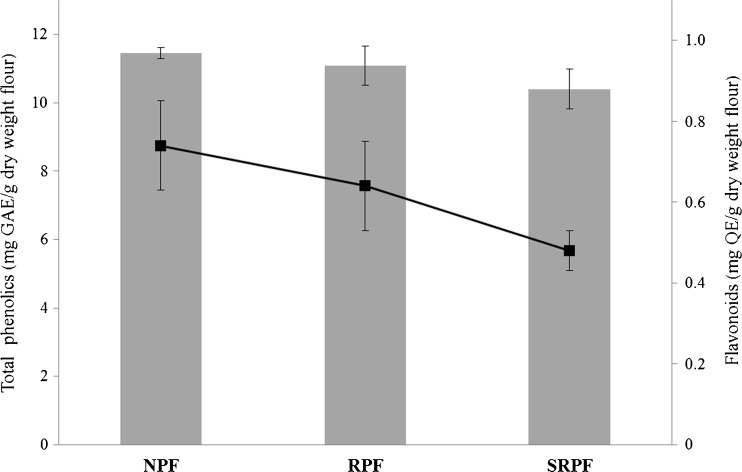
Total phenolic (TP) (grey bars) and flavonoids (FT) (dot) content of acidified methanolic pistachios flours extracts (NPF: natural pistachio flour, RPF: roasted pistachio flour, SRPF: salted roasted pistachio flour). Results are expressed as mean ± SD (standard deviation). Different letters indicate significant difference among treatments, Duncan (P < 0.05)
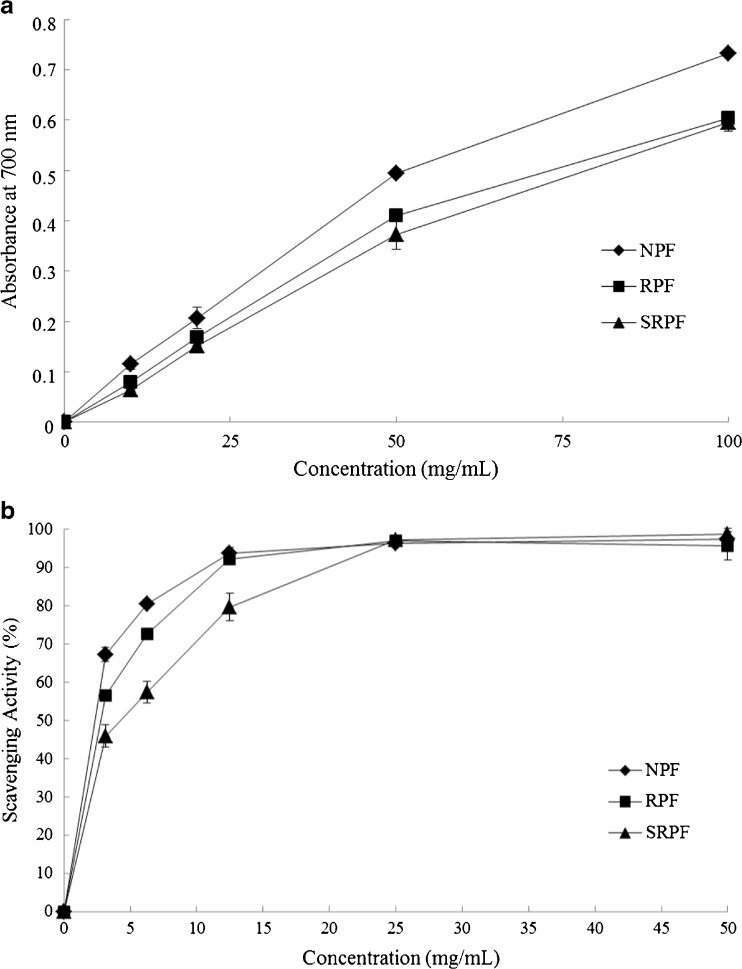
Otherwise, the scavenging effect on DPPH radicals assay showed concentration-dependent activity, and similar trends were observed for NPF and RPF extracts (Fig. (Fig.3a).3a). The EC50 values in NPF extract had the lowest value (2.2 mg/mL), followed by RPF (2.7 mg/mL) and SRPF (4.1 mg/mL). The correlation between antioxidant activity and TP content was evaluated applying simple correlation analysis. A positive significant Pearson’s correlations was found between DPPH activity and TP (r = 0.69, P < 0.01). Also, a major statistically significant correlation was observed between FT content and DPPH antioxidant capacity (r = 0.78, P < 0.01). The Fig. Fig.3b3b shows that the reducing antioxidant power (FRAP assay) of pistachio flour extracts have a direct relation with extracts concentration being NPF the one that have the highest reducing power. TP and FL content are also directly correlated with FRAP assay results (r = 0.68 and r = 0.72, respectively, at P < 0.01). The roasting process not diminish total phenolic (TP) and flavonoids (FL) content significantly compared to natural pistachio flour (NPF), even so reduced the DPPH antioxidant capacity (approximately 20 %) and antioxidant power in the roasted pistachio flour (RPF). Furthermore, salted roasted pistachio flour (SRPF) showed a slight and significant decrease on TP and FL content in relation to the others samples.
Identification and quantification of pistachio flour phenolics
The results of the HPLC-PDA-ESI-MS and MS/MS assays of pistachio flours extract were summarized in Table Table3.3. In all samples, a total of 13 compounds belonging to the family of phenolic acids and flavonoids, and also 2 anthocyanins were identified and quantified. The total amount of polyphenolic was a little higher in NPF (173 ± 18 μg/g PF d.w.) compared with SRPF (155 ± 15 μg/g PF d.w.) and RPF (129 ± 11 μg/g PF d.w.) (Table (Table3).3). Results of the Folin-Ciocalteu were compared with HPLC findings and no relation was found among the results obtained. It is well know that the Folin-Ciocalteu reagent is not specific and detects all phenolic groups found in extracts. However, this assay still provides a very useful index for phenolic content, but it would not be expected to correlate with the weight of phenolics quantified by HPLC.
Table 3
Polyphenols content corresponding to each acidified methanolic pistachio flours extracts
| Compounds | NPFa | RPFa | SRPFa |
|---|---|---|---|
| Gallic acida | 36a ± 2 | 23b ± 3 | 30ab ± 2 |
| Procyanidin dimerb,c | 15a ± 1 | 10a ± 7 | 13a ± 3 |
| (+)-catechina | 65.5a ± 0.1 | 38c ± 2 | 54b ± 3 |
| (−)-epicatechina | 7.7a ± 0.7 | 5a ± 2 | 8.4a ± 0.8 |
| Eriodictyol-O-hexosideb,d | 0.88a ± 0.09 | 0.8a ± 0.1 | 1.0a ± 0.1 |
| Eriodictyol-O-hexosideb,d | 0.70a ± 0.03 | 0.75a ± 0.02 | 0.69a ± 0.06 |
| Quercetin-O-hexosideb,e | 2.2a ± 0.7 | 3a ± 1 | 3a ± 1 |
| Isoquercetrina | 5a ± 2 a | 5a ± 2 | 6a ± 2 |
| Myricetina | 0.5a ± 0.02 | 0.46a ± 0.07 | 0.32a ± 0.02 |
| Eriodictyolb,d | 11.3a ± 0.3 | 13a ± 5 | 9a ± 1 |
| Quercetina | 4.2a ± 0.6 | 6a ± 3 | 5a ± 1 |
| Naringenina | 0.13b ± 0.02 | 0.27a ± 0.03 | 0.20ab ± 0.02 |
| Luteolinb,f | 1.8b ± 0.2 | 3.0a ± 0.2 | 1.98b ± 0.04 |
| Cyanidin-O-galactosideb,g | 21a ± 6 | 21a ± 11 | 23a ± 2 |
| Cyanidin-O-glucosideb,g | 0.9a ± 0.1 | 1.1a ± 0.3 | 0.81a ± 0.06 |
| Total phenolics | 173 ± 18 | 129 ± 11 | 155 ± 15 |
amean ± standard deviation (n = 3). Mean values in each column followed by different superscript letters present significant differences, Duncan (P < 0.05) among natural, roasted and salted roasted pistachios. Procedures employed for identification or tentative identification: a, co-analysis relative to a pure compound showing identical retention and mass data
bcomparison with literature MS, MS/MS and UV data. Quantification was made using a calibration curve of the corresponding standard, except were indicated
cquantified as catechin
dquantified as naringenin
equantified as isoquercitrin
fquantified as apigenin
gquantified as malvidin-O-glucoside
The major polyphenol identified in pistachio flours was (+)-catechin (38–65.5 μg/g PF d.w.), followed by gallic acid (23–36 μg/g PF d.w.), procyanidin dimer (10–15 μg/g PF d.w.) and eriodictyol (9–13 μg/g PF d.w.). Conversely, Mandalari et al. (2013) reported gallic acid as the phenolic present in major quantities in natural and roasted salted pistachio kernels (Pistachia vera L.) from California (USA).
In the present study, the treatments have different effects on the phenolics constituents of pistachio flour. According to Xu and Chang (2008) the thermal treatment applied to foods of plant origin by roasting causes evaporation of intracellular water, resulting in a greater availability of plant phenolic compounds in the matrix. Roasting caused a significant reduction of some phenolics in pistachio flour, gallic acid and (+)-catechin, and increased others, (naringenin and luteolin) (Table (Table3).3). On the other hand, a hypothesis that may explain the increase of the level of some phenolics compounds in salted-roasted pistachio flour is that during salting the integument (the outer skin) is in contact with a brine solution (NaCl 10 % w/v). Then, during moisture conditioning the proteins could be partly solubilized, resulting in increased levels of free proteins and phenolic compounds (gallic acid and naringenin) on the SRPF. The anthocyanins identified in PF extracts were cyanidin-3-O-galactoside and cyanidin-3-O-glucoside and content ranged from 21 to 23 to 0.81–1.1 μg/g PF d.w., respectively. In the current study roasting at 120 °C during 90 min did not influence significantly in the degradation of anthocyanins (Table (Table3).3). Mandalari et al. (2013) reported similar behaviour for certain compounds in sated roasted pistachios from Californian and Bonilla-Lemos et al. (2012) in Brazilian baru nuts roasted at 150 °C for 45 min.
Pistachio nuts analysed here present higher amounts of polyphenolic compounds in comparison with almonds, hazelnuts, peanuts, macadamia and pistachio nuts from other origins (Yang et al. 2009). Polyhydric phenols with high number of OH-groups, such as several compounds identified in pistachio methanolic extracts (Fig. (Fig.3),3), have been also recognized for their antioxidant activity in lipid peroxidation reactions owing to their capacity of hydrogen-atom transfer to lipid alkyl radicals. However, such effect must be interpreted with caution because such polyphenols are rather polar and hydrophilic substances and could have low solubility in oil. In summary, considering the whole set of compounds with potential antioxidant capacities, it is possible that tocopherols and carotenoids could be the main contributors to the radical scavenging activity observed in pistachio oil, with a minor contribution of polyphenolic compounds. Conversely, (+)-catechin and gallic acid could be the responsible of the bioactivity in pistachio flour extracts. These well known for their antioxidant activity in different trials. (+)-catechin is a flavonoid that has been indicated as a factor that reduce cardiovascular risk by lowering serum cholesterol levels, diminishing platelet aggregation and reducing blood pressure (Marinou et al. 2010). On the other hand, procyanidins are reported to be potent antioxidants. Human studies show that a diet rich in procyanidins decreases/inhibits lipid peroxidation of LDL cholesterol and increases free radical scavenging capacity (Natella et al. 2002).
Nutritional value
The nutritional importance of pistachio nuts is related to its kernel composition. Pistachio kernel mainly contains lipids, including triglycerides which are present in very high concentration. The pistachio composition is largely of unsaturated fatty acids, mainly oleic and linoleic acids. However, the elevated unsaturation level may result in a moderate oxidative stability of the oil (15 h average). The pistachio kernels contain a diverse array of phenolic compounds with strong antioxidant and radical-scavenging properties, even though protection against oxidative degradation seems to be limited mainly to tocopherol and carotenoids content. Additional work is necessary to evaluate antioxidants as well as packaging and storage conditions of pistachio oil aimed towards quality retention.
Actually, seed flour(s) have been used in innumerable bakery foods and therefore study their functional properties is of great interest. Flours obtained after pistachio oil extraction are rich in proteins and polyphenolic compounds, mainly flavonoids and gallic acid, which show strong antioxidant capacity. In the gastronomy, seeds flours have been used more frequently as an ingredient to improve flavour and nutritional value so that these results add relevant information to proximate composition of pistachio flour. Also fits to highlight, that phenolic compounds present in pistachio flour may be remove due they bind to proteins affecting protein solubility and possibly amino acids bioavailability (Martínez et al. 2010). This procedure should be considered to attempt successfully the commercial production of good quality pistachio flour. The processing conditions used to obtain roasted and salted-roasted pistachios did not affect the composition and activity of these natural substances.
Conclusions
The results of this work suggest that the high oil and essential fatty acids contents of pistachio kernel make it a good source for commercial production of edible oil. Pistachio oil can be extracted easily by screw pressing and at the same time the extraction method is suitable to get good oil yield without affecting the chemical quality and it may be consumed without refining. The pistachio flours obtained are rich in polyphenolic compounds could be considered as an ingredient in bakery foods. Instead, results suggest that Argentinian pistachio oil and flour could be considered as ingredients in the formulation of food products and serve as a way of incorporating some specific biologically active components present in these seed, whose intake helps to promote a healthy lifestyle.
DPPH 2,2-diphenyl-1-picrylhydrazyl, FRAP ferric-reducing antioxidant power, acidified methanol extract, MeOH-H+, TP total phenolics, FT flavonoids, GAE gallic acid equivalents, QE quercetin equivalents, NP natural pistachio, RP roasted pistachio, SRP salted roasted pistachio, NPF natural pistachios flour, RPF roasted pistachios and SRPF salted roasted pistachios flour.
Acknowledgments
Authors are grateful to CICITCA, Universidad Nacional de San Juan and Universidad Nacional de Córdoba, SECITI Gobierno de la Provincia de San Juan (IDeA Exp N° 1400-0107-2012), Argentina for the financial support. G.E.F., M.P.F., M.V.B., M.L.M., D.M.M. and D.A.W. are researchers from CONICET, Argentina. R.N.M.H. is fellow CONICET. We would like to express our gratitude to Piste´ S.R.L. for providing pistachio samples.
Footnotes
Marcela Lilian Martínez and María Paula Fabani have equal contribution.
Research highlights
The chemical quality of oil and flour from natural, roasted and salted-roasted pistachios are reported.
The pistachio oils are highlighted by the contents of tocopherols, oleic and linoleic acid.
Pistachio flour is a valuable natural product with potential to improve human health
The results suggest that oil and flour from pistachio are a novel approach of pistachio nut.
References
- AACC . Approved methods of the American association of cereal chemists. 10th. St. Paul Min: American Association of Cereal Chemist’s; 2003. [Google Scholar]
- Álvarez-Ortí M, Quintanilla C, Sena E, Alvarruiz A, Pardo JE. The effects of a pressure extraction system on the quality parameters of different virgin pistachio (Pistacia vera L. var. Larnaka) oils. Grasas y Aceites. 2012;63:260–266. 10.3989/gya.117511. [CrossRef] [Google Scholar]
- Anjum F, Anvar F, Jamil A, Iqbal M. Microwave roasting effects on the 15xcels-chemical composition and oxidative stability of sunflower seed oil. J Am Oil Chem Soc. 2006;83:777–784. 10.1007/s11746-006-5014-1. [CrossRef] [Google Scholar]
- AOCS . Official methods of analysis. 5th. Illinois, Washington DC: American Oil Chemists Society Champaign; 2009. [Google Scholar]
- Aparicio-Ruiz R, Mínguez-Mosquera MI, Gandul-Rojas B. Thermal degradation kinetics of chlorophyll pigments in virgin olive oils. 1. Compounds of series a. J Agric Food Chem. 2010;58:6200–6208. 10.1021/jf9043937. [Abstract] [CrossRef] [Google Scholar]
- Arena E, Campisi S, Fallico B, Maccarone E. Distribution of fatty acids and phytosterols as a criterion to discriminate geographic origin of pistachio seeds. Food Chem. 2007;104:403–408. 10.1016/j.foodchem.2006.09.029. [CrossRef] [Google Scholar]
- Arranz S, Cert R, Pérez-Jiménez J, Cert A, Saura-Calixto F. Comparison between free radical scavenging capacity and oxidative stability of nut oils. Food Chem. 2008;110:985–990. 10.1016/j.foodchem.2008.03.021. [Abstract] [CrossRef] [Google Scholar]
- Bonilla-Lemos MR, Machado de Almeida Siqueira E, Fernandes Arruda S, RC Z. The effect of roasting on the phenolic compounds and antioxidant potential of baru nuts [dipteryx alata Vog.] Food Res Int. 2012;48:592–597. 10.1016/j.foodres.2012.05.027. [CrossRef] [Google Scholar]
- Chiou RYY, Tsai TT. Characterization of peanut proteins during roasting as affected by initial moisture content. J Agric Food Chem. 1989;37:1377–1381. 10.1021/jf00089a037. [CrossRef] [Google Scholar]
- Fabani MP, Luna L, Baroni MV, Monferran MV, Ighani M, Tapia A, Wunderlin DA, Feresin GE. Pistachio (Pistacia vera var Kerman) from Argentinean cultivars. Natur prod Potential Improve Hum Health J Funct Foods. 2013;5:1347–1356. [Google Scholar]
- Gentile C, Tesoriere L, Butera D, Fazzari M, Monastero M, Allegra M, Livrea MA. Antioxidant activity of Sicilian pistachio (Pistacia vera L. Var. Bronte) nut extract and its bioactive components. J Agric Food Chem. 2007;55:643–648. 10.1021/jf062533i. [Abstract] [CrossRef] [Google Scholar]
- Goli AH, Barzegar M, Sahari MA. Antioxidant activity and total phenolic compounds of pistachio (pistachia Vera) hull extracts. Food Chem. 2005;92:521–525. 10.1016/j.foodchem.2004.08.020. [CrossRef] [Google Scholar]
- John JA, Shahidi F. Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia 16xcels) J Funct Foods. 2010;2:196–209. 10.1016/j.jff.2010.04.008. [CrossRef] [Google Scholar]
- Kim IH, Kim CJ, You JM, Lee KW, Kim CT, Chung SH, et al. Effect of roasting temperature and time on the chemical composition of rice germ oil. J Am Oil Chem Soc. 2002;79:413–418. 10.1007/s11746-002-0498-2. [CrossRef] [Google Scholar]
- Kornsteiner M, Wagner K, Elmadfa I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006;98:381–387. 10.1016/j.foodchem.2005.07.033. [CrossRef] [Google Scholar]
- Liu Y, Blumberg JB, Chen CY. Quantification and bioaccessibility of California pistachio bioactives. J Agric Food Chem. 2014;62:1550–1156. 10.1021/jf4046864. [Abstract] [CrossRef] [Google Scholar]
- Mandalari G, Bisignano C, Filocamo A, Chessa S, Saro M, Torre G, Faulks RM, Dugo P. Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion. Nutrition. 2013;29:338–344. 10.1016/j.nut.2012.08.004. [Abstract] [CrossRef] [Google Scholar]
- Marinou KA, Georgopoulou K, Agrogiannis G, Karatzas T, Iliopoulos D, Papalois A, et al. Differential effect of pistachio Vera extracts on experimental atherosclerosis in the rabbit animal model: an experimental study. Lipids Health Dis. 2010;73:1–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Martínez ML, Maestri DM. Oil chemical variation in walnut genotypes grown in Argentina. Eur J Lipid Sci Technol. 2008;110:1183–1189. 10.1002/ejlt.200800121. [CrossRef] [Google Scholar]
- Martínez ML, Mattea MA, Maestri DM. Varietals and crop year effects on lipid composition of walnut (Juglans regia L.) genotypes. J Am Oil Chem Soc. 2006;83:791–796. 10.1007/s11746-006-5016-z. [CrossRef] [Google Scholar]
- Martínez ML, Mattea MA, Maestri DM. Pressing and supercritical carbon dioxide extraction of walnut oil. J Food Eng. 2008;88:399–404. 10.1016/j.jfoodeng.2008.02.026. [CrossRef] [Google Scholar]
- Martínez ML, Labuckas DO, Lamarque AL, Maestri DM. Walnut (Juglans regia L.): genetic resources, chemistry, by-products. J Sci Food Agric. 2010;90:1959–1967. [Abstract] [Google Scholar]
- Martínez ML, Penci MC, Ixtaina V, Ribotta PD, Maestri DM. Effect of natural and synthetic antioxidants on the oxidative stability of walnut oil under different storage conditions. LWT Food Sci Technol. 2013;51:44–50. 10.1016/j.lwt.2012.10.021. [CrossRef] [Google Scholar]
- Minguez-Mosquera MI, Rejano L, Gandul B, Sanchez AH, Garrido J (1991) Color-pigment correlation in virgin olive oil. J AmOil Chem Soc 68:322–336. 10.1007/BF02657688
- Natella F, Belelli F, Gentili V, Ursini F, Scaccani C. Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. J Agric Food Chem. 2002;50:7720–7725. 10.1021/jf020346o. [Abstract] [CrossRef] [Google Scholar]
- Nikzadeh V, Sedaghat N. Physical and sensory changes in pistachio nuts as affected by roasting temperature and storage. J Agric Environ Sci. 2008;4:478–483. [Google Scholar]
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986;44:307–315. 10.5264/eiyogakuzashi.44.307. [CrossRef] [Google Scholar]
- Penci MC, Martínez ML, Fabani MP, Feresin GE, Tapia A, Ighani M, Ribotta PD, Wunderlin DA. Matching changes in sensory evaluation with physical and chemical parameters. A case study: Argentinean pistachio nuts (pistachia Vera L. cv Kerman) Food Bioproc Technol. 2013;6:3305–3316. 10.1007/s11947-012-0993-4. [CrossRef] [Google Scholar]
- Rajaei A, Barzegar M, Mobarez AM, Sahari MA, Esfahani ZH. Antioxidant, anti-microbial and antimutagenicity activities of pistachio (pistachia Vera) green hull extract. Food Chem Toxicol. 2010;48:107–112. 10.1016/j.fct.2009.09.023. [Abstract] [CrossRef] [Google Scholar]
- Shakerardekani A. Factors affecting production, sensory properties and oxidative stability of nut butters and nut. Spreads-A Rev Am J Food Sci Nut Res. 2015;2:83–88. [Google Scholar]
- Singh J, Bargale PC. Development of a small capacity double stage compression screw press for oil expression. J Food Eng. 2000;43:75–82. 10.1016/S0260-8774(99)00134-X. [CrossRef] [Google Scholar]
- Tapia A, Rodriguez J, Theoduloz C, Lopez S, Feresin G, Schmeda-Hirschmann G. Free radical scavengers and antioxidants from baccharis grisebachii. J Ethnopharmacol. 2004;95:155–161. 10.1016/j.jep.2004.06.035. [Abstract] [CrossRef] [Google Scholar]
- Tsantili E, Takidelli C, Christopoulos MV, Lambrinea E, Rouskas D, Roussos PA. Physical, compositional and sensory differences in nuts among pistachio (pistachia Vera L.) varieties. Sci Hortic. 2010;125:562–568. 10.1016/j.scienta.2010.04.039. [CrossRef] [Google Scholar]
- US Department of Agriculture, Agricultural Research Service. USDA nutrient database for standard reference, Release 28. Nutrient data laboratory. Available at: http://www.ars.usda.gov/nutrientdata. Accessed 4 Jan 2015.
- Xu B, Chang SKC. Total phenolics, phenolic acids, isoflavones, and antioxidant properties of yellow and black soybeans as affected by thermal processing. J Agric Food Chem. 2008;56:7165–7175. 10.1021/jf8012234. [Abstract] [CrossRef] [Google Scholar]
- Yang J, Liu RH, Halim L. Antioxidant and antiproliferative activities of common edible nut seeds. Food Sci Technol. 2009;42:1–8. [Google Scholar]
Articles from Journal of Food Science and Technology are provided here courtesy of Springer
Full text links
Read article at publisher's site: https://doi.org/10.1007/s13197-016-2184-1
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4921076?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s13197-016-2184-1
Article citations
Study of volatile compounds in Greek pistachio (Pistacia vera L. 'Aegina' cultivar) oils using Soxhlet and ultrasound assisted extraction.
Heliyon, 9(5):e15623, 20 Apr 2023
Cited by: 0 articles | PMID: 37153392 | PMCID: PMC10160742
Study of the Quality Parameters and the Antioxidant Capacity for the FTIR-Chemometric Differentiation of Pistacia Vera Oils.
Molecules, 25(7):E1614, 01 Apr 2020
Cited by: 2 articles | PMID: 32244701 | PMCID: PMC7181075
Subcritical Fluid Extraction of Antioxidant Phenolic Compounds from Pistachio (Pistacia vera L.) Nuts: Experiments, Modeling, and Optimization.
J Food Sci, 84(5):963-970, 23 Apr 2019
Cited by: 2 articles | PMID: 31012966
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins.
Biochimie, 92(9):1115-1122, 11 Apr 2010
Cited by: 82 articles | PMID: 20388531
Effects of different roasting methods on formation of acrylamide in pistachio.
Food Sci Nutr, 8(6):2875-2881, 12 May 2020
Cited by: 4 articles | PMID: 32566205 | PMCID: PMC7300066
Composition and properties of virgin pistachio oils and their by-products from different cultivars.
Food Chem, 240:123-130, 19 Jul 2017
Cited by: 12 articles | PMID: 28946247
Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects.
Plants (Basel), 11(1):18, 22 Dec 2021
Cited by: 27 articles | PMID: 35009022 | PMCID: PMC8747606
Review Free full text in Europe PMC