Abstract
Free full text

The Gut Microbiome Composition Associates with Bipolar Disorder and Illness Severity
Abstract
The gut microbiome is emerging as an important factor in regulating mental health yet it remains unclear what the target should be for psychiatric treatment. We aimed to elucidate the complement of the gut-microbiome community for individuals with bipolar disorder relative to controls; and test for relationships with burden of disease measures. We compared the stool microbiome from individuals with bipolar disorder (n=115) and control subjects (n=64) using 16S ribosomal RNA (rRNA) gene sequence analysis. Analysis of molecular variance (AMOVA) revealed global community case-control differences (AMOVA p=0.047). Operational Taxonomical Unit (OTU) level analysis revealed significantly decreased fractional representation (p<0.001) of Faecalibacterium after adjustment for age, sex, BMI and false discovery rate (FDR) correction at the p<0.05 level. Within individuals with bipolar disorder, the fractional representation of Faecalibacterium associated with better self-reported health outcomes based on the Short Form Health Survey (SF12); the Patient Health Questionnaire (PHQ9); the Pittsburg Sleep Quality Index (PSQI); the Generalized Anxiety Disorder scale (GAD7); and the Altman Mania Rating Scale (ASRM), independent of covariates. This study provides the first detailed analysis of the gut microbiome relationships with multiple psychiatric domains from a bipolar population. The data support the hypothesis that targeting the microbiome may be an effective treatment paradigm for bipolar disorder.
INTRODUCTION
Accumulated evidence over the past decade supports a bi-directional relationship between the modifiable gut microbiome, mood and cognitive dysfunction. Schizophrenic and bipolar subjects show increased markers of bacterial tanslocation from the intestinal lumen (compared to controls), which may underlie increased inflammation (1) known to be a component of these disorders. In depressed subjects compared to controls, a survey of the stool microbiota showed increased Bacteroidetes, Proteobacteria and Actinobacteria and reduced Firmicutes Faecalibacterium, the latter of which negatively associated with severity of depression (2, 3). Intervention studies in humans suggest a benefit of microbiome manipulation for clinical psychiatric outcomes. Supplementation of L. helviticus and Bifobacterium longum, which reduce anxiety-like behavior in rats, reduced psychological distress in humans (4). Patients with gastrointestinal disorder, which is highly comorbid with psychiatric conditions, who consumed fermented milk that contained B.bifidum, showed improvement in gastrointestinal and psychological symptoms (5). In a small, randomized control trial of a multi-species pro-biotic in depressed adults, the pro-biotic treatment significantly reduced cognitive activity toward depressed mood when compared to placebo (6). These studies suggest that targeted manipulation of the microbiome can be beneficial for mood state in a psychiatric population.
Animal studies support a neurochemical mechanism for the effect of microbiome manipulation on behavior. Treating mice with pre-biotics (7) or antibiotics (8) influenced brain derived neurotrophic factor activity, NMDA glutamate receptors, oxytocin, vasopressin and central tryptophan metabolism. Diet induced disruption of the mouse microbiome is concomitant with changes in anxiety-like behavior (9, 10), depressive-like behaviors (10), and cognitive performance (9, 11). Disruption of the mouse enteric microbiota by infection can mediate stress-induced memory dysfunction and this can be normalized by probiotic (12) and prebiotic treatment (13). Taken together, these studies demonstrate that the gut microbiome is responsive to many physiological stimuli associated with the induction of affective disorders and relevant central circuitry, and that targeting the integrity of the microbiome with prebiotic and probiotic therapies may attenuate or prevent dysregulation.
In the current study we surveyed the stool microbiome of bipolar and control subjects from the Prechter Longitudinal Study of Bipolar Disorder, housed at the University of Michigan. This is a deep phenotyping longitudinal study for which we collect bi-monthly self-report measures of physical and mental health, allowing us to compare the taxonomical complement of the stool microbiome with self-reported burden of disease measures.
METHODS AND MATERIALS
Human Subjects
All subjects in the current study were recruited from the Heinz C. Prechter Longitudinal Study of Bipolar Disorder at the University of Michigan Depression Center (14). All individuals were diagnosed using the Diagnostic Interview for Genetic Studies (DIGS) (15) and included in this current study are bipolar individuals adhering to the DSM IV diagnostic criteria (16) and healthy unaffected controls who were willing to return stool samples as described below. Most bipolar subjects were taking more than one psychiatric medication, This study was not designed to specifically test for medication effects, which for the current analyses are viewed as a component of the illness. Limitations of the conclusions regarding the lack of separation of medication effects are discussed in the Discussion Section of the manuscript. The Internal Review Board for Human Studies at the University of Michigan approved all protocols.
Collection of Self-Reports
Longitudinal participants completed self-rated questionnaires by mail every 2 months from the time of their enrollment in the longitudinal study. Due to the dynamic temporal nature of these responses, for the current study we used the means of summary scores from each questionnaire to most robustly estimate the chronic burden of disease. Limitations of this strategy are discussed in the Discussion Section at the end of the manuscript. These questionnaires used include the Patient Health Questionnaire-9 (PHQ-9), the Altman Self-Rating Mania Scale (ASRM), the Short Form Health Survey (SF-12), the Generalized Anxiety Disorder Assessment (GAD-7), and the Pittsburg Sleep Quality Index (PSQI). The PHQ-9 defines severity and frequency of depressive states, while the ASRM defines severity and frequency of manic states. Similarly, the GAD-7 defines severity and frequency of anxiety symptoms. Scores greater than or equal to 5 for the PHQ-9, ASRM, and GAD-7 indicate a clinically significant depressive, manic, or anxious mood state, respectively, however for the current analyses these were treated as continuous variables without thresholds. The SF-12 scores are sub-scaled into composite scores for both physical (PCS) and mental (MCS) health. SF-12 scores are standardized on a scale of 0 to 100, where 0 indicates lowest level of health and 100 indicates the highest level of health. The PSQI measures sleep quality where higher scores indicate lower sleep quality. For primary statistical analyses, PSQI measures were condensed to a single summary score by the method of Buysse et al. (17). However, analysis of the sub components are also discussed.
Stool Microbiota Analysis
Home stool collection kits (DNA Genotek, Ontario CA), were mailed to 825 active Prechter Longitudinal Study subjects with a return pre-paid mailer, opt-in consent form and request to participate in the microbiome analysis. Returned stool samples were frozen at -80C until aliquoted into 96 well plates for DNA extraction. DNA was isolated with a PowerMag Microbiome RNA/DNA Isolation Kit (Mo Bio Laboratories, Inc.) using an epMotion 5075 liquid handling system. The V4 region of the bacterial 16S rRNA gene was amplified using a dual-indexing sequencing strategy and sequenced on the Illumina MiSeq platform as described previously (18). Negative control wells including all PCR reagents with water replacing sample template DNA were included across the plates and showed no visible bands upon gel analysis. Sequences were processed and analyzed using the software package mothur (v.1.36.1) according to the MiSeq SOP (http://www.mothur.org/wiki/MiSeq_SOP)(19). Briefly, after sequence processing and alignment to the SILVA reference alignment (20), sequences were binned into operational taxonomic units (OTUs) based on 97% sequence similarity using the average neighbor method. Clustering sequences by the average neighbor method produces high-quality OTUs that include all sequences regardless of their database representation (21, 22). After discarding 11 samples with low sequence counts (<100 per sample) and 1 sample with a sequence count of 1187 sequences, 3692 sequence reads remained as the least common denominator and were subsampled for direct comparison of the remaining 179 samples used in this analysis. Relative abundances of OTUs were calculated. Analysis of molecular variance (AMOVA) (23) of θYC distances (a metric that takes relative abundances of both shared and non-shared OTUs into account) (24) between communities was used to determine if there were statistically significant differences between the microbiota from the bipolar and control groups. Principle coordinates analysis (PCoA) was used to visualize the θYC distances between samples. We also investigated the taxonomic composition of the bacterial communities by classifying sequences within mothur using a modified version of the Ribosomal Database Project (RDP) training set (25, 26). In a few cases, OTUs that were unidentified were retrieved by manual BLAST search.
Statistical Analyses
For analysis of case-control differences of OTU level data, we used logistical regressions, adjusting for age, sex, and BMI (Table 2b). To determine associations between OTUs and self-reported health measures, we used linear regression analyses with the intra-individual means of self-report summary scores as the outcome measures and fractional representation of each OTU as the main predictor, controlling for age, sex, and BMI. For these regressions we used only data from individuals with bipolar disorder to eliminate potential confounders associated with a bipolar diagnosis. Standardized beta coefficients for the OTU – self-report associations were extracted along with the p-values for associations, which were adjusted using false-discovery rate (FDR) multiple testing correction originally described by Benjamini and Hochberg, using a tool developed by Pike et al. (27) to determine significance at the FDR p<0.05 level.
Table 2b
Microbiome OTU Fractional Representation
| OTU | Phylum | Genus | Bipolar | Control | p-value |
|---|---|---|---|---|---|
| OTU00001 | Bacteroidetes | Bacteroides | 11.1(8.9) | 11.5(8.2) | 0.288 |
| OTU00002 | Bacteroidetes | Bacteroides | 5.9(9.7) | 5.6(4.7) | 0.391 |
| OTU00003 | Firmicutes | Faecalibacterium | 5.1(4.3) | 7.7(5.0) | 0.001 |
| OTU00004 | Bacteroidetes | Prevotella | 4.5(11.6) | 1.9(6.1) | 0.408 |
| OTU00006 | Bacteroidetes | Bacteroides | 3.9(3.9) | 3.4(3.0) | 0.929 |
| OTU00007 | Bacteroidetes | Bacteroides | 2.7(5.6) | 2.5(4.7) | 0.836 |
| OTU00008 | Firmicutes | Roseburia | 2.6(2.6) | 3.0(2.5) | 0.176 |
| OTU00009 | Verrucomicrobia | Akkermansia | 1.9(3.1) | 1.3(2.3) | 0.916 |
| OTU00010 | Bacteroidetes | Alistipes | 1.7(2.0) | 2.1(3.3) | 0.226 |
| OTU00012 | Actinobacteria | Bifidobacterium | 1.7(3.1) | 1.6(2.6) | 0.538 |
| OTU00013 | Bacteroidetes | Parabacteroides | 1.9(2.0) | 1.4(2.1) | 0.330 |
| OTU00014 | Firmicutes | Blautia | 1.7(1.9) | 1.5(1.6) | 0.857 |
| OTU00015 | Firmicutes | Phascolarctobacterium | 1.4(1.8) | 1.8(2.6) | 0.425 |
| OTU00016 | Bacteroidetes | Bacteroides | 1.2(2.6) | 0.9(2.2) | 0.630 |
| OTU00017 | Bacteroidetes | Alistipes | 1.1(1.3) | 1.4(1.6) | 0.143 |
| OTU00018 | Actinobacteria | Collinsella | 1.1(1.9) | 1.0(2.6) | 0.206 |
| OTU00019 | Firmicutes | Ruminococcus | 1.1(1.9) | 1.1(1.4) | 0.051 |
| OTU00020 | Bacteroidetes | Bacteroides | 1.0(3.2) | 0.8(1.5) | 0.178 |
| OTU00021 | Firmicutes | Blautia | 0.9(1.1) | 0.8(1.0) | 0.803 |
| OTU00022 | Proteobacteria | unclassified* | 1.0(2.9) | 0.6(2.6) | 0.319 |
| OTU00023 | Firmicutes | Streptococcus | 0.6(2.8) | 0.6(1.4) | 0.307 |
| OTU00024 | Firmicutes | Anaerostipes | 0.8(1.2) | 1.0(1.2) | 0.019 |
| OTU00025 | Firmicutes | unclassified* | 0.6(1.0) | 1.1(1.2) | 0.002 |
| OTU00026 | Firmicutes | unclassified* | 0.9(1.4) | 0.8(0.8) | 0.061 |
| OTU00027 | Bacteroidetes | Parabacteroides | 0.7(0.8) | 0.7(1.1) | 0.761 |
| OTU00028 | Firmicutes | Lachnospiracea incertae sedis | 0.8(0.9) | 0.7(0.9) | 0.341 |
| OTU00029 | Firmicutes | unclassified* | 0.7(1.0) | 0.5(0.6) | 0.996 |
| OTU00030 | Proteobacteria | Parasutterella | 0.6(1.2) | 0.7(1.3) | 0.273 |
| OTU00031 | Firmicutes | Acidaminococcus | 1.0(2.3) | 0.3(1.0) | 0.067 |
Table 2b gives the mean and (SD) values for OTU fractional representations (percent of total) for all OTUs greater than 1% total in either group. P-values derive from logistical regressions with diagnosis (1,0) as the outcome measure and OTU as the predictor, adjusting for age, gender and BMI. Values in bold remained significant following FDR correction at the p<0.05 level.
RESULTS
Of the 825 stool collection kits mailed out to our longitudinal study participants with request to opt-in to the current sub study, 247 were returned and submitted for microbiome analysis. Quality 16S rRNA-encoding gene sequence data was generated from 233 (a 5.7% failure to generate quality sequence data) and 179 were used in the current analysis. The remaining 54 were excluded with their diagnosis yet to be confirmed (n=38) or a non-bipolar DSM IV diagnosis of Schizo Affective, 295.70M (n=5); Other Affective, 555.00A (n=2); or Other Non-Affective, 555.00N (n=9) and not used for this analysis. Table 1a shows the study subject demographics, separated by diagnostic group. Age was not significantly different between cases and controls, however percent females and body mass index (BMI) was significantly higher in the bipolar group, all of which were added as covariates to the analyses or further explored as described below. Table 1b summarizes the confirmed diagnostic codes for the included subjects.
Table 1a
Demographics
| Control | Bipolar | p-value | |
|---|---|---|---|
| Total Number | 64 | 115 | NA |
| Number Female (%) | 40 (62.5%) | 83 (72.2%) | 0.019 |
| Mean Age (SD) | 48.6 (16.6) | 50.2 (12.8) | 0.194 |
| Mean BMI (SD) | 26.0 (4.6) | 29.3 (7.2) | <0.001 |
Table 1a details basic demographic information by diagnosis for all study subjects included in the analyses. P-values are given for group comparisons using ttests (for age and BMI) and a binomial test (for % female, setting control value as the expected).
Table 1b
Subject Diagnoses
| DSMIV code | Diagnosis | Number |
|---|---|---|
| 296.70A | Bipolar I Disorder | 76 |
| 296.8 | Bipolar NOS | 10 |
| 296.89R | Bipolar II Disorder | 29 |
| V71.09 | No Diagnosis (controls) | 64 |
Table 1b details the expanded diagnostic categories for the subjects included in the current study.
The microbiota of bipolar and control subjects were significantly different based on AMOVA of θYC distances (p=0.047). Principal coordinate analysis (PCoA) was performed to visualize group differences, (Fig 1). A Scree plot of the PCoA axes is shown in Fig 1a, with the variance accounted for by the major axis and the group difference significance level of each. Axis 1 explained 12.3% of the variance with a trend toward different representation by bipolar and control groups (p=0.067). Axis 3 and 5 represented 5.5% and 3.2% of the total variance and were significantly different between bipolar and control groups with p=0.026 and p=0.035, respectively. Figure 1b shows a visual representation of the PCoA results with the most significant axis plotted. These data suggest moderate global differences at the community level exist between individuals with bipolar disorder and controls.
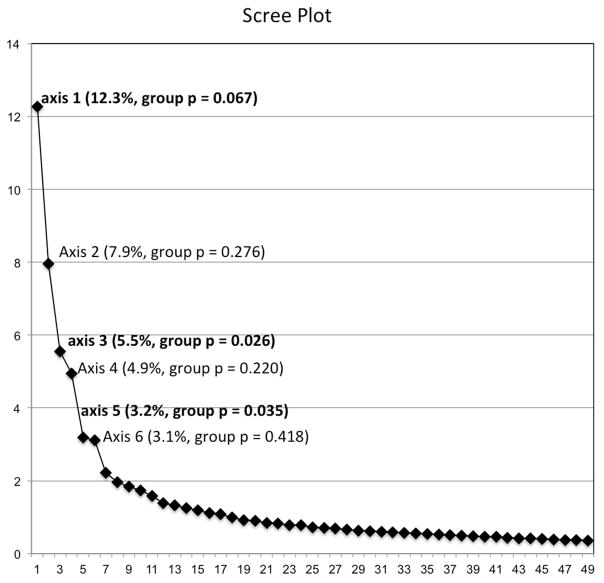
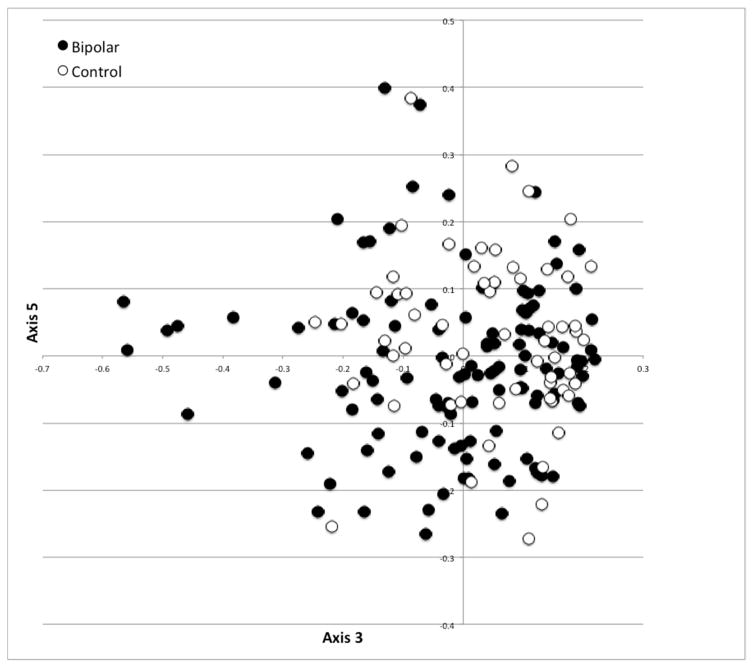
Scree plot (a) with each axis point above the inflection denoted with the variance explained and p-value for group differences between bipolar and control subjects; and (b) scatter plot of the significant axes 3 and 5 showing a partial separation of bipolar and control subjects (AMOVA p=0.047).
Table 2a and and2b2b give the summary scores from the self-report questionnaires and the fractional representation of OTUs, respectively, for all OTUs with at least 1% fractional representation in either group. As shown in Table 2a, we found strong significant differences between cases and controls on mean summary scores from self-reported functional physical health (PCS), mental health (MCS), sleep quality (PSQI), depression (PHQ9), anxiety (GAD7), and mania (ASRM); all with case-control differences significant at p<0.001. Table 2b gives the fractional representation of the OTUs by diagnostic group and the significance value for mean group differences, after adjusting for age, sex and BMI using logistical regression. OTU00003, Faecalibacterium (34% lower fractional representation in BPD, p<0.003), and OTU00025, unidentified (45% lower fractional representation in BPD, p=0.002) remained significantly different between groups following FDR correction at the corrected p<0.05. Although OTU00025 was not identified at the genus level, a manual blast search identified it is as a member of the Ruminococcaceae family of the Firmicutes phylum.
Table 2a
Self Report Health Questionnaire Scores
| Control | Bipolar | p-value | |
|---|---|---|---|
| Self-Reports | |||
| SF12 PCS | 50.0 (3.2) | 46.7 (7.2) | <0.001 |
| SF12 MCS | 45.2 (5.2) | 29.2 (9.2) | <0.001 |
| PSQI | 4.00 (1.94) | 7.74 (3.38) | <0.001 |
| PHQ9 | 1.2 (1.2) | 8.0 (4.8) | <0.001 |
| GAD7 | 0.83 (1.4) | 7.5 (5.3) | <0.001 |
| ASRM | 1.4 (1.5) | 3.0 (2.2) | <0.001 |
Table 2a gives the mean and (SD) for the self-report questionnaires for bipolar and control subjects and the 2-sided t-test p-value for mean group differences.
Table 3 reports significant associations between OTU fractional representation and mean summary self-reported health measures. We used regression analyses with the self-report measures as outcomes and the OTUs as primary predictor variables, adjusting for age, sex, and BMI in bipolar subjects only. These analyses revealed several significant associations, showing that representation of specific OTUs associated with severity of disease burden in subjects with bipolar disorder, as assessed by these self-reports. After FDR multiple testing correction, OTU00003 (Faecalibacterium) associated with improved physical health, depression, and sleep quality scores; OTU00024 (Anaerostipes) and OTU00025 (Ruminococcaceae family, unresolved at genus level) associated with improved PCS, while an unclassified genus from the family OTU00022 (Enterobacteriaceae family, unresolved at the genus level) associated with worse PCS scores. Significance levels and standardized β coefficients are given in Table 3. To further explore the relationship between Faecalibacterium and sleep quality we reanalyzed the PSQI data at the subscale level. This revealed that Components 1 (Overall Sleep Quality Rating), 2 (Sleep Latency), 6 (Sleep Medication Use) and 7 (Daytime Sleepiness/Lethargy) explained the associations between Faecalibacterium with PSQI. The remaining Components 3 (Sleep Duration), 4 (Percent Sleep Duration) and 5 (Awakening Events) were not significantly associated with fractional representation of Faecalibacterium.
Table 3
Regression Analyses of OTUs with Self-Report Measures.
| standardized β coefficient
| |||||||
|---|---|---|---|---|---|---|---|
| OTU | Predictor(Genus Level) | PCS B | MCS B | PSQI B | GAD7 B | PHQ9 B | ASRM B |
| Otu00003 | Faecalibacterium | 0.328**** | 0.174 | −0.329**** | −0.2* | −0.297**** | −0.203* |
| Otu00008 | Roseburia | 0.223* | 0.101 | −0.18 | −0.16 | −0.133 | 0.002 |
| Otu00013 | Parabacteroides | 0.016 | −0.078 | 0.203* | 0.111 | 0.122 | 0.067 |
| Otu00015 | Phascolarctobacterium | 0.187* | −0.052 | −0.016 | −0.05 | −0.051 | 0.039 |
| Otu00016 | Bacteroides | 0.083 | −0.291*** | 0.104 | 0.202* | 0.078 | 0.083 |
| Otu00017 | Alistipes | 0.227* | 0.027 | −0.137 | −0.191* | −0.209* | −0.078 |
| Otu00019 | Ruminococcus | 0.168 | 0.139 | −0.224* | −0.241* | −0.224* | −0.144 |
| Otu00022 | unclassified | −0.306**** | 0.018 | 0.237* | 0.116 | 0.202* | 0.132 |
| Otu00024 | Anaerostipes | 0.282*** | 0.086 | −0.242** | −0.162 | −0.133 | 0.008 |
| Otu00025 | unclassified | 0.298**** | 0.08 | −0.248** | −0.224* | −0.248** | −0.042 |
| Otu00028 | Lachnospiracea-incertae-sedis | 0.087 | 0.207* | −0.121 | −0.143 | −0.129 | 0.017 |
| Otu00030 | Parasutterella | 0.119 | 0.111 | −0.155 | −0.262** | −0.191* | −0.147 |
| Otu00031 | Acidaminococcus | −0.09 | −0.082 | 0.004 | 0.142 | 0.082 | 0.248** |
Table 3 shows standardized β coefficients from regression analyses that included self report questionnaire scores (top row) as the outcome variable and microbial OTUs (first column) as the primary predictor variable after correcting for age, gender, and BMI in each model. Only OTUs with at least one significant association are shown, all others from Table 2b were not significantly associated with any clinical outcomes measured. Also, only data from individuals with bipolar disorder were used for this analysis. Significance levels are indicated by asterisks as *p<0.05, **p<0.01, ***p<0.005, p<0.001. Bold values highlight associations that remained significant following false discovery rate correction at the p<0.05 level. ASRM = Altman Scale for Rating Mania, GAD = General Anxiety Disorder 7, LFQ = Life Functioning Questionnaire, PHQ9 = Patient Health Questionnaire, MCS = Mental Health Composite Score from the Short Form Health Survey (SF12), and PCS = Physical Health Composite Score from the SF12. Two OTUs were unclassified at the genus level but are identified at the family level as Enterobacteriaceae (OTU00022) and Ruminococcaceae (OTU00025).
DISCUSSION
In the current study we report an analysis of stool microbiome samples collected from bipolar individuals and healthy controls who are participants of the Prechter Longitudinal Study of Bipolar Disorder at the University of Michigan. We found significant differences between the global microbiome communities and specific OTUs in individuals with bipolar disorder, compared to controls. We also found significant relationships between fractional representation of several OTUs and self-reported burden of disease measures. These data represent the first survey of the microbiome of a relatively large number of bipolar individuals and the first analysis of the associations between the human microbiome and psychiatric burden of disease measures in this population.
The most striking finding from the current study was the group difference and the clinical outcome associations for Faecalibacterium (OTU0003). Faecalibacterium is a Gram-positive butyrate-producing gut bacterium (28) in large abundance in the human gut (29). Faecalibacterium has previously been shown to be lower in many human conditions. Hospitalized (30) and frail elderly (31), compared to healthy elderly, have lower gut Faecalibacterium. Decreased representation of this genus in inflammatory bowel syndrome (32, 33) Crohn’s disease (34, 35), ulcerative colitis (35–37), colorectal cancer (38) chronic idiopathic diarrhea (39), have also been reported. Other studies have found decreases in diabetic patients (40) and glucose intolerance (41), non-alcoholic steatohepatitis (42), patients with cholesterol gallstones (43), association with high hepatic fat content (44) and an inverse association with severity of acute appendicitis (45). These studies all suggest potential effects of Faecalibacterium on organ systems outside the gut as well. Furthermore, Faecalibacterium has been shown to have anti-inflammatory properties in vitro and in vivo (46, 47). Taken together studies examining the gut presence of Faecalibacterium suggest that higher fractional representation associates with a healthier state.
One study, relevant to the current report, found decreased fractional representation of Faecalibacterium in a small sample of actively depressed patients relative to controls that was not evident in treatment responsive depressed patients (3). This consistency adds strength to our current finding of decreased Faecalibacterium in bipolar subjects and suggests that the lower gut levels may be native to the illness and relevant to a depressed state. Furthermore, our current reports of associations between self-reported mental health scales and this gut bacteria within bipolar subjects, suggest that increasing gut levels of Faecalibacterium may have value for psychiatric outcomes.
Methods to increase Faecalibacterium may include dietary approaches. Dietary studies have found that, in healthy subjects, Faecalibacterium increased secondary to prebiotic supplementation, including, inulin (48), raffinose (49), polydextrose (50), soluble corn fiber (50) high amylose maize starch (51), resistant maltodextrin (52), and the pro-biotic, Bacillus coagulans (53). However, bipolar subjects have a high incidence of comorbid gastrointestinal disorders, which are sensitive to the many of the above supplementations so other dietary approaches should be investigated for this population. In a subset of subjects from our current study we also have habitual dietary information published previously (54). Leveraging these data we found significant positive associations between habitual intake of several polyunsaturated fatty acids (PUFA) and Feacalibacterium fractional representation and launched a PUFA dietary intervention study, which may be much better tolerated by individuals with gastrointestinal disorders.
The current analysis is limited by the cross-sectional design, which prevents any causative conclusions. We chose to use mean summary scores from the self-report scales, rather than the single scores collected nearest the stool collection to minimize the acute variation inherent in these data. We justify this approach since analysis of the questionnaire data reveals wide swings, in individual scores without clear trajectories of increasing or decreasing significantly over time. Thus any given measure may inaccurately reflect the burden of disease for individual subjects, whereas the regression to the mean of data collected over time likely provides a better indicator. Furthermore, the temporal relationship between changes in the microbiome composition and changes in clinical outcome measures or behavior are unclear. There may or may not be substantial lag period (as there is for several pharmaceutical treatments), if indeed there is a causal effect of the microbiome composition on psychiatric outcomes. Research with repeated measured stool and self-report sampling pre- and post- intervention strategies, including dietary approaches, are necessary to address this limitation of the current study.
The current analysis is further limited by the inability to control for medication use and compliance. The study was not designed to separate the effects of psychiatric medication use from the effects of disease on the gut microbiome composition or its relationships with clinical outcomes. This was a naturalistic study of individuals with bipolar disorder, relative to controls, and medication use was neither used as inclusion or exclusion criteria. Therefore, substantial polypharmacy use by individuals with bipolar disorder precludes us from drawing conclusions related to how psychiatric medication use may effect the reported observations. Studies designed specifically to address this question are necessary.
In summary, we found several OTUs, especially Faecalibacterium to associate with bipolar illness and negatively associate with self-reported burden of disease measures in bipolar individuals, independent sex, age and BMI. These data suggest that therapeutically increasing Faecalibacterium in bipolar patients may be beneficial to reduce disease burden, however this hypothesis needs to be tested with the appropriate study design.
Acknowledgments
The authors would like the thank the team at the ARC, http://arc.umich.edu/flux-and-otherhpc-resources/flux/citations/, for their technical assistance with the flux computational power used for analysis of microbial genetic data.
ROLE OF FUNDING SOURCES:
This work is supported by the Heinz C. Prechter Bipolar Research Fund and the Richard Tam Foundation at the University of Michigan Depression Center; NIH Grant # 5-K01-MH- 093708-04 (Evans); and The University of Michigan Medical School Host Microbiome Initiative.
Footnotes
CONTRIBUTORS
SJE designed the study, organized the collection of data and wrote the manuscript; CMB performed the microbiome analysis assays, aided in the bioinformatics analysis and edited the manuscript; RH performed the bioinformatics analyses, SA aided in the secondary statistical analyses of processed data and edited the manuscript; SAF performed the analysis of psychiatric medication effects and edited the manuscript; MBK aided in subject recruitment; VBY aided supervised the bioinformatics and microbiome assays and edited the manuscript; VEE supervised the psychiatric medication analysis and edited the manuscript; MGE supervised the interaction with psychiatric subjects and sample collection and edited the manuscript.CONFLICT OF INTEREST
Dr. McInnis reports having served as a consultant for Janssen Pharmaceuticals. Dr. Young reports having received consulting fees from Merck and Vedanta, and research funding from MedImmune. No other authors have anything to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.jpsychires.2016.12.007
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc5336480?pdf=render
Citations & impact
Impact metrics
Article citations
Insomnia, OSA, and Mood Disorders: The Gut Connection.
Curr Psychiatry Rep, 14 Oct 2024
Cited by: 0 articles | PMID: 39400694
Review
A Cross Talking between the Gut Microbiota and Metabolites of Participants in a Confined Environment.
Nutrients, 16(11):1761, 04 Jun 2024
Cited by: 0 articles | PMID: 38892694 | PMCID: PMC11175105
The emerging roles of microbiota-derived extracellular vesicles in psychiatric disorders.
Front Microbiol, 15:1383199, 08 Apr 2024
Cited by: 0 articles | PMID: 38650872 | PMCID: PMC11033316
Review Free full text in Europe PMC
The Potential Role of the Ketogenic Diet in Serious Mental Illness: Current Evidence, Safety, and Practical Advice.
J Clin Med, 13(10):2819, 10 May 2024
Cited by: 1 article | PMID: 38792361 | PMCID: PMC11122005
Review Free full text in Europe PMC
Food for thought: Making the case for food produced via regenerative agriculture in the battle against non-communicable chronic diseases (NCDs).
One Health, 18:100734, 20 Apr 2024
Cited by: 0 articles | PMID: 38711478 | PMCID: PMC11070632
Review Free full text in Europe PMC
Go to all (118) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Epigenetics of the molecular clock and bacterial diversity in bipolar disorder.
Psychoneuroendocrinology, 101:160-166, 10 Nov 2018
Cited by: 25 articles | PMID: 30465968
Effects of Atypical Antipsychotic Treatment and Resistant Starch Supplementation on Gut Microbiome Composition in a Cohort of Patients with Bipolar Disorder or Schizophrenia.
Pharmacotherapy, 39(2):161-170, 03 Feb 2019
Cited by: 34 articles | PMID: 30620405 | PMCID: PMC6386623
Plasma linoleic acid partially mediates the association of bipolar disorder on self-reported mental health scales.
J Psychiatr Res, 68:61-67, 14 Jun 2015
Cited by: 7 articles | PMID: 26228402 | PMCID: PMC4522046
The Gut Microbiome and Mental Health: Implications for Anxiety- and Trauma-Related Disorders.
OMICS, 22(2):90-107, 02 Aug 2017
Cited by: 65 articles | PMID: 28767318
Review
Funding
Funders who supported this work.
NIDDK NIH HHS (1)
Grant ID: P30 DK089503
NIMH NIH HHS (1)
Grant ID: K01 MH093708





