Abstract
Importance
The use of palliative care programs and the number of trials assessing their effectiveness have increased.Objective
To determine the association of palliative care with quality of life (QOL), symptom burden, survival, and other outcomes for people with life-limiting illness and for their caregivers.Data sources
MEDLINE, EMBASE, CINAHL, and Cochrane CENTRAL to July 2016.Study selection
Randomized clinical trials of palliative care interventions in adults with life-limiting illness.Data extraction and synthesis
Two reviewers independently extracted data. Narrative synthesis was conducted for all trials. Quality of life, symptom burden, and survival were analyzed using random-effects meta-analysis, with estimates of QOL translated to units of the Functional Assessment of Chronic Illness Therapy-palliative care scale (FACIT-Pal) instrument (range, 0-184 [worst-best]; minimal clinically important difference [MCID], 9 points); and symptom burden translated to the Edmonton Symptom Assessment Scale (ESAS) (range, 0-90 [best-worst]; MCID, 5.7 points).Main outcomes and measures
Quality of life, symptom burden, survival, mood, advance care planning, site of death, health care satisfaction, resource utilization, and health care expenditures.Results
Forty-three RCTs provided data on 12 731 patients (mean age, 67 years) and 2479 caregivers. Thirty-five trials used usual care as the control, and 14 took place in the ambulatory setting. In the meta-analysis, palliative care was associated with statistically and clinically significant improvements in patient QOL at the 1- to 3-month follow-up (standardized mean difference, 0.46; 95% CI, 0.08 to 0.83; FACIT-Pal mean difference, 11.36] and symptom burden at the 1- to 3-month follow-up (standardized mean difference, -0.66; 95% CI, -1.25 to -0.07; ESAS mean difference, -10.30). When analyses were limited to trials at low risk of bias (n = 5), the association between palliative care and QOL was attenuated but remained statistically significant (standardized mean difference, 0.20; 95% CI, 0.06 to 0.34; FACIT-Pal mean difference, 4.94), whereas the association with symptom burden was not statistically significant (standardized mean difference, -0.21; 95% CI, -0.42 to 0.00; ESAS mean difference, -3.28). There was no association between palliative care and survival (hazard ratio, 0.90; 95% CI, 0.69 to 1.17). Palliative care was associated consistently with improvements in advance care planning, patient and caregiver satisfaction, and lower health care utilization. Evidence of associations with other outcomes was mixed.Conclusions and relevance
In this meta-analysis, palliative care interventions were associated with improvements in patient QOL and symptom burden. Findings for caregiver outcomes were inconsistent. However, many associations were no longer significant when limited to trials at low risk of bias, and there was no significant association between palliative care and survival.Free full text

Association Between Palliative Care and Patient and Caregiver Outcomes
Associated Data
Abstract
IMPORTANCE
The use of palliative care programs and the number of trials assessing their effectiveness have increased.
OBJECTIVE
To determine the association of palliative care with quality of life (QOL), symptom burden, survival, and other outcomes for people with life-limiting illness and for their caregivers.
DATA SOURCES
MEDLINE, EMBASE, CINAHL, and Cochrane CENTRAL to July 2016.
STUDY SELECTION
Randomized clinical trials of palliative care interventions in adults with life-limiting illness.
DATA EXTRACTION AND SYNTHESIS
Two reviewers independently extracted data. Narrative synthesis was conducted for all trials. Quality of life, symptom burden, and survival were analyzed using random-effects meta-analysis, with estimates of QOL translated to units of the Functional Assessment of Chronic Illness Therapy–palliative care scale (FACIT-Pal) instrument (range, 0–184 [worst-best]; minimal clinically important difference [MCID], 9 points); and symptom burden translated to the Edmonton Symptom Assessment Scale (ESAS) (range, 0–90 [best-worst]; MCID, 5.7 points).
MAIN OUTCOMES AND MEASURES
Quality of life, symptom burden, survival, mood, advance care planning, site of death, health care satisfaction, resource utilization, and health care expenditures.
RESULTS
Forty-three RCTs provided data on 12 731 patients (mean age, 67 years) and 2479 caregivers. Thirty-five trials used usual care as the control, and 14 took place in the ambulatory setting. In the meta-analysis, palliative care was associated with statistically and clinically significant improvements in patient QOL at the 1- to 3-month follow-up (standardized mean difference, 0.46; 95%CI, 0.08 to 0.83; FACIT-Pal mean difference, 11.36] and symptom burden at the 1- to 3-month follow-up (standardized mean difference, −0.66; 95%CI, −1.25 to −0.07; ESAS mean difference, −10.30). When analyses were limited to trials at low risk of bias (n = 5), the association between palliative care and QOL was attenuated but remained statistically significant (standardized mean difference, 0.20; 95%CI, 0.06 to 0.34; FACIT-Pal mean difference, 4.94), whereas the association with symptom burden was not statistically significant (standardized mean difference, −0.21; 95%CI, −0.42 to 0.00; ESAS mean difference, −3.28). There was no association between palliative care and survival (hazard ratio, 0.90; 95%CI, 0.69 to 1.17). Palliative care was associated consistently with improvements in advance care planning, patient and caregiver satisfaction, and lower health care utilization. Evidence of associations with other outcomes was mixed.
CONCLUSIONS AND RELEVANCE
In this meta-analysis, palliative care interventions were associated with improvements in patient QOL and symptom burden. Findings for caregiver outcomes were inconsistent. However, many associations were no longer significant when limited to trials at low risk of bias, and there was no significant association between palliative care and survival.
Improving quality of life (QOL) in serious illness is an international priority.1,2 Palliative care focuses on improving QOL and reducing suffering for seriously ill patients and their families.3 More than 65%ofUS hospitals have an inpatient palliative care program.4 Community- and outpatient-based models of palliative care delivery are increasing.5
A 2008 systematic review6 and a 2011 narrative review7 both reported mixed evidence for the association between palliative care and patient, family, and health care utilization outcomes, as well as methodological shortcomings in the evidence. Since 2011, additional randomized clinical trials (RCTs) have reported that palliative care improves outcomes such as QOL,8–11 symptom burden,8–10 and survival.12,13 As a result, palliative care has been included in international policy and guidelines.14,15
The aims of this study were to conduct a systematic review of palliative care RCTs to provide an up-to-date summary of palliative care outcomes and to perform meta-analyses to estimate the association of palliative care with patient QOL, symptom burden, and survival.
Methods
This protocol-based systematic review and meta-analysis (PROSPERO ID: CRD42014013696)16 was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions.17
Identification and Selection of Studies
We searched MEDLINE, EMBASE, CINAHL, and Cochrane Library’s CENTRAL from inception to July 22, 2016. A health sciences librarian (M.K-F.) developed, piloted, and executed the searches (eText 1 in the Supplement). Searches excluded pediatric and non–English-language articles.
Study Eligibility
Two reviewers (D.K. and L.H.) independently evaluated all records for eligibility (eTable 1 in the Supplement). Disagreements were resolved by consensus with 2 other authors (J.C. and Y.S.). The RCTs investigating palliative care interventions targeting adult patients (≥18 years) with life-threatening illness that reported on at least 1 of 9 patient-level outcomes were included: QOL, symptom burden, mood, survival, advance care planning, site of death, resource utilization, health care expenditures, and satisfaction with care. Interventions were included if they comprised at least 2 of 8 possible domains of palliative care, as defined by the National Consensus Project for Quality Palliative Care.18 Interventions that treated a single symptom (eg, opioids for dyspnea), targeted only one palliative care domain (eg, advance care planning only), or did not target patients (eg, caregiver-only interventions) were excluded. Trials with usual care, waitlist, or attention control comparators were included.
Data Extraction and Risk of Bias Assessment
Two of 4 investigators (D.K., J.C., N.C.E., J.H.) used structured, customized forms to extract information from each trial’s primary and secondary reports. Risk of bias was independently rated by 2 investigators (D.K., J.N.D-O.) using the Cochrane Collaboration’s tool.17 Within each trial, risk of bias was evaluated separately for subjective (eg, patient-reported outcomes) and objective (eg, survival) outcomes. Therefore, each trial has 2 summary risk-of-bias judgments, 1 regarding subjective outcomes and 1 for objective outcomes. Detailed information regarding risk of bias assessment is provided in eText 2 in the Supplement. Trial authors were contacted to provide additional detail necessary to render high or low judgments.
Synthesis
A narrative synthesis was conducted for all trials. In addition, patient QOL, symptom burden, and survival outcomes were selected a priori for meta-analysis. Quality of life and symptom burden are considered to be primary targets of palliative care interventions. However, the association of palliative care and survival has been of considerable interest.12,19,20 Due to the variety of instruments used to evaluate QOL and symptom burden, pooled effects were summarized as standardized mean differences (SMDs), calculated using a Hedges adjusted g estimator to correct for small sample bias.21 If necessary, individual study results were corrected for directionality such that higher QOL scores represented better QOL, and lower symptom scores indicated less symptom burden. Pooled SMDs were reexpressed as units of familiar instruments by multiplying SMDs by the among-person SDs of the Functional Assessment of Chronic Illness Therapy–palliative care scale (FACIT-Pal)22 for QOL, and the Edmonton Symptom Assessment Scale (ESAS)23 for symptom burden (eText 3 in the Supplement).24 Translations are provided to assist with interpretation of results; however, due to differences in study variances, inferences regarding statistical significance of findings should be interpreted from SMD calculations. The FACIT-Pal scores range from 0 (worst) to 184 (best). Although the minimal clinically important difference (MCID) is unknown for the FACIT-Pal, it has been suggested that MCIDs for total Functional Assessment of Cancer Therapy (FACT) scores, including the FACIT-Pal, are 4% to 6% of a measure’s overall score.25 A midrange bound of 5% equals 9 points on the FACIT-Pal. Edmonton Symptom Assessment Scale scores range from 0 (best) to 90 (worst). The MCID for improvement in the ESAS total score is 5.7 points using the conservative within-patient change approach.26
Given heterogeneity across trials, DerSimonian-Laird random effects models were constructed using Stata version 13 (StataCorp). All significance tests were 2-tailed, with P < .05 considered statistically significant. The proportion of variability in point estimates attributable to between-study heterogeneity was quantified by the I2 statistic21 and interpreted qualitatively as low (25%–50%), moderate (50%–75%), and high (75%–100%).27 Heterogeneity was also examined using τ2 and Cochrane Q statistics. All studies included in the meta-analysis had comparable baseline characteristics between intervention and control groups or outcome measurements adjusted by baseline scores.
To account for variability in the timing of study end points, clinically relevant follow-up periods of 1 to 3 and 4 to 6 months were used. For studies that reported outcomes at more than one time point within the same 1- to 3- or 4- to 6–month window, the last time point was analyzed. Outcomes reported between 2 time points were categorized with the earlier month. Hazard ratios (HRs)were used as the treatment effect for survival. Hazard ratios were imputed when they were not provided using the log-rank approach.28,29
Sensitivity analyses were conducted to evaluate the influence of risk of bias, the use of follow-up time windows (vs 3- or 6-month discrete follow-up time points), and imputation of HRs. Post hoc analyses were conducted to assess whether associations varied according to setting and disease (cancer only, noncancer only, or mixed-disease samples). Univariable meta-regression was used to explore associations between estimated effect sizes and publication year and intervention intensity. Publication bias was assessed through funnel plots and Egger tests. Statistical heterogeneity was explored by modeling study-level characteristics using univariable meta-regression.
Results
Study Characteristics
Searches identified 6158 unique records, of which 200 were potentially relevant based on initial screening (Figure 1). Fifty-six articles were ultimately included, describing 43 trials that involved 12 731 patients (mean age, 67 years) and 2479 caregivers (eTables 2–4 in the Supplement). Thirty trials (69.7%) included patients with cancer and 14 trials (32.5%) included patients with heart failure, both of which diseases represent the diagnoses most commonly requiring palliative care. Thirty-one trials (72.0%)were conducted in the United States. Fourteen trials (32.5%) were in ambulatory settings; 18 (41.8%), home-based; and 11 (25.6%), hospital-based. Regarding subjective outcomes, 24 trials (55.8%)were judged as having high risk, 11 (25.6%) as unclear risk, and 7 (16.3%) as low risk of bias. One trial did not evaluate subjective outcomes (eTables 5–6 in the Supplement). Regarding objective outcomes, 19 trials (44.1%) were judged as having high risk, 10 (23.2) as unclear risk, and 3 (6.9%) as low risk of bias; 11 trials (25.6%) did not evaluate objective outcomes.

The specific reasons for exclusion of 5958 records at the title and abstract screening stage were not recorded.
Interventions addressed a median of 5 (range, 2–7) of 8 palliative care components.18 Forty-two trials addressed physical and 39 trials addressed psychological aspects of care. No interventions explicitly described cultural assessment as an aspect of the intervention or reported using culturally sensitive materials (eFigure 1 in the Supplement).
Fifteen RCTs evaluated caregiver outcomes. One had a separate, yet concurrent caregiver-focused intervention.30 Four included the patient and caregiver as a unit of care in a single intervention,31–34 5 invited but did not require a caregiver to participate in a patient-focused intervention,35–39 and 5 collected caregiver data only, without a caregiver-focused intervention.40–44
Thirty-nine studies used parallel-group designs (35 with a usual-care comparator, 2 with active comparators, and 2 with attention controls). Five studies used waitlist designs,20,43,45–47 with delay intervals ranging from 2 to 12 weeks. Most trials randomized patients; 5 used cluster randomization.8,48–51
Patient QOL
Quality of life was assessed in 24 studies (55.8%) (4576 patients), of those 129,10,20,32,35,44,48,51–55 were at high risk; 5,11,13,31,42,56 unclear risk; and 7,8,12,33,57–60 low risk of bias. Sixteen trials (67%) exclusively comprised patients with cancer. Twelve trials (50%) evaluating QOL reported statistically significant improvements related to palliative care. Of the 7 trials at low risk of bias, 5 (71%) reported statistically significant improvements.8,12,33,57–60 Six (85.7%) of which were conducted in the ambulatory setting,8,12,33,58–60 and 5 (71.4%) involved patients with cancer,8,33,57,59,60 with 2 of those involving outpatient specialist palliative care interventions.8,60
Fifteen trials evaluating QOL at the 1- to 3-month follow-up could be pooled in meta-analysis; of these, 11 exclusively comprised patients with cancer, and 8 used ambulatory interventions. Among these 15 trials, palliative care was associated with statistically significant and clinically meaningful improvement in QOL at 1 to 3 months (SMD,0.46; 95%CI,0.08 to 0.83; Figure 2; mean difference in FACIT-Pal units, 11.36; heterogeneity, I2 = 94.8%). There was no association of palliative care and QOL among 12 trials pooled with 4- to 6-month follow-up (Figure 3).
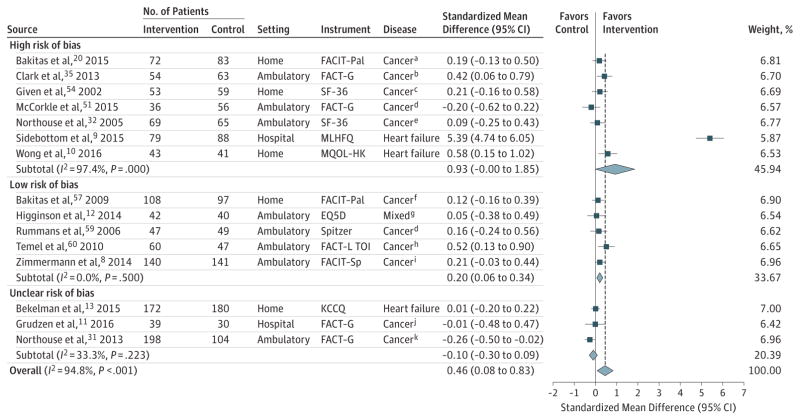
For all trials, the P value for the pooled standardized mean difference (SMD) was .02; τ2, 0.52; and Q, 268.18. For trials at low risk of bias, the P value for the pooled the SMD was .01; τ2, <0.0001; and Q, 3.36. For trials at high risk of bias, the P value for the pooled SMD was .05; τ2, 1.52; and Q, 233.84. For trials at unclear risk of bias, the P value for the pooled SMD was .31; τ2, 0.01; and Q, 3.00. Sample sizes in the figure are the number of patients analyzed at the specific time points.
Error bars represent 95%CIs. The size of the shaded squares indicates study weight. Diamonds represent pooled SMDs and 95%CIs. The vertical dashed line indicates the pooled effect estimate, and the solid vertical line depicts a null effect.
SF-36 indicates Short Form-36; EQ5D, EuroQol 5 Dimensions Questionnaire; FACIT-Pal, Functional Assessment of Chronic Illness Therapy–Palliative; FACT-L TOI, Functional Assessment of Cancer Therapy–Lung Treatment Outcome Index; FACT-G, Functional Assessment of Cancer Therapy- General; FACIT-Sp, Functional Assessment of Chronic Illness Therapy-Spirituality; KCCQ, Kansas City Cardiomyopathy Questionnaire; MLHFQ, Minnesota Living With Heart Failure Questionnaire; and MQOL-HK, McGill Quality of Life Questionnaire–Hong Kong adaptation.
aSolid or hematological cancers.
bBrain, gastrointestinal, head-neck, lung, and other cancers.
cBreast, colon, lung, and gynecological cancers, and lymphoma.
dNot further specified.
eBreast cancer.
fGastrointestinal, lung, genitourinary, and breast cancers.
gCancer, chronic obstructive pulmonary disease, interstitial lung disease, and motor neuron disease.
hNon–small cell lung cancer.
iLung, gastrointestinal, genitourinary, breast, and gynecological cancers.
jBreast, colon, lung, and other cancers.
kBreast, colon, lung, and prostate cancers.
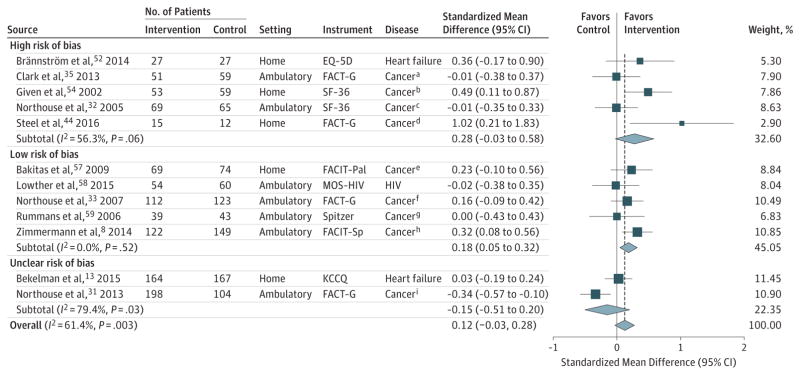
For all trials, the P value for the pooled standardized mean difference (SMD) was .12; τ2, 0.04; and Q, 28.51. For trials at high risk of bias, the P value for the pooled the SMD was .07; τ2, <0.06; and Q, 9.15. For trials at low risk of bias, the P value for the pooled SMD was .01; τ2 <0.0001; Q, 3.20. For trials at unclear risk of bias, the P value for the pooled SMD was .41; τ2, 0.05; and Q, 4.86. Sample sizes in the figure are the number of patients analyzed at the specific time points.
Error bars represent 95%CIs. The size of the shaded squares indicates study weight. Diamonds represent pooled SMDs and 95%CIs. The vertical dashed line indicates the pooled effect estimate, and the solid vertical line depicts a null effect.
EQ-5D indicates EuroQol 5 Dimensions Questionnaire; FACT-G, Functional Assessment of Cancer Therapy-General; FACIT-Pal, Functional Assessment of Chronic Illness Therapy-Palliative; FACIT-Sp, Functional Assessment of Chronic Illness Therapy-Spirituality; HIV, human immunodeficiency virus; MOS-HIV, Medical Outcomes Study-HIV scale; KCCQ, Kansas City Cardiomyopathy Questionnaire; and SF-36, Short Form-36.
aBrain, gastrointestinal, head-neck, lung, and other cancers.
bBreast, colon, lung, and gynecological cancers, and lymphoma.
cBreast cancer.
dUpper gastrointestinal cancers.
eGastrointestinal, lung, genitourinary, and breast cancers.
fProstate cancer.
gNot further specified.
hLung, gastrointestinal, genitourinary, breast, and gynecological cancers.
iBreast, colon, lung, and prostate cancers.
In sensitivity analyses restricted to trials at low risk of bias, palliative care was associated with improved QOL at the 1- to 3-month follow-up, but the point estimate was attenuated (SMD, 0.20; 95% CI, 0.06–0.34; 5 trials; I2 = 0.0%; Figure 2; mean difference in FACIT-Pal units, 4.94 points) and at the 4- to 6-month follow-up (SMD, 0.18; 95% CI, 0.05–0.32; 5 trials; I2 = 0.0%; Figure 3; mean difference in FACIT-Pal units, 2.96). Additional post hoc analyses related to disease or study setting demonstrated no associations between palliative care and QOL (eFigures 2 and 3). Analyses using discrete time points vs windows demonstrated a statistically significant association at 3 months (eFigure 4), but not at 6 months (eFigure 5). Evidence of publication bias was detected by an Egger test (P = .03), and from visual examination of an asymmetrical funnel plot (eFigure 6). Heterogeneity was explainable by study setting, with hospital-based palliative care interventions showing stronger associations with improved QOL (P = .04; eTable 7 in the Supplement).
Physical Symptoms
Of the 29 trials involving 10105 patients and assessing physical symptoms, 17 had a high risk of bias,9,10,20,43–45,47–49,51,52,54,55,61–64 5 had an unclear risk of bias,37,42,46,50,65 and 7 had a low risk8,12,33,57–60 of bias. Ten trials reported statistically significant reductions in specific physical symptoms or a composite symptom indicator.8–10,46–48,59–61,63 Of the 7 trials at low risk of bias, 3 reported statistically significant reductions in symptom burden.8,59,60 All 3 included only patients with cancer and reported findings as multisymptom composites; 2 of them used specialist outpatient palliative care interventions.8,60
Ten trials involving 1813 participants were pooled in a meta-analysis regarding symptom burden at the 1- to 3-month follow-up8–10,12,20,43,46,54,57,60; 4 trials were judged as having a low risk,8,12,57,60 1 as unclear,46 and 5 as high risk of bias.9,10,20,43,54 Palliative care was associated with a statistically and clinically significant reduction in symptom burden at the 1- to 3-month follow-up, but the analysis had extremely high heterogeneity (SMD, −0.66; 95% CI, −1.25 to −0.07; I2 = 96.1%; Figure 4; mean difference in ESAS units, −10.30). At the 4- to 6-month follow-up, palliative care was associated with improved symptom burden (SMD, −0.18; 95% CI, −0.31 to−0.05; I2 = 0.0%; Figure 5; mean difference in ESAS units, −2.80).
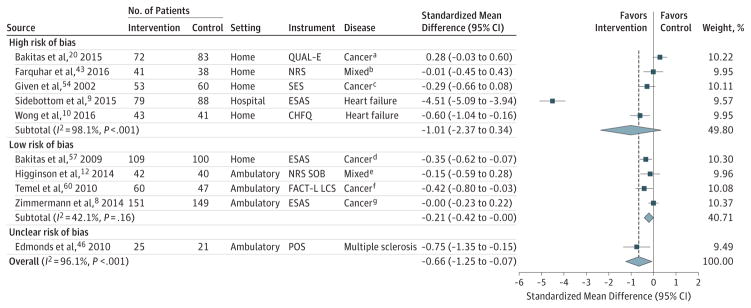
For all trials, the P value for the pooled standardized mean difference (SMD) was .03; τ2, 0.86; and Q, 230.90. For trials at high risk of bias, the P value for the pooled the SMD was .14; τ2, 2.34; and Q, 215.72. For trials at unclear risk of bias, the P value for the pooled SMD was .01; τ2, <0.0001; and Q, 230.90. Sample sizes in the figure are the number of patients analyzed at the specific time points.
Error bars represent 95%CIs. The size of the shaded squares indicates study weight. Diamonds represent pooled SMDs and 95%CIs. The vertical dashed line indicates the pooled effect estimate, and the solid vertical line depicts a null effect.
CHFQ indicates Chronic Heart Failure Questionnaire; COPD, chronic obstructive pulmonary disease; ESAS, Edmonton Symptom Assessment Scale; FACT-L LCS, Functional Assessment of Cancer Therapy-Lung Lung Cancer Scale; NRS SOB, Numerical Rating Scale Shortness of Breath; POS, Palliative Outcomes Scale; QUAL-E, Quality of Life at the End of Life; and SES, Symptom Experience Scale.
aSolid or hematological cancers.
bCOPD or other source of dyspnea.
cBreast, colon, lung, and gynecological cancers, and lymphoma.
dGastrointestinal, lung, genitourinary, and breast cancers.
eCancer, COPD, heart failure, interstitial lung disease, motor neuron disease.
fNon–small cell lung cancer.
gLung, gastrointestinal, genitourinary, breast, and gynecological cancers.

For all trials, the P value for the pooled standardized mean difference (SMD) was .01; τ2, <0.0001; and Q, 3.97. For trials at low risk of bias, the P value for the pooled the SMD was .06; τ2, <0.0001; and Q, 0.62. For trials at high risk of bias, the P value for the pooled SMD was .01; τ2, <0.0001; and Q, 0.31. Sample sizes in the figure are the number of patients analyzed at the specific time points.
Error bars represent 95%CIs. The size of the shaded squares indicates study weight. Diamonds represent pooled SMDs and 95%CIs. The vertical dashed line indicates the pooled effect estimate, and the solid vertical line depicts a null effect.
APOS indicates African Palliative Outcomes Scale; BPI, Brief Pain Inventory; ESAS, Edmonton Symptom Assessment Scale; HF, heart failure; HIV, human immunodeficiency virus; MS, multiple sclerosis; OSQ, Omega Symptom Questionnaire; and SES, Symptom Experience Scale.
aBreast, colon, lung, and gynecological cancers and lymphoma.
bUpper gastrointestinal cancers.
cGastrointestinal, lung, genitourinary, and breast cancers.
dProstate cancer.
eLung, gastrointestinal, genitourinary, breast, and gynecological cancers.
In sensitivity analyses limited to the 4 trials at low risk of bias, palliative care was not associated with change in symptom burden at the 1- to 3-month follow-up (SMD, −0.21; 95% CI, −0.42 to 0.00; I2 = 42.1%; Figure 4; mean difference in ESAS units, −3.28; 4 trials). Nor was it associated with change in symptom burden at the 4- to 6-month follow-up (SMD, −0.13; 95% CI, −0.27 to 0.01; I2 = 0.0%; Figure 5; mean difference in ESAS units, −2.03, 4 trials). Additional post hoc analyses related to disease, setting, or discrete time point assessment revealed no associations between palliative care and symptom burden (eFigures 7–9). There was no evidence of publication bias (eFigure 10). Heterogeneity was largely explained by study setting, with hospital-based palliative care interventions showing stronger associations with improved symptom burden (P < .001; eTable 7 in the Supplement).
Survival
Survival was assessed in 17 trials involving 8196 patients;10 trials were judged as having high risk of bias,9,20,34,39,49,52,55,60,67,68 5 as unclear risk,11,13,50,65,66 and 2 as low risk.12,57 One trial specified survival as a primary outcome.20 The 2 trials at low risk of bias reported conflicting findings. A telepalliative care intervention for patients with advanced cancer reported no effect on survival (HR, 0.82; 95% CI, 0.64–1.07),20 whereas a trial of integrated palliative and respiratory care for dyspnea, which included survival as a safety outcome, reported greater survival at 6 months(94%vs 75%,P = .048).12 Three additional trials(2 at high risk of bias,20,60 1 at unclear risk13) reported statistically significant improvements in survival.
Seven trials involving 2184 patients that assessed survival were pooled in a meta-analysis.9,11,13,50,57,60,65 One trial was rated as having low risk of bias, summarized above.12 There was no association between palliative care and survival (HR, 0.90; 95% CI, 0.69–1.17; I2 = 75.3%; Figure 6). Post hoc analyses related to disease, setting, or imputation of HRs identified no significant associations of palliative care and survival (eFigures 11–13). No evidence of publication bias was detected (eFigure 14). Heterogeneity of estimates could not be explained by study-level characteristics (eTable 7 in the Supplement).
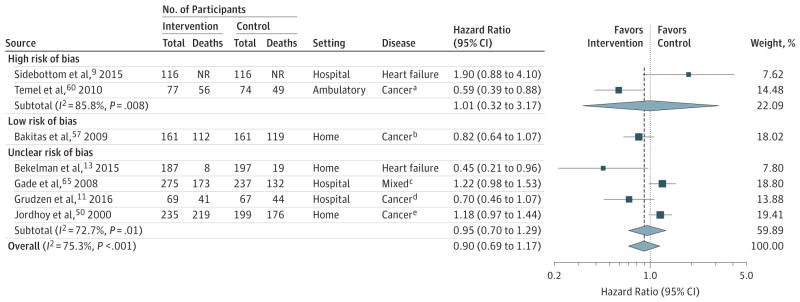
For all trials, the P value for the pooled hazard ratio (HR) was .44; τ2, 0.08; and Q, 24.29. For trials at low risk of bias, the P value for the pooled the HR was .14; τ2, <0.0001; and for Q, <0.0001. For high risk of bias, the P value for the pooled HR was .99; τ2, <0.59; and Q, 7.03. For unclear risk of risk of bias the P value for the pooled HR was .74; τ2, 0.06; and Q, 10.98. Sample sizes in the figure are the number of patients analyzed at the specific time points.
Error bars represent 95%CIs. The size of the shaded squares indicates study weight. Diamonds represent pooled HRs and 95%CIs. The vertical dashed line indicates the pooled effect estimate, and the solid vertical line depicts a null effect (ie, HR, 1).
aNon–small cell lung cancer.
bGastrointestinal, lung, genitourinary, and breast cancers.
cCancer, heart failure, chronic obstructive pulmonary disease, end-stage renal disease, stroke, and dementia.
dBreast, colon, lung, and other cancers.
eGastrointestinal, lung, breast, gynecological, genitourinary, kidney, lymphoma, skin, and other cancers.
Patient Mood
There was mixed evidence from 23 trials involving 4175 patients regarding the association of palliative care with mood. Of the 23 trials, 13 were judged as high risk,9,20,35,43–45,47,48,51,55,62–64 5 as unclear risk,11,13,37,42,56 and 5 as low risk of bias.12,57–60 Overall, 7 trials reported statistically significant improvements in mood related to palliative care9,13,48,57–60; of these, 4 were at low risk of bias.57–60 Of the 5 trials at low risk of bias,12,57–60 4 reported statistically significant improvements in mood.57–60
Advance Care Planning
Advance care planning was assessed in 10 trials involving 6525 patients; 7 trials were judged as having high risk of bias,9,39,48,49,55,61,68 2 as unclear risk,65,66 and 1 as low risk.60 Among the 5 trials that reported statistically significant improvements,9,39,61,65,66 3 were at high risk of bias9,39,61 and 2 were of unclear risk of bias.65,66 One trial at low risk of bias, a trial of early specialist palliative care in patients with lung cancer, demonstrated no association with documentation of resuscitation preferences (P = .05).60
Site of Death
Eight trials involving 1556 patients assessed site of death with mixed results; of these, 5 trials were judged as having high risk of bias20,48,67,69,70 and 3 as unclear risk.50,66,71 Three trials reporting statistically significant increases in at-home death,50,67,72 tested home-based interventions. Of these, 2 were large trials involving 744 patients were at unclear risk of bias,50,71 and 1 was a medium-sized trial that involved 167 patients was at high risk of bias.67
Resource Utilization and Expenditures
Twenty-four trials involving 4794 patients assessed resource utilization9–13,20,34,37,38,42,48,50,52,55,57,60,61,63,65,66,68,69,71,73; of these, 11 reported significantly decreased utilization among palliative care recipients.10,34,37,38,48,50,52,65,66,71,73
Hospital utilization was assessed in 20 trials involving 4329 patients; of these, 11 trials were judged as having high risk of bias,9,10,20,34,48,52,55,61,63,67,69 7 as unclear risk,11,13,37,42,65,66,71 and 2 as lowrisk.12,57 Neither of 2 trials at low risk of bias demonstrated statistically significant differences in length of stay.12,57 Five trials, all of home-based interventions involving either heart failure or mixed-disease samples, reported significant reductions in hospital utilization10,34,52,67,71; of these, 4 were judged at high risk of bias,10,34,52,67 and 1 at unclear risk.71
Six trials involving 1360 patients assessed hospice use; of these, 3 trials were judged as having high risk of bias9,55,68 and 3 as unclear risk.11,65,71 Overall, 1 trial involving 517 participants and judged as having an unclear risk of bias that assessed inpatient specialist palliative care consultation reported significantly longer hospice stays among intervention patients (median, 24 vs 12 days; P = .04), although the overall percentage of patients admitted to hospice did not differ between groups (P = .50).65
Four trials involving 704 patients evaluated the use of intensive nonpalliative services (eg, chemotherapy within the last 14 days of life, no hospice care, or admission to hospice ≤3 days before death), of which 1 trial was judged as having high risk of bias,20 2 as unclear risk,37,66 and 1 as lowrisk.60 The trial at low risk reported no association between palliative care and intensive, nonpalliative services (P = .05).60
Twelve trials involving 6892 patients assessed health care expenditures; of these 7 trials were judged as having high risk of bias,34,39,43,47–49,67 4 as unclear risk,37,46,65,71 and 1 as low risk.12 Only 1 trial was considered at low risk of bias, a multidisciplinary palliative intervention for patients with refractory dyspnea. This trial reported no differences in 6-weekmean costs (£1402 vs £1408).12 Of the 4 trials that reported significant reductions in expenditures favoring the intervention, 2 were at high risk34,67 and 2 were at unclear risk of bias.65,71 None of the trials in this review demonstrated increased overall health care expenditures related to palliative care.
Satisfaction With Care
Patient satisfaction with care was assessed in 11 trials involving 2690 patients; of these 6 trials were judged as having high risk of bias,10,34,39,48,64,67 4 as unclear risk,37,42,65,71 1 as lowrisk.8 Overall, 7 trials reported a significant improvement in satisfaction among palliative care recipients,8,34,37,39,65,71 including 1 trial that assessed and was judged at low risk of bias.8
Caregiver Outcomes
Fifteen trials involving 2479 caregivers with 8 trials judged as having high risk, 4 as unclear risk, and 3 as low risk of bias included subjective caregiver outcomes. Of 7 trials assessing caregiver QOL,30–33,35,41,44 three31,33,44 showed benefit in 1 or more QOL domain at 1 or more time point. However, only 1 trial was at low risk of bias.33 Of 5 studies assessing caregiver mood,30,35,37,43,44 two30,37 showed benefit at 1 or more time points. Of these, one was at high risk of bias30 and the other had an unclear risk of bias.37 Out of 7 studies30–33,36,40,41 evaluating caregiver burden, three30,32,33 reported benefit in at least 1 domain at 1 or more time points, although only 1 was at low risk of bias.33 Caregiver satisfaction was measured in 5 studies. Of these,34,37–39,42 four34,37–39 showed higher scores among intervention groups; however, 3 were at high risk of bias34,38,39 and 1 was at unclear risk.37
Discussion
In this meta-analysis, palliative care interventions were associated with significant improvements in QOL and symptom burden but not in 1- to 3-month survival. However, because of marked heterogeneity among trials in methodological quality and rigor, there was weak evidence for these associations. When sensitivity analyses were restricted to trials at low risk of bias, associations between palliative care and QOL remained statistically significant but not clinically important and associations with symptom burden were no longer statistically significant. Of the outcomes narratively synthesized, palliative care was associated with improved advance care planning, greater patient and caregiver satisfaction with care, and lower health care utilization. There was mixed evidence of associations of palliative care with site of death; patient mood; health care expenditures; and caregiver QOL, mood, or burden.
This study adds to the literature by (1) including 23 trials published since a 2008 systematic review6 and 29 trials not included in the 2011 narrative review,7 (2) by evaluating risks of bias and methodological limitations in each trial, and (3) by conducting a systematic review that includes a meta-analysis of 3 important outcomes. Although these analyses provide increased evidence for the association of palliative care with several patient and caregiver outcomes, particularly for patients with advanced cancer, the results should be interpreted cautiously given persistent methodological limitations. High-quality palliative care studies with innovative and context-specific methods are needed that are responsive to the complexities of conducting research in seriously ill populations are needed.74,75
Although all included trials involved patients with life-limiting illness, there was wide variability across samples. This is consistent with the concept that palliative care is appropriate at any stage of life-limiting illness, including patients less severely ill.2 However, the effects of palliative care may be more difficult to demonstrate among people with less symptom burden or QOL impairment. Future meta-analyses should account for this diversity between studies, to avoid ceiling and floor effects.
Survival was reported as an outcome in recent trials, although improving survival is not an aim of palliative care.2 Only one trial specified survival as a primary end point.20 Given that some clinicians and members of the lay public view palliative care negatively due to an unfounded belief that it may shorten survival,76,77 it is important to note that no trial showed a decrease in survival from palliative care.
The association of palliative care with caregiver outcomes was mixed. Three explanations may clarify these seemingly discrepant findings. First, many of the reviewed interventions did not specifically target caregivers. Included trials were typically patient focused. Second, of palliative care interventions that targeted caregivers, there was considerable variability in their type and delivery. Third, care needs of patients with life-limiting illness change as patient health deteriorates. Hence, despite training in coping skills, caregivers may feel burdened by having to adapt to these changing needs. Because we excluded caregiver-focused interventions, the outcomes presented reflect only caregiver outcomes of patient-focused palliative care interventions.
Strengths and Weaknesses
This review used a broad search for palliative care RCTs to detect interventions consistent with the philosophy or components of palliative care, including interventions that may not be explicitly described as palliative care. Consequently, this review includes a wide spectrum of palliative care delivery models, with interventions ranging from interdisciplinary specialized palliative care to those in which palliative care domains were delivered by a nonpalliative care specialist. Although all interventions met our prespecified definition of “palliative care,” their diversity likely introduced heterogeneity into the meta-analysis.78 The use of a random-effects model measures variability between trials, weighting each study’s contribution within the pooled effect. This review regards palliative care as a philosophy of care. Insufficient data were available to identify the associations between specific models of palliative care (eg, specialist vs generalist palliative care training)and patient and caregiver outcomes.
This review has several limitations. First, several trials could not be included in meta-analyses, typically, because missing data remained even after contacting authors. Second, the review excluded quasi-experimental studies, several of which have demonstrated benefits of palliative care.79,80 Third, post hoc analyses including meta-regressions and tests for publication bias should be interpreted cautiously given that these statistical tests may have been underpowered. Fourth, trial duration and attrition rates were not uniformly reported in studies and are therefore excluded from this review. Fifth, this review did not distinguish between early palliative care interventions vs those at the end-of-life, reflecting the prevailing view that palliative care is appropriate at any point in the disease trajectory.2 Sixth, risk of bias assessment is subjective, and the Cochrane Risk of Bias tool is not designed to account for the intricacies of conducting behavioral interventions among seriously ill populations. Given these limitations, results of this systematic review and meta-analysis should be interpreted cautiously.
Unanswered Questions and Future Research
Several gaps remain regarding palliative care. First, this review could not discern the association between specific palliative care processes and outcomes. Future research should aim to identify the efficacious component(s) of palliative care. Second, future studies should assess patient-reported outcomes using a core set of standardized and validated measures appropriate for seriously ill patients at similar time points. Third, additional studies are needed to evaluate the role of palliative care in chronic nonmalignant illnesses (eg, heart failure, chronic obstructive pulmonary disease, renal disease). Fourth, among subgroups for which the efficacy of palliative care has been established (eg, oncology), future trials should consider active controls to investigate the comparative effectiveness of different palliative care strategies. Finally, trials are needed to establish optimal models of palliative care delivery that help caregivers in addition to patients.
Conclusions
In this meta-analysis, palliative care interventions were associated with improvements in patient QOL and symptom burden. Findings for caregiver outcomes were inconsistent. However, many associations were no longer significant when limited to trials at low risk of bias, and there was no significant association between palliative care and survival.
Acknowledgments
Funding/Support: Dr Kavalieratos receives research support from the Agency for Healthcare Research and Quality (K12HS022989), the National Heart, Lung, and Blood Institute (K01HL13346), the Cystic Fibrosis Foundation, as well as a Junior Faculty Career Development Award from the National Palliative Care Research Center. Dr Schenker is supported by the National Institutes of Health under award KL2TR000146. Dr Dionne-Odom receives support from the National Institute of Nursing Research (K99NR015903), the American Cancer Society, and the National Palliative Care Research Center.
Role of the Funder/Sponsor: The funders and supporters had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or AHRQ.
Additional Contributions: We thank Adelina Malito, BA, Greer Tiver-Baskin, MPH, and Laura Obregon, University of Pittsburgh, for their administrative assistance. Mss Malito and Obregon were compensated for their contribution. Ms Tiver-Baskin was not compensated and no longer works at the University of Pittsburgh. We also thank Daniel Siconolfi, PhD, MPH, University of Pittsburgh, and R. Sean Morrison, MD, Icahn School of Medicine at Mount Sinai, for their comments on earlier drafts and Sydney Dy, MD, MSc, Johns Hopkins University, for her guidance regarding risk of bias assessment. None of the latter 3 were compensated for their contributions.
Author Contributions: Dr Kavalieratos had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Kavalieratos, Corbelli, Klein-Fedyshin, Zimmermann, Morton, Arnold, Heller, Schenker.Acquisition, analysis, or interpretation of data: Kavalieratos, Corbelli, Zhang, Dionne-Odom, Ernecoff, Hanmer, Hoydich, Ikejiani, Klein-Fedyshin, Zimmermann, Morton, Heller, Schenker.
Drafting of the manuscript: Kavalieratos, Corbelli, Zhang, Ikejiani, Klein-Fedyshin, Schenker.
Critical revision of the manuscript for important intellectual content: all authors.
Statistical analysis: Kavalieratos, Zhang, Hanmer, Hoydich, Morton.
Administrative, technical, or material support: Kavalieratos, Corbelli, Hoydich, Ikejiani, Klein-Fedyshin, Heller.
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jama.2016.16840
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc5226373?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1001/jama.2016.16840
Article citations
Management of pulmonary hypertension in special conditions.
Eur Respir J, 64(4):2401180, 31 Oct 2024
Cited by: 2 articles | PMID: 39209477 | PMCID: PMC11525332
Review Free full text in Europe PMC
Telehealth vs In-Person Early Palliative Care for Patients With Advanced Lung Cancer: A Multisite Randomized Clinical Trial.
JAMA, 11 Sep 2024
Cited by: 0 articles | PMID: 39259563
Experiences of patients with advanced chronic diseases and their associates with a structured palliative care nurse visit followed by an interprofessional case conference in primary care - a deductive-inductive content analysis based on qualitative interviews (KOPAL-Study).
BMC Prim Care, 25(1):323, 04 Sep 2024
Cited by: 0 articles | PMID: 39232658 | PMCID: PMC11373434
Conducting Comparative Effectiveness, Multisite Palliative Care and Advance Care Planning Trials: Lessons Learned and Future Directions From PCORI-Funded Studies.
Med Care, 62(10):671-679, 06 Sep 2024
Cited by: 0 articles | PMID: 39245815 | PMCID: PMC11373889
Healthcare Professionals' Views of Supportive Care Needs for Chinese, Korean, and Vietnamese Americans with Metastatic Cancer.
Asian Am J Psychol, 15(3):233-245, 01 Sep 2024
Cited by: 0 articles | PMID: 39430037
Go to all (429) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association of Receipt of Palliative Care Interventions With Health Care Use, Quality of Life, and Symptom Burden Among Adults With Chronic Noncancer Illness: A Systematic Review and Meta-analysis.
JAMA, 324(14):1439-1450, 01 Oct 2020
Cited by: 66 articles | PMID: 33048152 | PMCID: PMC8094426
Review Free full text in Europe PMC
Effects of Palliative Care for Progressive Neurologic Diseases: A Systematic Review and Meta-Analysis.
J Am Med Dir Assoc, 24(2):171-184, 05 Dec 2022
Cited by: 4 articles | PMID: 36481217
Review
Effect of Inpatient Palliative Care on Quality of Life 2 Weeks After Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial.
JAMA, 316(20):2094-2103, 01 Nov 2016
Cited by: 189 articles | PMID: 27893130 | PMCID: PMC5421101
The effectiveness of patient navigation programs for adult cancer patients undergoing treatment: a systematic review.
JBI Database System Rev Implement Rep, 14(2):295-321, 01 Feb 2016
Cited by: 30 articles | PMID: 27536800
Review
Funding
Funders who supported this work.
AHRQ HHS (1)
Grant ID: K12 HS022989
NCATS NIH HHS (2)
Grant ID: KL2 TR000146
Grant ID: L30 TR001226
NHLBI NIH HHS (1)
Grant ID: K01 HL133466
NINR NIH HHS (1)
Grant ID: K99 NR015903





