Abstract
Free full text

Molecular characterization of a 23-kilodalton major antigen secreted by Toxoplasma gondii.
Abstract
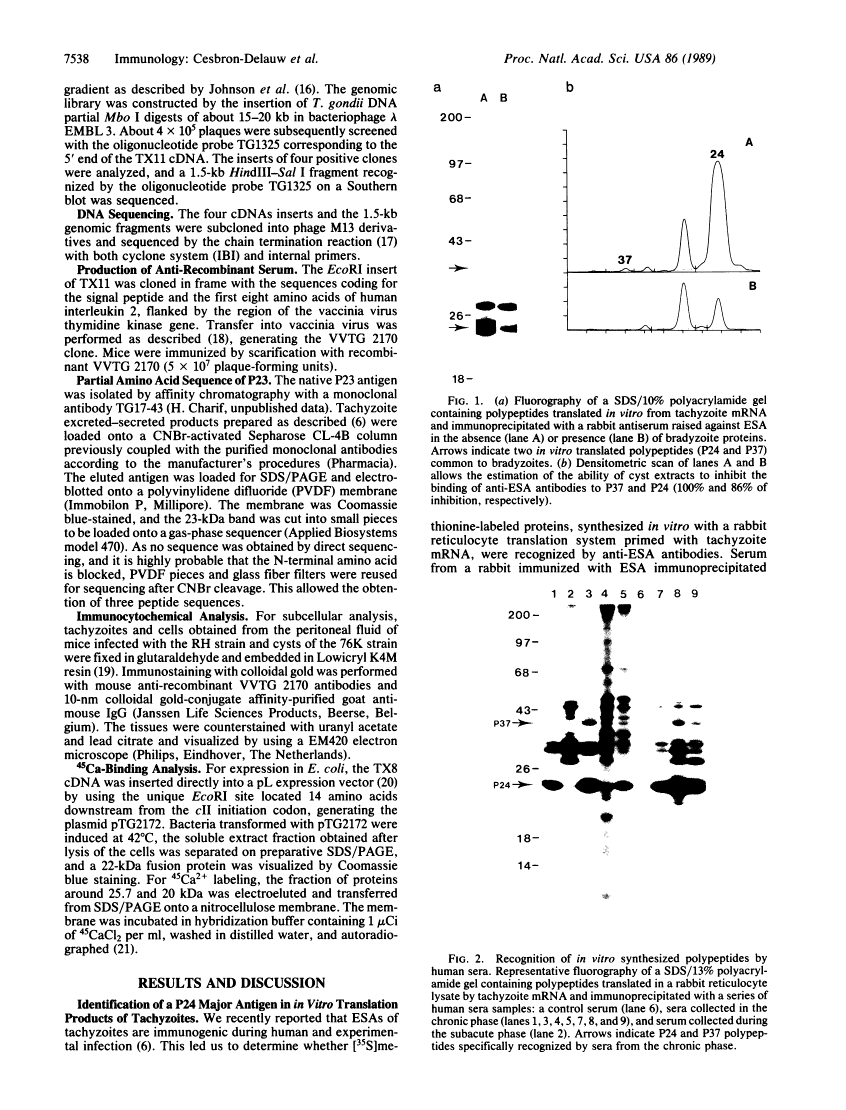
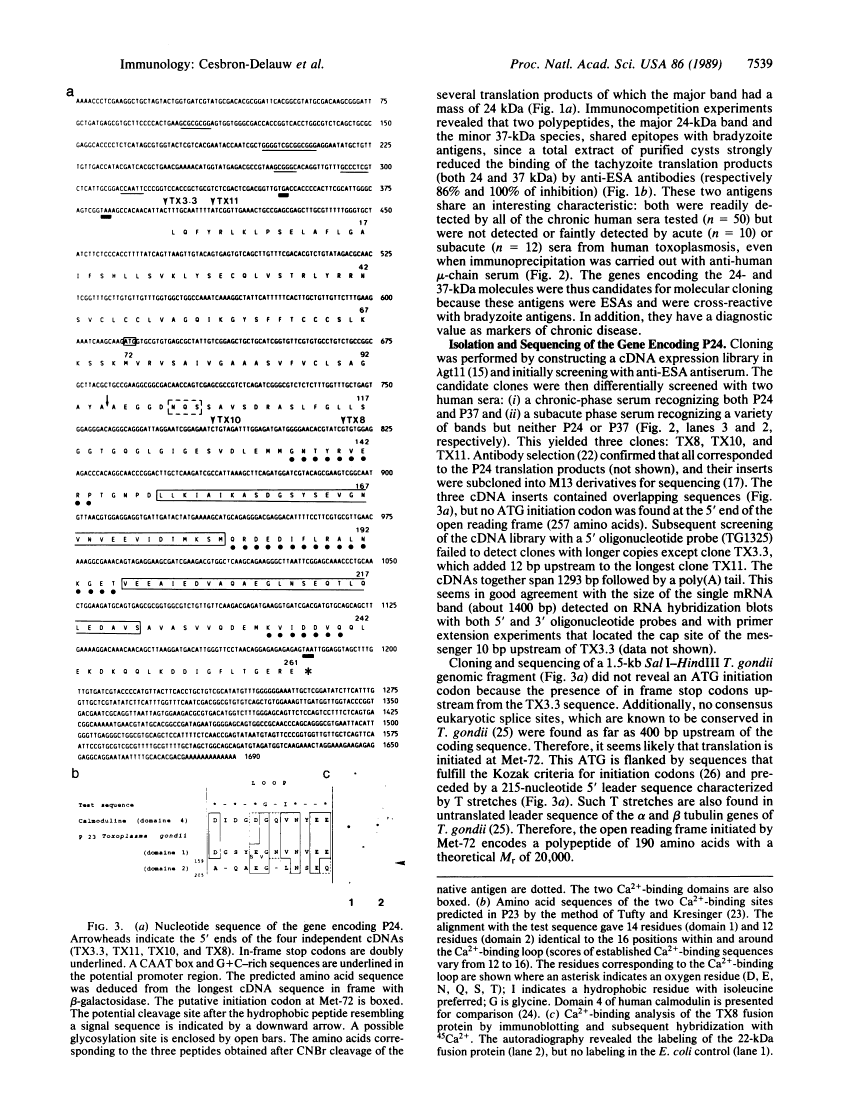
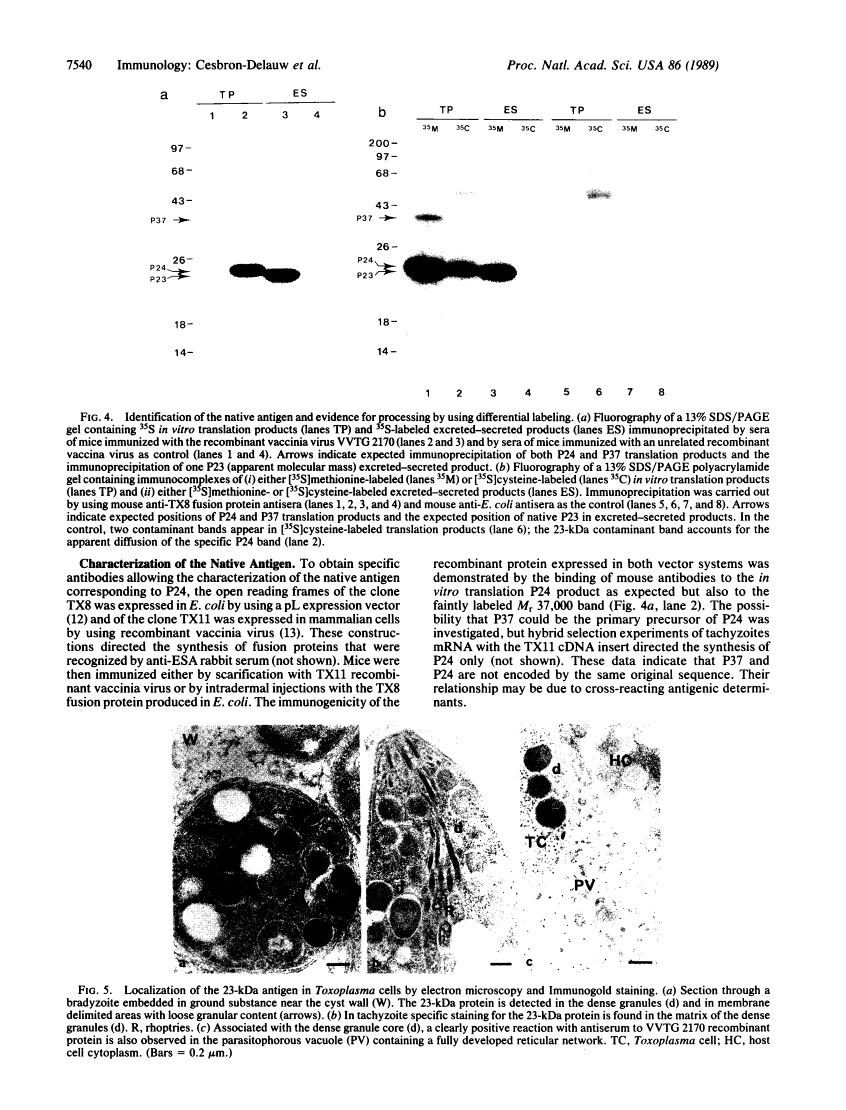
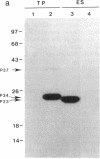
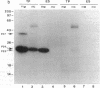
The strategy chosen for cloning potential vaccine antigens of Toxoplasma gondii was based on the hypothesis that the definitive protection observed in natural infection is due to the presence of encysted bradyzoite forms in host tissues throughout life. The antigens released by the bradyzoites would maintain an immune response against the invading tachyzoites. This led us to identify in tachyzoite in vitro translation products a polypeptide of 24 kDa that is an excreted-secreted antigen (ESA) and is cross-reactive with bradyzoites. In addition, the detection of anti-P24 IgG antibodies is correlated with the chronic infection in man. The gene encoding P24 has been isolated, sequenced, and expressed in Escherichia coli and eukaryotic cells. The recombinant proteins were immunogenic in mice, producing anti-native P23 antibodies. Immunocytochemical analysis located the native antigen in the dense granules of both tachyzoite and bradyzoite forms and showed that it is secreted within host-cell-modified phagosome. Moreover 45Ca2+ labeling as well as regional homologies indicate that this protein has Ca2+-binding properties, suggesting its physiological importance in host-cell invasion. P23 is of diagnostic interest as a marker of chronic toxoplasmosis and is proposed as a vaccine component.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.6M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley JK. Toxoplasmosis in animals. Vet Rec. 1976 Aug 14;99(7):123–127. [Abstract] [Google Scholar]
- Luft BJ, Brooks RG, Conley FK, McCabe RE, Remington JS. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984 Aug 17;252(7):913–917. [Abstract] [Google Scholar]
- Frenkel JK. Pathophysiology of toxoplasmosis. Parasitol Today. 1988 Oct;4(10):273–278. [Abstract] [Google Scholar]
- Capron A, Dessaint JP. Vaccination against parasitic diseases: some alternative concepts for the definition of protective antigens. Ann Inst Pasteur Immunol. 1988 Jan-Feb;139(1):109–117. [Abstract] [Google Scholar]
- Darcy F, Deslee D, Santoro F, Charif H, Auriault C, Decoster A, Duquesne V, Capron A. Induction of a protective antibody-dependent response against toxoplasmosis by in vitro excreted/secreted antigens from tachyzoites of Toxoplasma gondii. Parasite Immunol. 1988 Sep;10(5):553–567. [Abstract] [Google Scholar]
- Auffray C, Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. [Abstract] [Google Scholar]
- Aviv H, Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. [Europe PMC free article] [Abstract] [Google Scholar]
- Pelham HR, Jackson RJ. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. [Abstract] [Google Scholar]
- Dissous C, Dissous C, Capron A. Isolation and characterization of surface antigens from Schistosoma mansoni schistosomula. Mol Biochem Parasitol. 1981 Aug;3(4):215–225. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Laugier M, Quilici M. Intérêt expérimental d'une souche de Toxoplasme peu pathogène pour la souris. Ann Parasitol Hum Comp. 1970 Jul-Aug;45(4):389–403. [Abstract] [Google Scholar]
- Young RA, Davis RW. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson AM, Dubey JP, Dame JB. Purification and characterization of Toxoplasma gondii tachyzoite DNA. Aust J Exp Biol Med Sci. 1986 Aug;64(Pt 4):351–355. [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Kieny MP, Lathe R, Drillien R, Spehner D, Skory S, Schmitt D, Wiktor T, Koprowski H, Lecocq JP. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984 Nov 8;312(5990):163–166. [Abstract] [Google Scholar]
- Ohno S, Emori Y, Imajoh S, Kawasaki H, Kisaragi M, Suzuki K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984 Dec 6;312(5994):566–570. [Abstract] [Google Scholar]
- Courtney M, Buchwalder A, Tessier LH, Jaye M, Benavente A, Balland A, Kohli V, Lathe R, Tolstoshev P, Lecocq JP. High-level production of biologically active human alpha 1-antitrypsin in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(3):669–673. [Europe PMC free article] [Abstract] [Google Scholar]
- Maruyama K, Mikawa T, Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984 Feb;95(2):511–519. [Abstract] [Google Scholar]
- Olmsted JB. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [Abstract] [Google Scholar]
- Tufty RM, Kretsinger RH. Troponin and parvalbumin calcium binding regions predicted in myosin light chain and T4 lysozyme. Science. 1975 Jan 17;187(4172):167–169. [Abstract] [Google Scholar]
- Roth J, Bendayan M, Carlemalm E, Villiger W, Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. [Abstract] [Google Scholar]
- Nagel SD, Boothroyd JC. The alpha- and beta-tubulins of Toxoplasma gondii are encoded by single copy genes containing multiple introns. Mol Biochem Parasitol. 1988 Jun;29(2-3):261–273. [Abstract] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. [Europe PMC free article] [Abstract] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. [Abstract] [Google Scholar]
- Jones TC, Yeh S, Hirsch JG. The interaction between Toxoplasma gondii and mammalian cells. I. Mechanism of entry and intracellular fate of the parasite. J Exp Med. 1972 Nov 1;136(5):1157–1172. [Europe PMC free article] [Abstract] [Google Scholar]
- Sibley LD, Krahenbuhl JL, Adams GM, Weidner E. Toxoplasma modifies macrophage phagosomes by secretion of a vesicular network rich in surface proteins. J Cell Biol. 1986 Sep;103(3):867–874. [Europe PMC free article] [Abstract] [Google Scholar]
- Chou PY, Fasman GD. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. [Abstract] [Google Scholar]
- Werk R. How does Toxoplasma gondii enter host cells? Rev Infect Dis. 1985 Jul-Aug;7(4):449–457. [Abstract] [Google Scholar]
- Entzeroth R, Dubremetz JF, Hodick D, Ferreira E. Immunoelectron microscopic demonstration of the exocytosis of dense granule contents into the secondary parasitophorous vacuole of Sarcocystis muris (Protozoa, Apicomplexa). Eur J Cell Biol. 1986 Aug;41(2):182–188. [Abstract] [Google Scholar]
- Hughes HP, Connelly CA, Strangeways JE, Hudson L. Antigen specific lymphocyte transformation induced by secreted antigens from Toxoplasma gondii. Clin Exp Immunol. 1984 Dec;58(3):539–547. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.86.19.7537
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc298100?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.86.19.7537
Article citations
Egress Regulatory Factors: How Toxoplasma Exits from Infected Cells?
Pathogens, 12(5):679, 04 May 2023
Cited by: 1 article | PMID: 37242349 | PMCID: PMC10221845
Review Free full text in Europe PMC
Dense granule biogenesis, secretion, and function in Toxoplasma gondii.
J Eukaryot Microbiol, 69(6):e12904, 01 Apr 2022
Cited by: 16 articles | PMID: 35302693 | PMCID: PMC9482668
Review Free full text in Europe PMC
Characterization and evaluation of a recombinant multiepitope peptide antigen MAG in the serological diagnosis of Toxoplasma gondii infection in pigs.
Parasit Vectors, 14(1):408, 17 Aug 2021
Cited by: 4 articles | PMID: 34404476 | PMCID: PMC8369689
Toxoplasma Mechanisms for Delivery of Proteins and Uptake of Nutrients Across the Host-Pathogen Interface.
Annu Rev Microbiol, 74:567-586, 17 Jul 2020
Cited by: 20 articles | PMID: 32680452 | PMCID: PMC9934516
Review Free full text in Europe PMC
Review on the Current Trends of Toxoplasmosis Serodiagnosis in Humans.
Front Cell Infect Microbiol, 10:204, 08 May 2020
Cited by: 39 articles | PMID: 32457848 | PMCID: PMC7227408
Review Free full text in Europe PMC
Go to all (104) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Molecular characterization of a 65-kilodalton Toxoplasma gondii antigen expressed abundantly in the matrix of tissue cysts.
Mol Biochem Parasitol, 66(2):283-296, 01 Aug 1994
Cited by: 75 articles | PMID: 7808478
Identification and biochemical characterization of antigens of tachyzoites and bradyzoites of Toxoplasma gondii with cross-reactive epitopes.
Parasitol Res, 76(6):473-478, 01 Jan 1990
Cited by: 13 articles | PMID: 1696376
Toxoplasma gondii: differential location of antigens secreted from encysted bradyzoites.
Exp Parasitol, 77(1):13-22, 01 Aug 1993
Cited by: 36 articles | PMID: 8344403
[Antigens of Toxoplasma gondii with a diagnostic and potential immunoprophylactic value: new strategies of identification].
Ann Biol Clin (Paris), 47(7):451-457, 01 Jan 1989
Cited by: 2 articles | PMID: 2479305
Review