Abstract
Background
The primary objective of this meta-analysis is aimed at determining whether β-lactams prolonged infusion in patients with nosocomial pneumonia (NP) results in higher cure rate and improved mortality compared to intermittent infusion.Materials and methods
Relevant studies were identified from searches of MEDLINE, EMBASE, and CENTRAL from inception to September 1st, 2015. All published articles which evaluated the outcome of extended/continuous infusion of antimicrobial therapy versus intermittent infusion therapy in the treatment of NP were reviewed.Results
A total of ten studies were included in the analysis involving 1,051 cases of NP. Prolonged infusion of β-lactams was associated with higher clinical cure rate (OR 2.45, 95% CI, 1.12, 5.37) compared to intermittent infusion. However, there was no significant difference in mortality (OR 0.85, 95% CI 0.63-1.15) between the two groups. Subgroup analysis for β-lactam subclasses and for severity of illness showed comparable outcomes.Conclusion
The limited data available suggest that reduced clinical failure rates when using prolonged infusions of β-lactam antibiotics in critically ill patients with NP. More detailed studies are needed to determine the impact of such strategy on mortality in this patient population.Free full text

Prolonged versus Intermittent Infusion of β-Lactams for the Treatment of Nosocomial Pneumonia: A Meta-Analysis
Abstract
Background
The primary objective of this meta-analysis is aimed at determining whether β-lactams prolonged infusion in patients with nosocomial pneumonia (NP) results in higher cure rate and improved mortality compared to intermittent infusion.
Materials and Methods
Relevant studies were identified from searches of MEDLINE, EMBASE, and CENTRAL from inception to September 1st, 2015. All published articles which evaluated the outcome of extended/continuous infusion of antimicrobial therapy versus intermittent infusion therapy in the treatment of NP were reviewed.
Results
A total of ten studies were included in the analysis involving 1,051 cases of NP. Prolonged infusion of β-lactams was associated with higher clinical cure rate (OR 2.45, 95% CI, 1.12, 5.37) compared to intermittent infusion. However, there was no significant difference in mortality (OR 0.85, 95% CI 0.63–1.15) between the two groups. Subgroup analysis for β-lactam subclasses and for severity of illness showed comparable outcomes.
Conclusion
The limited data available suggest that reduced clinical failure rates when using prolonged infusions of β-lactam antibiotics in critically ill patients with NP. More detailed studies are needed to determine the impact of such strategy on mortality in this patient population.
Introduction
Nosocomial pneumonia (NP) is one of the frequent hospital acquired infections with an incidence ranging from 6 to 52% [1,2]. It remains associated with increased mortality rates and high health care costs [3]. Despite our better understanding of the mechanisms underlying NP, successful treatment of these patients remains a difficult and complex undertaking as it is influenced by various factors such as lack of a gold standard for definitive diagnosis of NP, difficulty in differentiating colonization from active infection, frequent association with multidrug resistant pathogens, and altered pharmacokinetics in critically ill patients [4].
Successful management of antimicrobial therapy in cases of NP hinges on prescribing appropriate initial empiric therapy, maximizing antimicrobial pharmacokinetics and/or pharmacodynamics (PK/PD) profiles, and administering a short course of treatment. Beta-lactams, when administered at doses commonly used in NP, exhibit time dependent killing. The extent of bacterial death is correlated with the percent of the dosing interval that drug concentrations are maintained above the minimum inhibitory concentration (MIC) of the pathogen. In these instances, pharmacokinetic modeling and dosing simulations showed that pharmacodynamics targets were better achieved by extended or continuous infusion of beta-lactams [5,6,7]. Several meta-analyses have examined the comparison between extended or continuous versus short term infusion on clinical outcomes with conflicting results [8,9,10]. Inclusion of crossover studies and trials with small number of participants may have obscured clinical benefits. No prior meta-analysis, to our knowledge, has examined the clinical efficacy of extended infusion in patients with NP. The constant and sustainable antibiotic concentrations provided by extended or continuous infusion are particularly important for pathogens with high MIC values commonly observed in these settings. We performed a systematic review and meta-analysis of published clinical investigations investigating the efficacy of prolonged infusion beta-lactam therapy compared with intermittent infusion beta-lactam therapy with regards to mortality and clinical cure in patients with NP. The primary objective was to determine if prolonged infusion of beta-lactam antibiotics resulted in improved patient survival and clinical cure compared to intermittent dosing of beta-lactam antibiotics.
Materials and Methods
1. Information sources and search
An internet search was performed using MEDLINE, EMBASE and CINAHL from inception to September 1st, 2015 to identify all published articles which evaluated the outcome of extended/continuous infusion of antimicrobial therapy versus intermittent infusion therapy in the treatment of NP. The electronic search strategy included the terms "infusion", "extended", "prolonged", "continuous", "intermittent", "nosocomial", "ventilator-associated pneumonia", and "outcome". Terms were "exploded" and combined by using Boolean operators where appropriate. We also searched the reference lists of original reports and systematic review of studies involving NP to identify studies not yet included in the computerized databases. In addition, we reviewed the cited lists of eligible trials by Google Scholar to ensure that all appropriate studies were included. Case reports and case series including <10 patients were excluded. No language restrictions were applied.
2. Data extraction and quality assessment
Articles were identified in a staged process whereby titles were initially screened for potential eligibility by a single reviewer (PJ). Abstracts and full texts of those potentially eligible were then assessed by two reviewers (HES and AL) independently and were included if the comparative outcomes included patients treated with "extended or continuous" versus "short term or intermittent" infusion. For the purpose of the review, patients were allocated in 2 groups: the "prolonged infusion" group that included patients receiving either extended infusions of a beta-lactam lasting ≥3 hours or a 24-hour continuous infusion, and the "short-term infusion" group comprising patients receiving short-term intermittent drug regimens (ie, 20–60 minutes infusion). In cases where the same population studied was analyzed in more than one publication, the study's results were accounted for only once.
Two reviewers independently extracted the data. In case of disagreement between the two reviewers, a third reviewer extracted the data. Trial authors were contacted for clarification and to complete missing data. We assessed the methodologic quality of observational studies with the Newcastle-Ottawa Scale [11] and that of randomized controlled trials with the Jadad scale [12]. We collected the raw, unadjusted number of deaths among patients given prolonged infusion versus intermittent antibiotic treatment. We collected descriptive data on setting, study years, follow-up duration, patient characteristics, severity of illness, types of pathogens, antimicrobial agents and clinical and microbiological outcomes, emergence of resistance, adverse events and mortality.
3. Outcomes
The primary outcomes of the review were all-cause mortality and clinical cure at the end of the treatment. When data regarding outcomes were not provided, outcomes at test-of cure visit were used. Clinical cure was defined by the discretion of the authors of the publication because of the heterogeneous nature of the study population, and pathogens involved. If only clinical failure was reported, it was assumed that clinical success was achieved in all those patients who did not fail treatment.
4. Data analysis
Data were analyzed with the use of the Review Manager (RevMan version 5.3; Cochrane Collaboration, Oxford, UK). Statistical heterogeneity was assessed by employing both the χ2 test and I2 statistic; a χ2 test's P value lower than 0.10 and an I2 value higher than 50% were defined to note statistical significance. When P was higher than 0.10 and I2 was lower than 50% (i.e., statistically non-significant heterogeneity), pooled odds ratios (ORs) and 95% confidence intervals (CIs) for all outcomes were calculated by using both the Mantel-Haenszel fixed effect and the DerSimonian-Laird random effects models. This was done because the possible persistence of the statistical significance of our results even after the implementation of the conservative random effects model (along with the fixed effect model) presumably adds to the robustness of our findings. When χ2 test's P was lower than 0.10 and I2 was higher than 50% (i.e., statistically significant heterogeneity), OR and CI were calculated by using only the random effects model. Subgroup analysis were carried out by type of study design, β-lactam subclasses, and by severity of illness determined by the Acute Physiology and Chronic Health Evaluation (APACHE) II score. Small study effect was detected by the funnel plot method using Egger's test; a P-value lower than 0.05 denotes statistical significance.
Results
1. Study selection
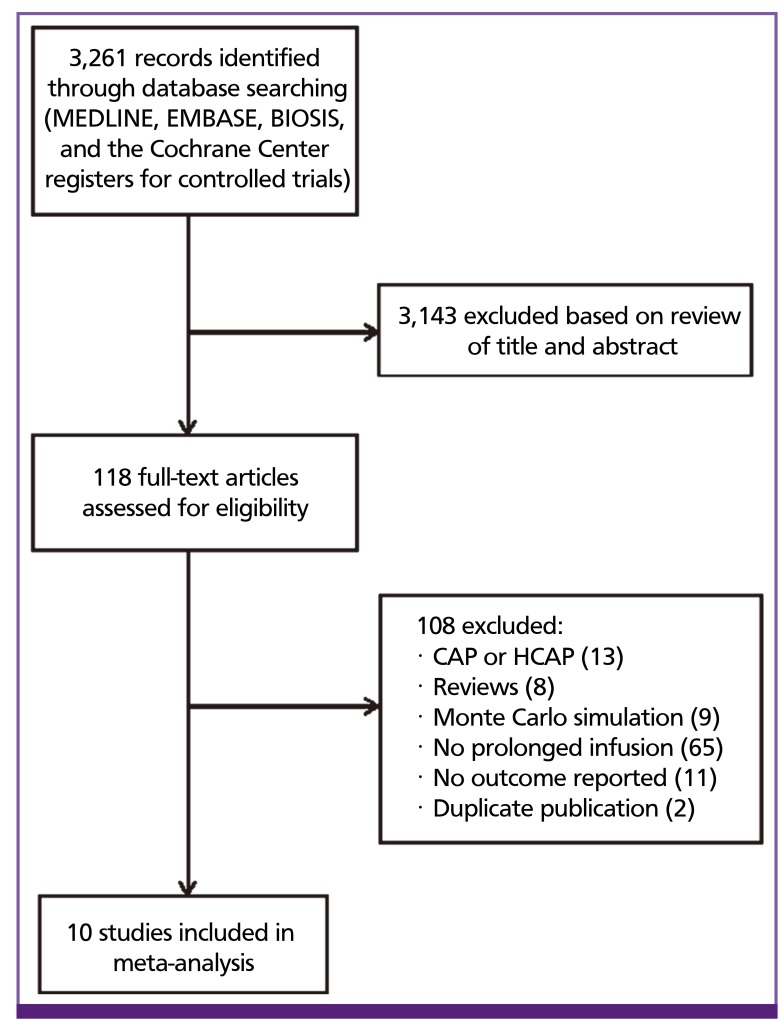
We identified 3261 potentially relevant published articles on review of MEDLINE, EMBASE, and CINHAL (Fig. 1). After removing 237 duplicates, 3,024 titles and abstracts were reviewed by two independent study team members. The majority of studies were excluded during initial screening because they were Monte Carlo simulation studies that did not involve patients, or were studies that did not have a comparator group. Thirty-four studies underwent full text review and 10 met criteria for inclusion [13,14,15,16,17,18,19,20,21,22] comprising 1,051 patients treated. Figure 1 shows the selection process of studies included in the meta-analysis.
2. Study characteristics
The characteristics of the eligible studies are presented in Table 1. Four studies were retrospective [16,17,18,20], one was prospective, parallel, non-randomized [22] and 5 randomized controlled trials (RCT)s [13,15,19,21,22]. The selected studies were performed on 4 continents (USA, Europe, Asia, and Australia) and included patients from medical, surgical, and mixed ICUs. All but two of the RCTs and all non RCTs were from a single center. Study sample sizes varied from 20 to 531. A mean/median APACHE II score ≥ 15 was observed in 6 studies (60%). Gram negative organisms were the predominant pathogens in all selected studies.
Table 1
| Author | Study year | Study design | Sample size | Pathogens | Mean/median APACHE II score (CI, EI) | Mean/median APACHE II score (II) | Jadad or Newcastle Ottawa scale |
|---|---|---|---|---|---|---|---|
| Hanes et al., [13] | 2000 | RCT | 31 | GNB | 14 | 11 | 2 |
| McNabb et al., [14] | 2001 | RCT | 35 | GNB | 13.9 | 15.5 | 3 |
| Georges et al., [15] | 2005 | RCT | 50 | GNB | N/S | N/S | 2 |
| Lorente et al., [16] | 2006 | R | 89 | GNB | 15 | 15 | 7 |
| Lorente et al., [17] | 2007 | R | 121 | GNB | 16 | 16 | 8 |
| Sakka et al., [21] | 2007 | RCT | 20 | GNB | 26 | 28 | 2 |
| Chastre et al., [19] | 2008 | RCT | 531 | GNB | N/S | N/S | 2 |
| Lorente et al., [18] | 2009 | R | 83 | GNB | 16.1 | 16.2 | 8 |
| Wang et al., [20] | 2009 | R | 30 | A. baumannii | 20 | 17 | 7 |
| Fahimi et al., [22] | 2012 | P | 61 | GNB | 19 | 20 | 7 |
RCT, randomized controlled trial; R, retrospective; P, prospective; GNB, Gram negative bacilli; CI, continuous infusion; EI, extended infusion; II, intermittent infusion; N/S, not specified.
Table 2 depicts the β-lactam antibiotic, dose and infusion schedule for each study. β-lactam antibiotics included cephalosporins [13,14,15,17], carbapenems [16,19,20,21] and piperacillin/tazobactam [18,22]. In the experimental arm, these agents were administered via either continuous infusion [13,14,16,17,18,21] or extended infusion [15,19,20,22]. In five of the six studies utilizing a continuous infusion of β-lactam delivery, a loading dose was administered initially to ensure that the time to maximum concentration of the drug is reached rapidly. Participants received additional non β-lactam antibiotics in 6 studies [14,15,16,17,19,23]. The medication in the study arms differed in one trial in which doripenem was compared to imipenem [19]. Because of the similar antimicrobial profile of these agents, this study was included. In several studies, β-lactam dosing was adjusted for renal insufficiency [14,19].
Table 2
| Author | Study antibiotic | Extended infusion/continuous infusion | Intermittent infusion | Concomitant antibiotic |
|---|---|---|---|---|
| Hanes et al., [13] | Ceftazidime | LD, 2 g (0.5-h infusion), then 60 mg/kg/day as Cl | 2 g q8 h (0.5-h infusion) | N/S |
| McNabb et al., [14] | Ceftazidime | 3 g/d as CI | 2 g/d q8 h | Tobramycin |
| Georges et al., [15] | Cefepime | 4 g/d as Cl | 2 g q12 h | Amikacin |
| Lorente et al., [16] | Meropenem | LD, 1 g over 0.5 h, then 1 g q6 h as CI | 1 g q6 h (0.5-h infusion) | Tobramycin |
| Lorente et al., [17] | Ceftazidime | LD, 1 g over 0.5 h, then 2 g q12 h as CI | 2 g q12 h (0.5-h infusion) | Tobramycin |
| Sakka et al., [21] | Imipenem | LD, 1 g over 40 min, then 2 g/24 h as CI for 3 days, then 1 g q8 h over 40 min | 1 g q8 h (40-min infusion) | N/S |
| Chastre et al., [19] | Doripenem/Imipenem | Doripenem 500 mg q8 h as 4-hr EI | Imipenem 500 mg q6 h | Aminoglycoside |
| Lorente et al., [18] | Piperacilin/tazobactam | LD, 4.5 g over 0.5 h, then 4.5 g q6 h as CI | 4.5 g q6 h (0.5-h infusion) | Tobramycin |
| Wang et al., [20] | Meropenem | 500 mg q6 h as 3-h EI | 1 g q8 h (1-h infusion) | N/S |
| Fahimi et al., [22] | Piperacillin/tazobactam | 3.375 g q8 for 4 h as CI | 3.375 g q6 for 30 min | N/S |
LD, loading dose; CI, continuous infusion; EI, extended infusion; N/S, not specified; q6, every 6 hours; q8, every 8 hours; q12, every 12 hours.
3. Quality appraisal of selected studies
The quality of RCTs selected in this study was moderate. Whilst randomization procedure was in place, few studies reported in detail randomization procedures and allocation concealment. Furthermore, none of the investigators were blinded to the mode of delivery. Non RCTs studies were of low to moderate quality. Several studies had a historic control design that compared patients receiving prolonged/continuous infusion with historical cohorts who were administered intermittent boluses per protocol implementation.
4. Mortality
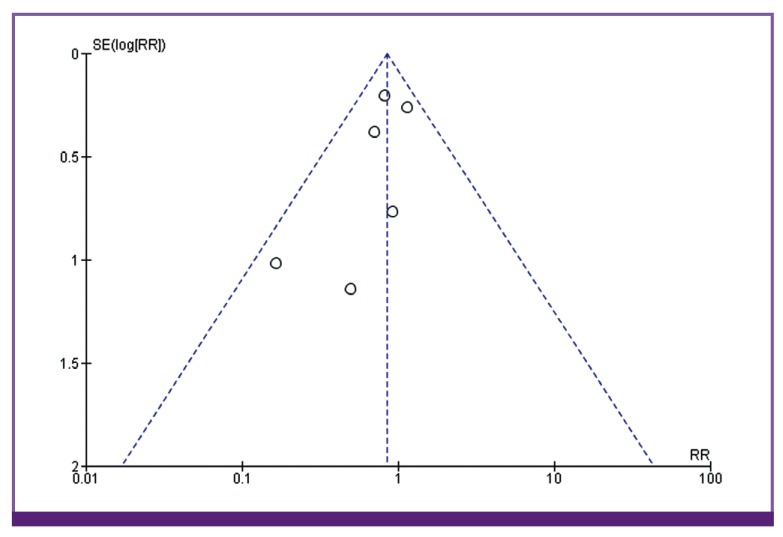
Six studies reported mortality as outcome (Fig. 2). Among the 368 patients enrolled in the continuous/extended infusion, there were 57 deaths compared to 69 deaths among the 377 in the intermittent infusion. The difference was not statistically significant with OR of 0.85 (95% CI 0.63–1.15). There was no significant heterogeneity among the identified studies evaluating mortality (I2 = 0). Visual inspection of the funnel plot comparing the effect measure for the primary outcome of mortality for each study with its precision did not suggest asymmetry (Fig. 3). Overall, based on qualitative and quantitative exploration, no conclusive evidence of reporting bias was found.
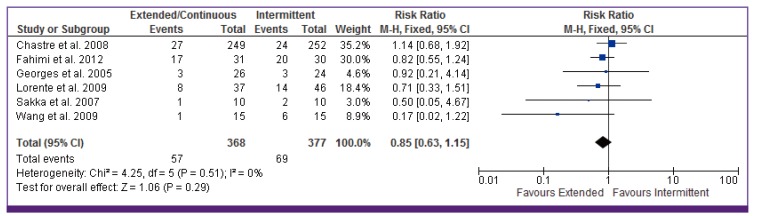
CI, continuous infusion.
5. Clinical cure
Pooling the outcomes of the eight studies that reported on clinical success showed that clinical cure was higher among patients who received extended or continuous infusion of a carbapenem, cephalosporin, or piperacillin/tazobactam than those who received intermittent (OR = 2.45 [95% CI, 1.12, 5.37]) (Fig. 4). Proportion of clinical cure ranged from 33% to 100%. There was significant heterogeneity among the selected studies evaluating clinical cure (I2 = 75%, P < 0.001). This is likely due to the varied definitions of clinical success among the studies. In the subgroup analysis (Table 3), no significant differences were noted by study design or β-lactam subclasses. However, clinical cure was higher in pneumonia patients receiving prolonged infusion with APACHE II score ≥ 15 versus intermittent infusion (OR 3.45, 95% CI 1.08–11.01).
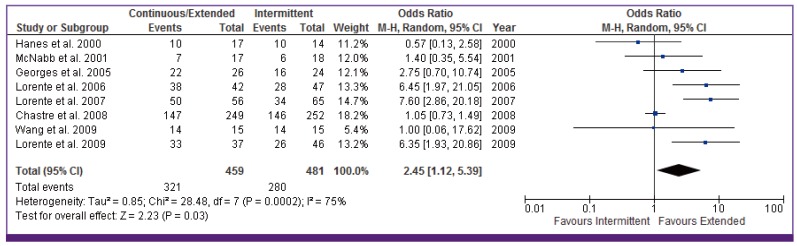
CI, continuous infusion.
Table 3
| Clinical cure | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of studies | Number of patients | Odds ratio (95% CI) | I2 | Number of studies | Number of patients | Odds ratio (95% CI) | I2 | |
| RCTs | 5 | 647 | 1.09 (0.79-1.51) | 0% | 3 | 571 | 1.07 (0.66-1.73) | 0% |
| Cephalosporins | 4 | 237 | 2.21 (0.71-6.86) | 68% | 1 | 50 | 0.92 (0.21-4.14) | - |
| Carbapenems | 3 | 620 | 2.01 (0.48-8.37) | 76% | 3 | 551 | 0.92 (0.57-1.47) | 47% |
| APACHE II ≥ 15 | 5 | 824 | 3.45 (1.08-11.01) | 85% | 4 | 634 | 0.86 (0.57-1.28) | 29% |
RCT, randomized controlled trial; CI, continuous infusion.
6. Microbiologic cure rate
Only two studies presented data on microbiologic cure rate [15,19]. There was significant overlap in the microbiological isolates seen in both studies. The proportion of patients in the microbiologically evaluable cohorts ranged from 59.3% to 79.9% of the entire sample size. There was no statistical heterogeneity with respect to results (I2 = 0). Although the point estimate for the pooled OR favored extended/continuous infusion over intermittent infusion this difference was not statistically significant (OR for cure, 1.50; 95% CI, 0.89–2.52).
7. Adverse events
Four studies reported on adverse events during antibiotic administration [14,15,19,21]. None were associated with mortality. Three cases of nephrotoxicity were reported in patients receiving ceftazidime (two in the intermittent infusion group and one in the continuous infusion group) [14]. These were attributed to concomitant tobramycin administration. C. difficile were described also in three patients who were treated successfully with oral metronidazole. In one study [19], seizures were more commonly observed in patients receiving imipenem (3.8%) than doripenem infusion (1.1%). Although the majority of these events occurred in patients with underlying brain injury or prior history of epilepsy, all but one did not appear related to study drug therapy. Gastrointestinal manifestations were minor and included nausea, vomiting, diarrhea, and transient elevation in liver enzymes. No significant differences between the study arms were discerned for each of the aforementioned adverse events.
Discussion
The first publication using continuous infusion as a method to administer β-lactam drugs to endocarditis patients dates back more than 65 years [24]. Since then there is growing evidence on the theoretical advantage of continuous β-lactam application, as a way to optimize the dose regimen and pharmacokinetics without increasing patient's drug exposure [25]. This review showed that there were significant clinical advantages associated with continuous and extended infusion of β-lactam in hospitalized patients with NP. Compared with intermittent boluses, prolonged infusion was associated with a higher clinical cure rate and a trend toward reduction in mortality.
Several meta-analyses have been conducted on this topic [8,9,26,27,28,29] with inconsistent results. These studies have included heterogeneous populations with different indications for antibiotic use, different study designs, and multitude of antimicrobial combinations that display dissimilar PK/PD properties. This review is the first to examine the role of prolonged versus intermittent infusion in nosocomial pneumonia. Despite the methodological differences of selected studies, patients who received prolonged over intermittent infusion rate displayed higher clinic cure rates than intermittent antibiotic infusion. In vitro and animal studies have demonstrated that the amount of time in which the free or non-protein bound drug concentration exceeds the MIC (fT > MIC) of the organism is the best predictor of clinical and microbiologic response for β-lactams [30,31,32]. Because critically ill patients are at high risk of acquiring pathogens with high MICs, it can be quite challenging to achieve adequate PK/PD targets with classical intermittent dosing regimens. The increased volume of distribution in this population leads frequently to a lower steady state concentration/MIC with subsequent risk of therapeutic failure or emergence of drug resistant pathogens [33]. This is well illustrated in a study by Chytra and colleagues [34] who demonstrated that administration of meropenem by continuous infusion was associated with higher eradication rate and lower bacterial persistence in comparison with higher intermittent dosage without difference in the rate of resistance, colonization, or superinfection. Similarly, two other studies documented an improved bacteriologic efficacy of continuous infusion of β-lactam over bolus dosing [35,36]. In 2009, a meta-analysis was published of all RCT from 1950 through November 2007 comparing the clinical benefits of continuous infusion regimens of β-lactam antibiotics with intermittent regimens [8]. No difference was found for mortality or clinical cure between the two groups. Yet, all but one of the included studies used a higher drug dose in the bolus group than in the continuous infusion group.
It is noteworthy that although clinical cure rate was significantly higher in patients who received extended or continuous infusions, the difference in mortality rate between the two groups did not reach statistical significance. The sample size of our meta-analysis may not have permitted detection of modest differences. To detect a significant difference in outcome, a 4-fold difference in mortality from baseline would have been necessary, or using the observed mortality between groups, more than 2,400 patients would have been required in each treatment group to achieve a power of 80% at a significance level of 0.05. In addition, the attributable mortality from nosocomial pneumonia (including ventilator associated pneumonia) has been reported to vary between 1.5% and 50% [37] depending on host characteristics, offending pathogens, and severity of illness. Due to the heterogeneity of the study population, the expected mortality of the selected studies was not uniform. The inclusion of low-risk patients could have resulted in a skewed mortality impact, and thus any mortality difference would have been extremely difficult to demonstrate. This explanation is further supported by the fact that infusion of β-lactams appears to be more effective in patients who have a higher acuity of illness [38]. This is illustrated in this study by the higher cure rate and improved survival of patients with APACHE >15. It is noteworthy to indicate that the subgroup analysis of RCTs did not reveal significant difference in clinical cure rate because the sample size of the RCTs is not powered for statistical significance. What is reassuring is that the mortality trend favors also the extended infusion. This analysis had only few retrospective studies.
Since the success of treatment depends upon antibiotic levels at the site of infection, prolonged infusion has been shown to include better penetration of β-lactam antibiotics into the lungs [35,39]. In patients with ventilator associated pneumonia, alveolar concentration exceeding the susceptibility breakpoint for Gram negative bacteria can be attained more reliably with prolonged infusion. This approach can be achieved with minimal side effects. Overall we have seen no differences in adverse events between the two administration groups in those studies that reported these complications.
Nonetheless, it has been suggested that continuous exposure to antimicrobial concentrations slightly in excess of the reported MIC could select subpopulations of resistant organisms that typically are not detected by MIC testing and usually are eliminated or inhibited by higher peak antimicrobial concentrations. However, in vitro studies could not corroborate this hypothesis. Using a hollow fiber infection model with Pseudomonas aeruginosa, Felton and coworkers [40] demonstrated that extended infusion resulted in comparable antibacterial activities and rates of emergence of antimicrobial resistance as by bolus infusion.
Several limitations of this analysis should be noted. First, the numbers of patients enrolled in the selected studies were relatively small and most of the RCT were single centered, unblinded, with only a minority reporting on quality indicators, such as allocation concealment, intention-to-treat analysis, and losses to follow-up after randomization. Second, the microbiology of these infectious events was not consistently provided and although the majority of these cases were related to Gram negative pathogens, the susceptibility profiles were not reported. Given the fact that inappropriate antibiotic regimens are linked to increased mortality, the presence of highly resistant organisms may have altered the mortality rates in few of these studies. Third, concomitant antibiotic therapy reduces the validity of conclusions about the impact of prolonged infusion on clinical outcomes particularly when the agents used possess different antimicrobial spectrum. Therefore, it is not possible to determine accurately how clinical cure and mortality relate to antibiotic exposure. Fourth, the clinical trial end point definitions are not homogeneously and consistently established across the ten selected studies, but represent reasonable options that are based on available data. Further attempts to evaluate clinical trials through combination of retrospective and prospective studies should be undertaken with caution and for the generation of hypotheses only.
In conclusion, the current evidence suggests that prolonged infusion of beta-lactams results in higher clinical cure rate. However, well-designed RCTs are warranted to validate these findings before such strategy can be widely applied in clinical practice.
Footnotes
Financial Support: Supported by a Merit Review Grant (CX000478) from the Department of Veterans Affairs (AES). None of the authors report any relevant conflict of interest. The views expressed in this review do not communicate an official position of the Department of Veterans Affairs.
Authors' Contributions: AES formulated the research question and was involved in manuscript preparation. All authors were involved in determining the search strategy, data collection for the review, and quantitative and qualitative analysis. All authors read and approved the final manuscript.
Conflicts of Interest: No conflicts of interest.
References
Articles from Infection & Chemotherapy are provided here courtesy of Korean Society of Infectious Diseases
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.3947/ic.2016.48.2.81
Article citations
Application of Monte Carlo simulation to optimise the dosage regimen of meropenem in patients with augmented renal clearance for Pseudomonas aeruginosa infection.
Heliyon, 10(12):e32600, 07 Jun 2024
Cited by: 1 article | PMID: 38975089
Usage of Meropenem Continuous Infusion for Treatment of Infectious Complications in Orthopedic Elderly Patients with Anemia: A Case Series.
Medicina (Kaunas), 60(6):929, 02 Jun 2024
Cited by: 0 articles | PMID: 38929546
Ten Issues to Update in Nosocomial or Hospital-Acquired Pneumonia: An Expert Review.
J Clin Med, 12(20):6526, 14 Oct 2023
Cited by: 2 articles | PMID: 37892664 | PMCID: PMC10607368
Review Free full text in Europe PMC
Extended infusion of β-lactams significantly reduces mortality and enhances microbiological eradication in paediatric patients: a systematic review and meta-analysis.
EClinicalMedicine, 65:102293, 02 Nov 2023
Cited by: 2 articles | PMID: 38021371 | PMCID: PMC10651452
β-Lactam Pharmacokinetic/Pharmacodynamic Target Attainment in Intensive Care Unit Patients: A Prospective, Observational, Cohort Study.
Antibiotics (Basel), 12(8):1289, 05 Aug 2023
Cited by: 3 articles | PMID: 37627709 | PMCID: PMC10451857
Go to all (15) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Does prolonged β-lactam infusions improve clinical outcomes compared to intermittent infusions? A meta-analysis and systematic review of randomized, controlled trials.
BMC Infect Dis, 11:181, 22 Jun 2011
Cited by: 52 articles | PMID: 21696619 | PMCID: PMC3141415
Review Free full text in Europe PMC
Loading dose and efficacy of continuous or extended infusion of beta-lactams compared with intermittent administration in patients with critical illnesses: A subgroup meta-analysis and meta-regression analysis.
J Clin Pharm Ther, 46(2):424-432, 02 Nov 2020
Cited by: 9 articles | PMID: 33135261
Review
Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. A Meta-analysis of Individual Patient Data from Randomized Trials.
Am J Respir Crit Care Med, 194(6):681-691, 01 Sep 2016
Cited by: 190 articles | PMID: 26974879
Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis.
Intensive Care Med, 42(10):1535-1545, 11 Jan 2016
Cited by: 161 articles | PMID: 26754759
Funding
Funders who supported this work.
CSRD VA (1)
Grant ID: I01 CX000478
U.S. Department of Veterans Affairs (1)
Grant ID: CX000478

 1,2,3
1,2,3