Abstract
Free full text

Sarcopenia and sarcopenic obesity
Abstract
Sarcopenia is an age-associated loss of muscle mass and decline in muscle strength; it is common in older adults and is associated with significant morbidity and mortality. Despite its prevalence, there is currently no universally adopted definition of sarcopenia. In addition to low muscle mass measurements, recent research has recognized the importance of muscle strength and physical performance. Aging induces changes in body composition, such as an increase in visceral fat and reduced muscle mass. Recently, the new concept of sarcopenic obesity has emerged, reflecting a combination of sarcopenia and obesity. The rapidly increasing prevalence and serious consequences of sarcopenic obesity are recognized as a critical public health risk in the aging society. Sarcopenia and obesity share several pathophysiological mechanisms, and they may potentiate each other. The present paper reviews the definitions and techniques used to measure sarcopenia, as well as the health outcomes of sarcopenic obesity. It also highlights the role of diminished muscle mass and strength in cardiometabolic disease mortality. Additional research may be needed to promote the identification and management of sarcopenia and sarcopenic obesity in the elderly population.
INTRODUCTION
One of the dramatic changes associated with human aging is the progressive decline of skeletal muscle mass. Several prospective studies have suggested that muscle mass decreases by approximately 6% per decade after mid-life [1]. Interestingly, results from the Health Aging, and Body Composition (ABC) study showed that the decreased muscle strength is predominantly due to a lower muscle mass [2]. There are significant differences among individuals in peak muscle mass, the age at which muscle loss begins, and the amount of muscle that is lost over time [3]. Rosenberg first proposed the term “sarcopenia,” which originated from the Greek words sarx (flesh) and penia (loss), to refer to the age-related loss of skeletal muscle mass [4]. At the cellular level, sarcopenia is accompanied by a loss of innervation and adaptive changes in the proportions of slow and fast motor units, as well as in the cross-sectional area of muscle fibers [5]. Primary sarcopenia is the term used to define sarcopenia that is caused by aging itself, whereas secondary sarcopenia describes sarcopenia that is caused by disuse (immobility or physical inactivity), disease (advanced organ failure, malignancy, neurodegenerative, or endocrine diseases), and inadequate nutrition [6]. Sarcopenia is closely associated with frailty, physical disability, hospitalization, osteoporosis, osteoarthritis, and even mortality [7,8].
DEFINITIONS OF SARCOPENIA
Several different definitions of sarcopenia have been suggested, although no consensus definition has been adopted [9]. In 1998, Baumgartner et al. [10] first defined sarcopenia as an appendicular skeletal muscle mass (ASM) (kg)/height2 (m2) of less than two standard deviations (SDs) below the mean of a young reference group. Janssen et al. [11] adopted the skeletal muscle mass index (SMI = skeletal muscle mass/body mass × 100) to establish the prevalence of sarcopenia in older Americans. They considered subjects with an SMI within one to two SDs of young adult values to have class I sarcopenia, and those with an SMI below two SDs of young adult values to have class II sarcopenia. In the Health ABC study, Newman et al. [12] proposed an alternative definition of sarcopenia using appendicular lean mass (ALM) adjusted for height and body fat mass (residuals). They indicated that Baumgartner’s definition (ASM/height)2 is strongly correlated with body mass index (BMI), and therefore identifies fewer obese individuals as sarcopenic. To examine the prevalence of sarcopenia in Korean adults and explore its impact on health outcomes, we previously established a cohort study called the Korean Sarcopenic Obesity Study (KSOS) [13]. The prevalence of sarcopenia differed according to the definition applied, as well as age and sex.
Since then, the definition of sarcopenia has evolved to highlight muscle strength and physical performance. The European Working Group on Sarcopenia in Older People (EWGSOP) defined sarcopenia as the presence of both low muscle mass and low muscle strength or performance [6]. Recently, the Foundation for the National Institutes of Health (FNIH) sarcopenia project suggested using ALM with adjustment for BMI to define low muscle mass [14]. Using a dataset from nine large observational studies containing more than 25,000 participants, the FNIH sarcopenia project established ALM/BMI ratio cutoff values of < 0.789 for men and < 0.512 for women [14,15]. Recently, Kim et al. [16] compared skeletal muscle mass indices and described their clinical implications. Further studies might be needed to compare the definitions of sarcopenia with regard to their impact on disability, cardiometabolic risk profiles, and mortality.
TECHNIQUES FOR ASSESSING SARCOPENIA
Various methods can be used to assess muscle mass and strength (Table 1). Anthropometric measurements, such as mid-upper arm circumference, calf circumference, and skin fold thickness, are not recommended for diagnosing sarcopenia since they are prone to error [6]. Computed tomography (CT) and magnetic resonance imaging are able to effectively distinguish fat from other soft tissues, which makes these the standard techniques for evaluating muscle mass in research [6]. However, limited access, the high cost, and the risk of radiation (with CT) preclude the wider use of these techniques in clinical practice. Therefore, dual energy X-ray absorptiometry (DXA) is the preferred method for correctly evaluating body composition; it is widely used to assess muscle mass in research studies because of good precision and safety with relatively low radiation [7]. Therefore, measuring ALM using DXA has become a standard criterion in most current sarcopenia definitions. Bioimpedance analysis (BIA) is an inexpensive, easy to use, and reproducible method that is considered a portable alternative to DXA. However, neither DXA nor BIA can distinguish between extracellular and intracellular water, making these techniques prone to error depending on the hydration status of the patient [7].
Table 1.
Methods for measurement of muscle mass, muscle strength, and physical performance
| Muscle mass | Muscle strength | Physical performance |
|---|---|---|
| Anthropometry | Handgrip strength | Short physical performance battery |
| Computed tomography | Knee flexion/extension | Usual gait speed |
| Magnetic resonance imaging | Timed get-up-and-go test | |
| Dual energy X-ray absorptiometry | ||
| Bioimpedance analysis |
Muscle strength is commonly evaluated using handgrip strength, which is an easy, reliable, and inexpensive method for identifying elderly adults at risk for disability [17]. Based on the EWGSOP definition, cutoffs for grip strength are < 20 kg for women and < 30 kg for men [6]. In contrast, the FNIH sarcopenia project established cutoffs of < 16 kg for women and < 26 kg for men [14,15]. Knee flexion techniques are appropriate for research purposes; however, their use in clinical practice is limited by the need for specific machines. The short physical performance battery (SPPB) is used in both clinical and research settings to measure physical performance. It combines gait speed, chair-rise time, and balance assessment to generate a standard measurement [18]. Usual gait speed, which is part of the SPPB, might be adopted as a single parameter to provide a predictive value for disability [19]. Previous studies have demonstrated that a walking speed of less than either 1.0 or 0.8 m/sec is associated with adverse outcomes [20]. Timed get-upand-go examines the time needed to accomplish a series of functionally critical tasks; it can also serve as a performance measurement.
MECHANISMS OF SARCOPENIA
Several underlying mechanisms have been linked to the development of sarcopenia, although not all have been fully elucidated. The relative contribution of the different mechanisms may vary over time in an individual with sarcopenia [6]. Understanding the mechanisms underlying sarcopenia may provide strategies for intervention and disease improvement. Most mechanisms of sarcopenia are also associated with visceral obesity, which may lead to a vicious cycle of intricate interactions among risk factors. Insulin resistance plays an important role in muscle fiber atrophy and mitochondrial dysfunction [21]. Bijlsma et al. [22] demonstrated that insulin resistance is related distinctly to the different diagnostic criteria for sarcopenia; it is better reflected by relative muscle mass than by absolute muscle mass or muscle strength. Aging is related to changes in a variety of hormones, including testosterone, estrogen, growth hormone, insulin-like growth factor 1, and corticosteroids [23]. These hormonal changes may affect the anabolic and catabolic processes in skeletal muscle [24]. Reduced androgen and estrogen concentrations decrease muscle mass and strength. In addition, previous studies have suggested that sarcopenia is an inflammatory state that is driven by proinflammatory cytokines and oxidative stress [25]. Oxidative stress modulates the expression of transcription factors, such as nuclear factor-κB, which enhances proteolytic pathways and increases the production of proinflammatory cytokines [26]. Tumor necrosis factor α impairs protein synthesis in skeletal muscle by altering translation initiation, which may contribute to sarcopenia [27]. In a prospective, population-based study, higher levels of interleukin 6 and C-reactive protein were associated with a greater decline in muscle strength [28]. Another pivotal factor in the regulation of skeletal muscle mass is myostatin, also known as growth/differentiation factor 8, which inhibits muscle cell growth and differentiation [29]. Deletion of the myostatin gene causes a double-muscled phenotype in cattle [30]. Myostatin has emerged as a potential mediator of sarcopenia and a promising therapeutic target [31].
IMPLICATIONS OF THE EFFECTS OF SARCOPENIA ON CARDIOMETABOLIC RISK AND MORTALITY
Sarcopenia is independently associated with insulin resistance [32], and in the KSOS study, type 2 diabetes was independently associated with an increased risk of sarcopenia [33]. Diabetes has been suggested to be an intermediate step in the development of frailty in individuals with sarcopenia [34]. Furthermore, we previously found that individuals with a lower muscle mass have an increased risk of nonalcoholic fatty liver disease, which is now recognized as a feature of metabolic syndrome [35]. Low muscle mass is related to cardiovascular risk factors including hypertension and arterial stiffness [36,37]. The coexistence of sarcopenia and metabolic syndrome further aggravates the risk of cardiovascular risk factors such as type 2 diabetes, hypertension, and hyperlipidemia in adult Japanese women [38]. Previous prospective studies have consistently shown a relationship between low muscle mass or muscle strength and an increased risk of mortality [39-41]. In the British Regional Heart Study, Atkins et al. [42] reported that sarcopenia is associated with greater cardiovascular mortality and allcause mortality. Hirani et al. [43] showed that sarcopenia, as defined by the FNIH criteria, is associated with an increased risk of mortality, disability, and institutionalization in community-dwelling older men. Several studies have suggested that deteriorated muscle strength is a more important risk factor for mortality than a decline in muscle mass [44,45].
SARCOPENIC OBESITY
Obesity is an important health threat that is a major risk factor for metabolic and cardiovascular morbidity and mortality. The prevalence of obesity in middle-aged and older adults has doubled since 1980, and it continues to increase worldwide [9]. The major age-related changes in body composition include an increase in body fat and a decline in skeletal muscle, although BMI may remain relatively unchanged. Sarcopenic obesity was first defined by Baumgartner [46] as the co-presence of sarcopenia and obesity, as measured using DXA. We subsequently introduced the ratio of visceral fat to thigh muscle area (VMR), as measured using CT, as a single indicator of sarcopenic obesity [47]. We found that VMR values were independently associated with metabolic syndrome in Korean adults.
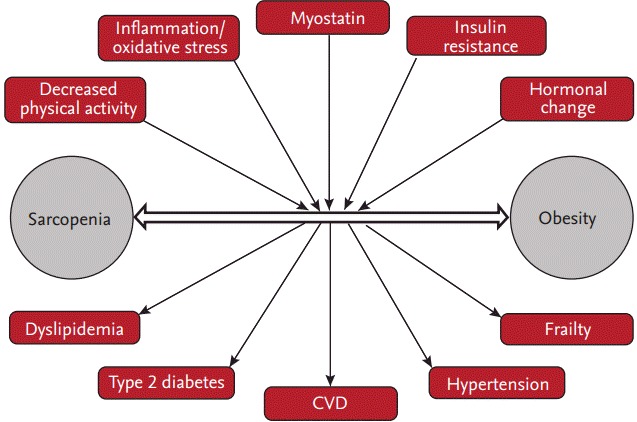
The complex interplay of common pathophysiological mechanisms, such as increased proinflammatory cytokines, oxidative stress, insulin resistance, and hormonal changes and decreased physical activity, underlie the close relationship between sarcopenia and obesity (Fig. 1). We reported that the homoeostasis model assessment of insulin resistance (HOMA-IR) and vitamin D levels are independently associated with sarcopenic obesity in men, whereas HOMA-IR and high-sensitivity C-reactive protein are associated with sarcopenic obesity in women [48]. A vicious cycle may exist between the accumulation of ectopic fat and the loss of skeletal muscle mass since they have a reciprocal influence on each other [49]. Sarcopenia reduces physical activity, which leads to decreased energy expenditure and increases the risk of obesity [50]. In contrast, an increase in visceral fat induces inflammation, which contributes to the development of sarcopenia [51]. In our longitudinal study, visceral obesity was independently associated with the future loss of skeletal muscle mass after adjusting for confounding factors [52].
IMPACT OF SARCOPENIC OBESITY ON MORBIDITY AND MORTALITY
Both sarcopenia and obesity are associated with metabolic disorders, morbidity, and mortality [50]. Thus, it has been hypothesized that sarcopenic obesity may have a greater impact on metabolic diseases and cardiovascular morbidity and mortality than either sarcopenia or obesity alone [9,53].
Recent studies have emphasized the influence of sarcopenic obesity on cardiometabolic risk and health outcomes [23,53,54]. Several cross-sectional studies in elderly Koreans have demonstrated that individuals with sarcopenic obesity have worse cardiovascular risk profiles, including hyperglycemia, hypertension, dyslipidemia, insulin resistance, and lower cardiorespiratory fitness [55-57]. Similarly, a Taiwanese study showed that sarcopenic obesity is associated with the highest risk of metabolic syndrome [58]. Furthermore, sarcopenia exacerbated obesity-associated dysglycemia and insulin resistance in a cross-sectional study from the National Health and Nutrition Examination Survey III (NHANES III) [32]. However, there have been conflicting results regarding whether patients with sarcopenic obesity have the worst risk profiles. Several cross-sectional studies have reported that obese individuals have more cardiovascular risk factors than those with sarcopenic obesity [59,60].
A limited number of studies have investigated the effects of sarcopenia and obesity on cardiovascular disease (CVD) and mortality. Stephen and Janssen [61] showed that sarcopenic obesity is associated with increased CVD risk based on muscle strength but not muscle mass. In the British Regional Heart Study, subjects with sarcopenic obesity had a significantly higher risk of mortality compared to nonsarcopenic, nonobese subjects [41]. In contrast, in a study using data from the NHANES III study, older women with sarcopenia had a higher risk of all-cause mortality, independent of obesity [62]. A recent meta-analysis demonstrated that sarcopenic obesity is associated with a 24% increase in the risk of all-cause mortality compared to patients without sarcopenic obesity, particularly in men [63].
CONCLUSIONS
Although BMI is a simple estimator of obesity, it cannot fully reflect muscle mass and body fat. Accumulating evidence underscores the need to consider muscle function and mass when evaluating the risk of obesity in elderly people. Visceral fat and muscle mass seem to have opposing influences on cardiometabolic morbidity and mortality. Sarcopenic obesity is a relatively novel concept that has become increasingly important in the aging population. There has been some evidence that sarcopenic obesity may be associated with an increased risk of mortality and cardiovascular risk factors compared to sarcopenia or obesity alone. However, several different definitions of sarcopenia limit the clinical application of sarcopenia and sarcopenic obesity with regard to metabolic disorders and CVD. A consensus definition of sarcopenia is needed to promote the standardized diagnosis and management of sarcopenic obesity. Furthermore, in addition to reducing body fat, increasing muscle mass and strength is required to promote healthy aging.
Acknowledgments
The present work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science and Technology (2015R1D1A1A09057389).
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
Articles from The Korean Journal of Internal Medicine are provided here courtesy of Korean Association of Internal Medicine
Full text links
Read article at publisher's site: https://doi.org/10.3904/kjim.2016.193
Read article for free, from open access legal sources, via Unpaywall:
http://kjim.org/upload/kjim-2016-193.pdf
Citations & impact
Impact metrics
Article citations
Analysis of body composition, functionality and muscle-specific strength of older women with obesity, sarcopenia and sarcopenic obesity: a cross-sectional study.
Sci Rep, 14(1):24802, 22 Oct 2024
Cited by: 0 articles | PMID: 39438648 | PMCID: PMC11496535
Association between systemic immune-inflammation index and sarcopenic obesity in middle-aged and elderly Chinese adults: a cross-sectional study and mediation analysis.
Lipids Health Dis, 23(1):230, 30 Jul 2024
Cited by: 1 article | PMID: 39080664 | PMCID: PMC11287930
Prediction of lymph node metastasis in T1 colorectal cancer based on combination of body composition and vascular invasion.
Int J Colorectal Dis, 39(1):84, 03 Jun 2024
Cited by: 0 articles | PMID: 38829434 | PMCID: PMC11147873
Timing, velocity, and magnitude of pubertal changes in body composition: a longitudinal study.
Pediatr Res, 11 Jun 2024
Cited by: 0 articles | PMID: 38862608
Exploration of a machine learning approach for diagnosing sarcopenia among Chinese community-dwelling older adults using sEMG-based data.
J Neuroeng Rehabil, 21(1):69, 09 May 2024
Cited by: 0 articles | PMID: 38725065 | PMCID: PMC11080130
Go to all (137) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association of physical activity with sarcopenia and sarcopenic obesity in community-dwelling older adults: the Fourth Korea National Health and Nutrition Examination Survey.
Age Ageing, 42(6):734-740, 11 Jun 2013
Cited by: 80 articles | PMID: 23761456
Sarcopenia and sarcopenic obesity among men aged 80 years and older in Beijing: prevalence and its association with functional performance.
Geriatr Gerontol Int, 14 Suppl 1:29-35, 01 Feb 2014
Cited by: 31 articles | PMID: 24450558
Health Consequences of Sarcopenic Obesity: A Narrative Review.
Front Endocrinol (Lausanne), 11:332, 21 May 2020
Cited by: 105 articles | PMID: 32508753 | PMCID: PMC7253580
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Ministry of Education, Science and Technology (1)
Grant ID: 2015R1D1A1A09057389