Abstract
Free full text

Elimination of proliferating cells from CNS grafts using a Ki67 promoter-driven thymidine kinase
Abstract
Pluripotent stem cell (PSC)-based cell therapy is an attractive concept for neurodegenerative diseases, but can lead to tumor formation. This is particularly relevant as proliferating neural precursors rather than postmitotic mature neurons need to be transplanted. Thus, safety mechanisms to eliminate proliferating cells are needed. Here, we propose a suicide gene approach, based on cell cycle-dependent promoter Ki67-driven expression of herpes simplex virus thymidine kinase (HSV-TK). We generated a PSC line expressing this construct and induced neural differentiation. In vitro, proliferating PSC and early neural precursor cells (NPC) were killed by exposure to ganciclovir. In vivo, transplantation of PSC led to tumor formation, which was prevented by early ganciclovir treatment. Transplanted NPC did not lead to tumor formation and their survival and neural maturation were not affected by ganciclovir. In conclusion, the cell cycle promoter-driven suicide gene approach described in this study allows killing of proliferating undifferentiated precursor cells without expression of the suicide gene in mature neurons. This approach could also be of use for other stem cell-based therapies where the final target consists of postmitotic cells.
Introduction
Pluripotent stem cell–based cell therapy is an attractive concept for regenerative medicine, however the progress toward clinical use has been slow.1 Various barriers have to be overcome, such as obtaining a pure population of cells with characteristics identical to those in the organs that need replacement and up-scaling of cell production from animal models to human use. Besides these challenges, the safety of pluripotent stem cell (PSC)-derived cells for transplantation is a concern. Human pluripotent stem cells, including embryonic stem cell (ESC) and induced pluripotent stem cell (iPSC), have the ability of self-renewal and therefore represent an unlimited source of cells for cell therapy. However, this advantage comes with a potential threat: there is a risk of tumor formation if transplanted cells continue to proliferate for a prolonged time period.2 PSC tumorigenicity can be divided into two different types: (i) teratoma formation from residual undifferentiated PSC, i.e., tumors containing cells of all three germ layers, and (ii) uncontrolled growth of PSC-derived partially differentiated cells. This potential tumor risk is particularly relevant with respect to transplantation of neurons. Indeed, mature neurons are large complex structures that poorly survive transplantation. Thus, for neural cell therapy, precursors rather than mature neurons need to be transplanted and as discussed above, precursor cells have the potential to proliferate and form tumors.3,4
To overcome tumor risk, suicide gene approaches have been evaluated. The most widely used suicide gene is Herpes simplex I thymidine kinase (TK), which renders cells sensitive to killing through ganciclovir. Indeed, TK phosphorylates ganciclovir, and phosphorylated ganciclovir is a nucleotide analog which upon integration into newly synthesized DNA leads to DNA breaks and ultimately to apoptosis. TK has mostly been used in the context of cancer therapy5,6 and graft versus host disease.7–9 There are suggestions that TK suicide gene system could also be useful for eliminating proliferating cells in the context of cell therapy. An in vitro study using PSC expressing TK under the control of the Oct4 promoter demonstrated upon ganciclovir treatment survival of PSC-derived neurons, but killing of undifferentiated PSC.10 Three studies have investigated the potential of TK as a suicide gene for cell therapy. Transplantation of hepatocytes expressing TK under the control of a tissue specific constitutive promoter11 have shown that ganciclovir efficiently eradicates transplanted cells. Two cell therapy studies have used PSC that express TK under the control of pluripotency promoters.12,13 Based on these studies, it appears that activity of pluripotency promoters decreases rapidly, and only immediate treatment (3 hours) with ganciclovir prevented tumor formation. In contrast, when mice were treated at later time points, tumor formation was not prevented.
Here, we propose a technology that allows selective expression of TK not only in pluripotent, but in all proliferating cells. For this purpose, we have generated a PSC line expressing TK under the control of the previously characterized cell cycle-dependent promoter Ki67.14 We show that ganciclovir kills proliferating PSC TK cells in vitro and in-vivo. In contrast, postmitotic neurons do not express TK and are resistant to killing by ganciclovir. We propose that the safety switch technology described in this manuscript might be a useful security feature in clinical studies using PSC.
Results
Generation of TK-PSC line
It was the ultimate aim of this study to develop a suicide gene approach toward the prevention of uncontrolled overgrowth of PSC-derived transplanted neural cells. For many reasons, ganciclovir and herpes simplex thymidine kinase is an attractive system for such a suicide gene technology. Indeed ganciclovir is widely used in patients and penetrates into the central nervous system. However, constitutive expression of HSV-TK would not be desirable, as this could lead to immune rejection of transplanted cells, and the use of ganciclovir could potentially lead to killing of functional transplanted neurons. We therefore decided to express HSV-TK under the control of a cell cycle-dependent Ki67 promoter fragment. For this purpose, the human pluripotent ESC (hPSC) line HS415 was transduced with a lentivector coding for the expression of HSV-TK under the control of the Ki67 promoter fragment.14 As the lentivector transduction efficiency of hPSC is in the range of 10–20%, a tool for selection of transduced cells was required. We thus used a construct that with the HSV-TK sequence fused to a zeocin resistance sequence (see Materials and Methods). HSV-TK-positive cells were selected by culturing transduced hESC in the presence of zeocin (Figure 1a). We thereby obtained a polyclonal HSV-TK-expressing PSC line, that will be referred to throughout the text as TK-PSC. Neural precursor cells and mature neurons were obtained from TK-PSC as described in Materials and Methods and will be referred to as TK-NPC and TK-neurons, respectively (Figure 1b,,cc).
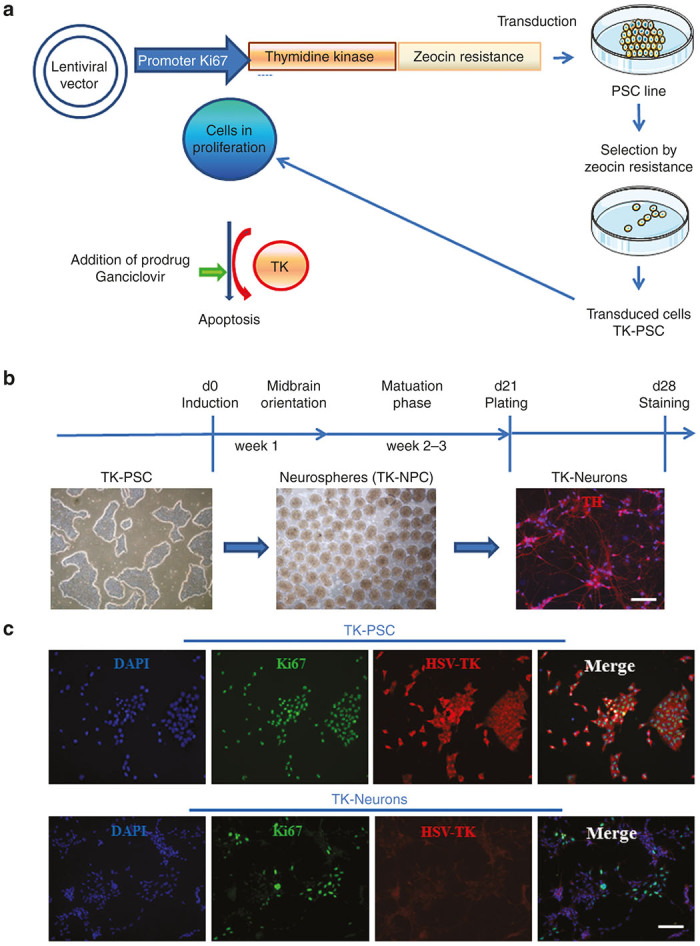
Elaboration of a pluripotent stem cell line expressing a suicide gene under the control of a cell cycle-dependent promoter. (a) thymidine kinase from herpes simplex virus (HSV-TK) was introduced into a lentiviral vector under the control of a Ki67 promotor fragment. The thymidine kinase used in this study was a fusion protein containing a C-terminally fused zeocin resistance (see Materials and Methods). The construct was transduced into the clinical grade human pluripotent stem cell line HS415 (TK-PSC). TK-PSC cells expressing this enzyme were selected by their resistance to zeocin. (b) Cell preparations used in this study: pluripotent stem cells (TK-PSC, left panel) were differentiated into neurospheres containing neuroprogenitors (TK-NPC, middle panel) and terminated maturation into neurons picture on the right). (c) Characterization of TK-PSC cells in undifferentiated state (upper panel) and upon differentiation into neurons (lower panel). Ki67 (green) and HSV-TK (red) were detected by immunostaining and nucleus by DAPI (blue). Scale bar B, C= 100 µm.
Highly proliferative cells expressing Ki67/TK are sensitive to ganciclovir in vitro
We next investigated TK expression in TK-PSC and in TK neurons. In TK-PSC, virtually 100% of cells expressed TK in concordance with high expression of Ki67 in these cells (Figure 1c, upper panel). In contrast, TK neurons did not express any TK, as predicted from them being postmitotic cells. It should however be mentioned that there were still Ki67-positive cells in the neuronal preparation (Figure 1c, lower panel). We do not think that these Ki67-positive cells reflect the presence of proliferating cells: many of the Ki67-positive cells in the neuronal preparation clearly showed a morphology of mature neurons with long neurite extension. We also investigated expression of Ki67, together with expression of the pluripotency markers nanog and Oct3/4 by flow cytometry. In PSC, virtually all cells were Ki67-positive, Nanog-positive, and Oct3/4-positive. NPC rapidly lost pluripotency markers (already after 1 week of neurosphere differentiation), while Ki67 expression decreased more slowly with levels slightly above background after 3 weeks of differentiation (Figure 2a).
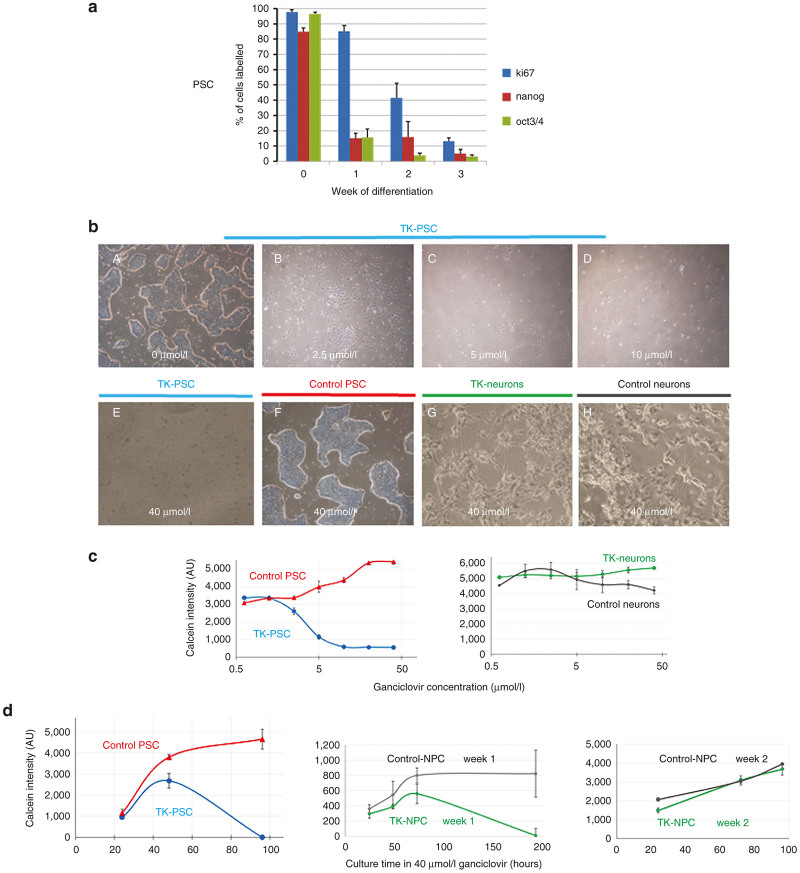
Effect of ganciclovir treatment in vitro. (a) Analysis of expression of proliferation markers (Ki67) and pluripotency markers (nanog, oct3/4) at different stages of the differentiation protocol described in Figure 1b. Protein expression was analyzed by flow cytometry (mean ± SEM of four independent experiments). (b) A–D: Undifferentiated pluripotent TK-PSC cells were exposed to increasing concentrations of ganciclovir. (b) E–F: Comparison of the effect of 40 µmol/l ganciclovir on TK-expressing and control PSC. Note that in control PSC, no ganciclovir toxicity is observed even with the highest concentration of ganciclovir (40 µmol/l). Upon neuronal differentiation, TK-expressing cells lose their sensitivity to ganciclovir as predicted by the lack of Ki67-driven TK expression in postmitotic neurons (see Figure 1c). (c) Dose response to ganciclovir: TK-PSC, control PSC; TK-neurons, and control neurons were exposed to increasing concentrations of ganciclovir. Cell viability was monitored using calcein. (d) Ganciclovir time course: TK and control PSC, 1 week NPC (TK and control), and 2 week NPC (TK and control) were exposed to 40 µmol/l ganciclovir and cell toxicity was monitored using calcein. Data from panel c and d are shown as triplicate determinations and are representative of three independent experiments. Error bars = ±SD, n = 3.
We next investigated the impact of ganciclovir on TK cells in vitro (Figure 2b–d). TK PSC were highly sensitive to ganciclovir exposure (96 hours) and already at ganciclovir concentrations of 2.5 µmol/l, loss of cells was observed, which was almost complete at 10 µmol/l (Figure 2b,,c).c). In contrast, regular PSC (not transduced with the TK construct) were resistant to ganciclovir even at concentrations of 40 µmol/l (Figure 2b, F). As expected from the lack of TK expression in TK neurons (see above), TK neurons were not affected by ganciclovir concentrations up to 40 µmol/l, similar as seen for control neurons.
We also investigated time course of the ganciclovir effects (Figure 2d). TK PSC were killed by Ganciclovir (40 µmol/l) within 4 days. TK-NPC after 1 week of neurosphere differentiation were still sensitive to Ganciclovir (40 µmol/l), however the time course of killing was markedly slowed down and complete killing was observed only after 8 days. TK-NPC after 2 weeks of neurosphere differentiation showed only little cell growth and were not killed by ganciclovir.
Early, but not late ganciclovir treatment prevents tumor formation upon transplantation of highly proliferative pluripotent stem cells
Given the encouraging results in vitro, we proceeded to investigate whether ganciclovir could prevent tumor formation in vivo (Figure 3). For this purpose, TK-PSC were transplanted into the striatum of NOD/SCID mice. In the absence of ganciclovir, mice, sacrificed 49 days post-transplantation, consistently developed tumors (Figure 4a), which consisted of human cells (HCM-positive) and showed abundant expression of Ki67 and Ki67 promoter-driven HSV-TK (Figure 5a,A). There was a moderate amount of mouse microglia invasion (Figure 5A,b). In contrast in mice that were treated for 15 days with ganciclovir (starting 4 days post-transplantation), no tumor was observed (Figure 4b). There are hardly any human cells left in the ganciclovir-treated animals, and the few surviving human cells were negative for HSV-TK and—to a large extent—also negative for Ki67, suggesting that the few surviving human cells had become postmitotic and therefore lost sensitivity to ganciclovir (Figure 5b,A). Thus, ganciclovir prevented tumor formation by transplanted TK-PSC. We next wanted to investigate whether ganciclovir could also be used to treat already established tumors. We therefore let tumor formation progress for 30 days (preliminary results had shown that within this time delay there was consistent tumor formation upon transplantation of PSC) and treated with ganciclovir (or PBS) for day 30–45 post-transplantation. Mice were sacrificed 30 days after the end of the ganciclovir treatment. Under these conditions, there was tumor formation in both, the PBS and the ganciclovir-treated animals (Figure 6). CD31 staining showed that tumors were vascularized, suggesting that lack of perfusion did not account for the absence of a ganciclovir therapeutic effect in established tumors. Similarly, many tumor cells showed high level expression of HSV-TK suggesting that downregulation of the transgene was not the explanation for the lack of a therapeutic response.
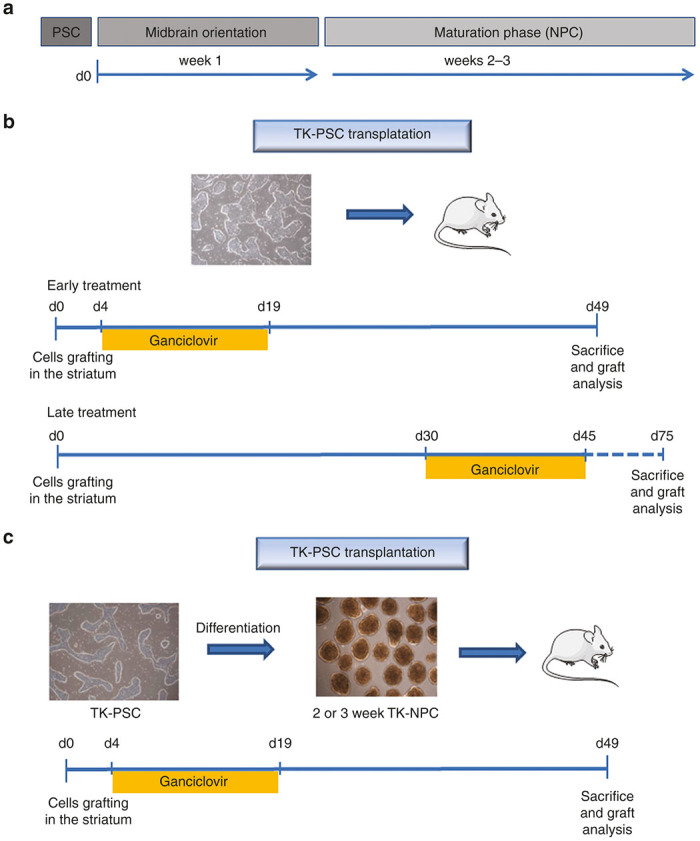
Schedule of cell transplantation and ganciclovir treatment. Different transplantation and ganciclovir treatment protocols were used. (a) Schematic representation of the neuronal differentiation protocol. Basically pluripotent stem cells were cultured as neurospheres for 1 week during midbrain orientation phase, followed by a 2- or 3-week maturation phase. (b) Transplantation of undifferentiated pluripotent stem cells with early or late ganciclovir treatment. Early treatment: undifferentiated pluripotent stem cells were transplanted and ganciclovir (or PBS as control) was injected i.p. daily from day 4 to day 19 post-transplantation. Late treatment: undifferentiated pluripotent stem cells were transplanted and ganciclovir (or PBS as control) was injected i.p. daily from day 30 to day 45 post-transplantation. (c) transplantation of 2 or 3 week NPC: NPC containing neurospheres were dissociated and transplanted into mice striatum. Ganciclovir (or PBS as control) treatment was injected i.p. daily from day 4 to day 19 post-transplantation. For all protocols, animals were sacrificed 1 month after termination of ganciclovir treatment.
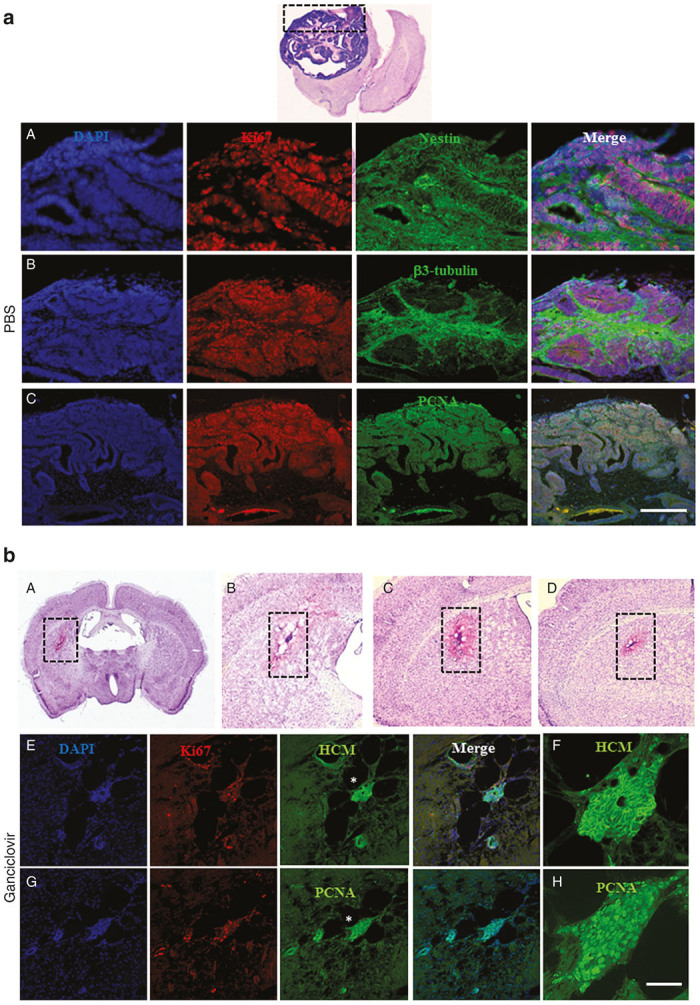
Early ganciclovir treatment prevents tumor formation after transplantation of HSV-TK-expressing pluripotent cells. (a) Transplantation of TK-PSC without ganciclovir treatment: tumor formation was consistently observed after transplantation of TK-PSC into PBS-treated mice Top image: Cresyl violet staining of a brain section showing tumor in the right hemisphere; A–C: Immunostaining of the graft. Tumor contains mainly neural tissue with expression of nestin (for immature neural cells), βIII tubulin (for mature neurons). Proliferative cells are stained with Ki67 and proliferating cell nuclear antigen (PCNA). (b) Transplantation of TK-PSC was followed by early ganciclovir treatment. Absence of tumor formation and cell proliferation in mice transplanted with TK-PSC (day 4 to 19 following transplantation); A–D: Cresyl violet coloration; E–H: Immunostaining with HCM, Ki67, and PCNA. HCM staining allows specific detection of human cytoplasmic proteins. Mice were sacrificed and immunohistochemistry was performed one month after termination of ganciclovir treatment. Asterix (*) tags the region displayed at a higher magnification in f and h. Scale bar: a A = 100 µm, a B,C = 400 µm, b A = 2 mm, b B,C,D = 900 µm, b E,G = 170 µm, b F,H = 45 µm.
mm, b B,C,D = 900 µm, b E,G = 170 µm, b F,H = 45 µm.
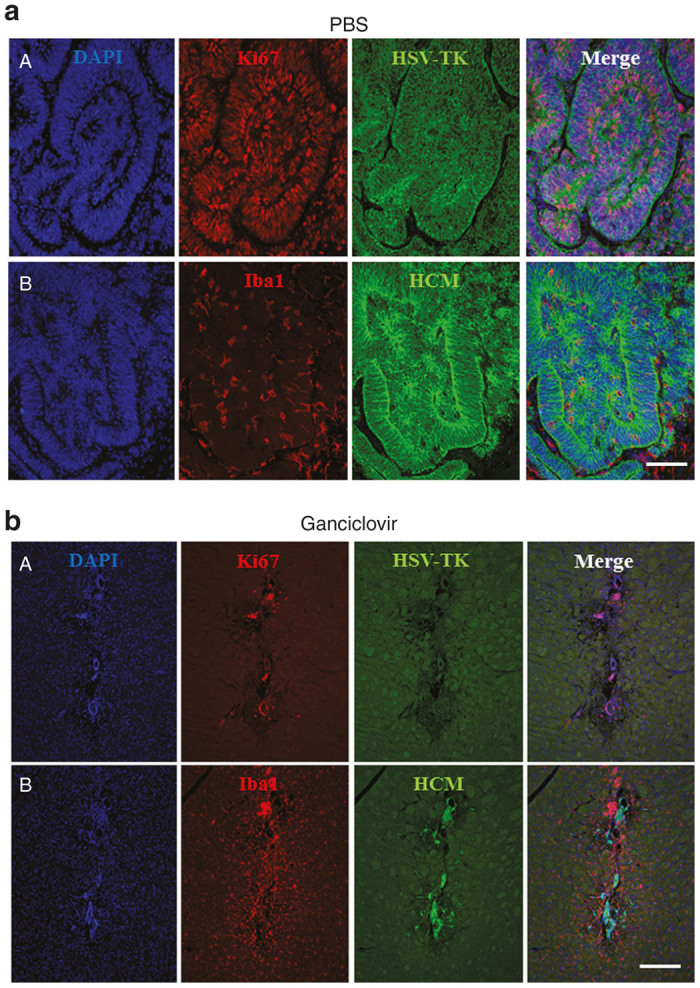
Early ganciclovir treatment and impact on Iba1 and Ki67-immunoreactive cells. (a) In the absence of ganciclovir treatment, there were abundant HSV-TK-immunoreactive and proliferating (Ki67-immunoreactive), human (HCM) cells; the tumors were infiltrated by microglia (Iba1-immunoreactive cells). (b) In ganciclovir-treated mice, a human cell graft (HCM) was barely detectable and virtually no Ki67 and HSV-TK immunoreactivity was observed in the graft. Iba1.immunoreactivity was detectable around the remaining human cell. Mice were sacrificed and immunohistochemistry was performed one month after termination of ganciclovir treatment. Stainings shown in this figure are representative of three to five mice per group. Scale bar: a = 100 µm, b = 200 µm.

Late ganciclovir treatment does not prevent tumor formation after transplantation of HSV-TK-expressing pluripotent cells. Mice were transplanted with TK-PSC and PBS treatment (a) or ganciclovir-treatment (b) was initiated one month after transplantation. Treatment was maintained for 15 days, followed by 1 month without treatment before sacrifice. Under these conditions, there was no difference between PBS-treated and ganciclovir-treated mice. The grafts have developed toward a tumor with predominantly neural tissue (βIII tubulin staining). Tumor cells expressed Ki67- and TK- but no Oct3/4-immunoreactivity. The grafts were vascularized, as evidenced by CD31 staining for endothelial cells. Scale bar: a A = 160 µm, a B = 2 mm, a C = 620 µm, a D, E = 80 µm, b A = 160 µm, b B = 320 µm, b C = 2
mm, a C = 620 µm, a D, E = 80 µm, b A = 160 µm, b B = 320 µm, b C = 2 mm, b D = 620 µm, b E, F = 80 µm.
mm, b D = 620 µm, b E, F = 80 µm.
A previous study15 had suggested that alternative splicing, due to cryptic splice donor and acceptor sites, can lead to formation of an inactive TK. Another study16 had suggested that various nonsense mutations may occur in the TK sequence in rapidly growing cells and might explain ganciclovir resistance. We therefore extracted mRNA from the tumors formed upon injection of undifferentiated pluripotent cells. From these mRNA preparations, we amplified five regions of the TK protein covering a total of 1,162 nucleotides, corresponding to 85% of the total TK sequence (Figure 7a). The sequenced regions also included the proposed cryptic splice sites (amplicon 3). We first directly sequenced the polymerase chain reaction (PCR) products (Figure 7b). The results of this sequencing allowed the following conclusions:
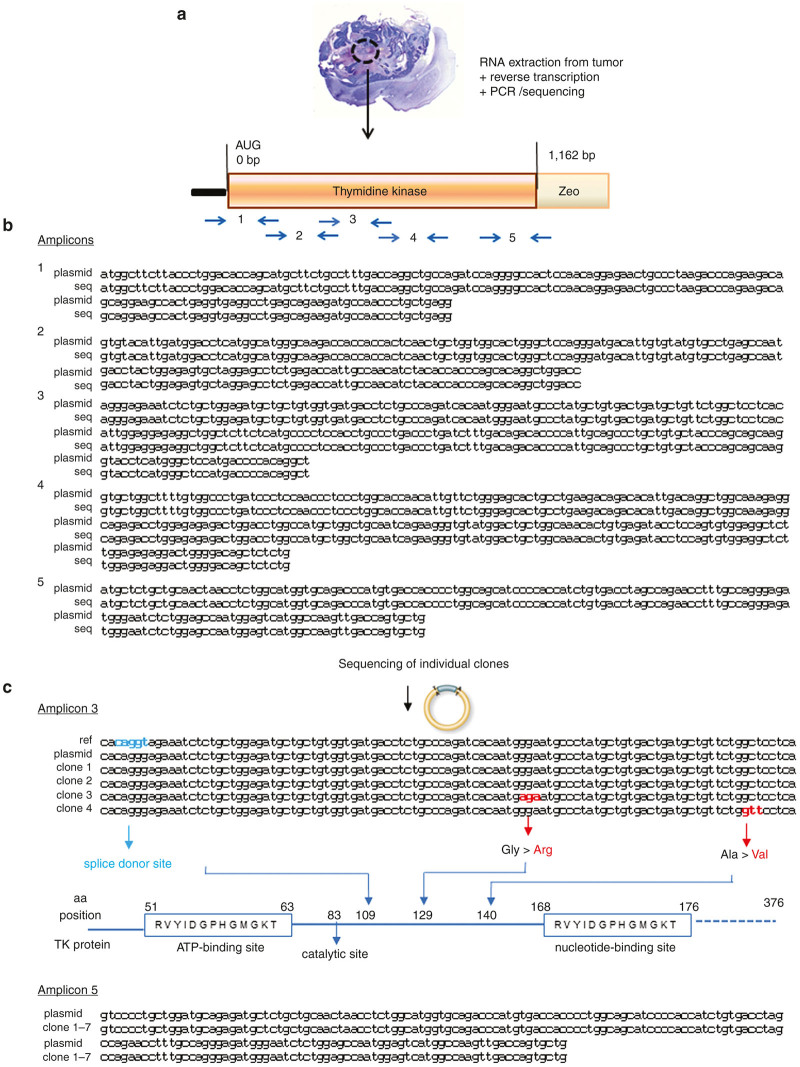
Sequencing of HSV-TK in pluripotent stem cell-derived tumors subjected to late ganciclovir treatment. Two weeks ganciclovir treatment was initiated 4 weeks after intrastriatal transplantation of pluripotent stem cells and mice were sacrificed 4 weeks after termination of treatment (as described in Figure 6). (a) DNA was extracted from paraformalgehyde-fixed, paraffin-embedded tumor samples and amplified using the indicated polymerase chain reaction (PCR) primers. (b) Direct sequencing of plasmid DNA and amplified sequences from tumor samples did not detect any mutations. (c) Sequencing of HSV-TK subclones from amplicon 3 and 5 (derived from PCR reactions described for panel b). amplicon 3: plasmid DNA used for cell transduction, as well as tumor-derived cDNA clones did not contain the splice donor site found in the HSV-TK reference sequence. However, cDNA clones 3 and 4 showed coding non-synonymous mutations. amplicon5: none of the cDNA clones showed any mutations.
The all over sequence found in the tumors was identical to the plasmid HSV-TK sequence, suggesting that—if mutations had occurred—they were not representing the majority of HSV-TK sequences within the tumor. We also directly inspected the sequencing results and did not observe evidence for ambivalent sequencing signals.
The cryptic splice sites described previously were not present in our version of the sequence (neither in the plasmid sequence nor in the tumor-derived sequences)
Accordingly no splice variant was detected in our sequencing results.
To investigate whether minority sequences with mutations had occurred within the tumor, we also subcloned two of the PCR amplicons and sequenced individual subclones. No mutation was observed in subclones of amplicon 5; in contrast, in two of the sublclones of amplicon 3, there was a single point mutation (Figure 7c) not located at critical sites of the TK enzyme (ATP and nucleotide-binding sites)17 (http://www.uniprot.org/uniprot/Q9QNF7).
Transplantation of TK-NPC produced mature neurons which were not sensitive to ganciclovir treatment
The in vivo results, shown so far, were obtained with transplantation of undifferentiated pluripotent stem cells. However, the final goal of neuronal cell therapy is the transplantation of neural precursor cells, which should differentiated into mature neurons and resist to ganciclovir treatment. We therefore transplanted 2–3 weeks-NPC containing neurospheres (Figure 3c and Figure 8). Under these conditions, no tumor formation was observed, even in the absence of ganciclovir treatment (Figure 8a). In line with these observations, the transplanted cells (assessed by immunofluorescence 7 weeks after transplantation) were abundantly positive for beta 3 tubulin (Figure 8b, upper panel), while a smaller fraction of cells was TH-positive (Figure 8b, lower panel). None of these markers were affected by ganciclovir treatment.
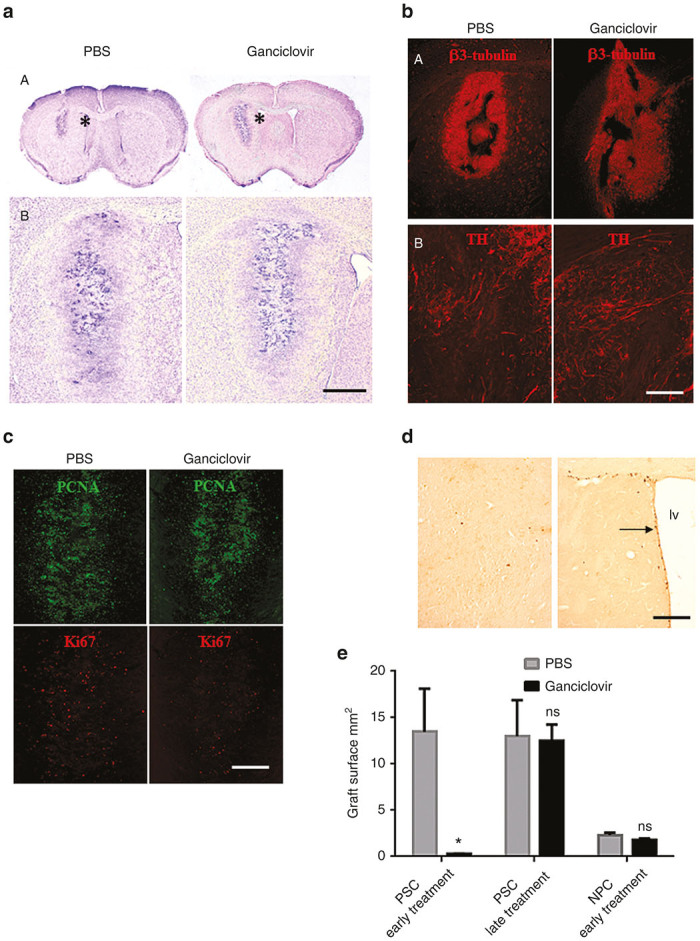
Graft development after transplantation of neural precursor cells (NPC). Mice were transplanted with NPC and treated with PBS or ganciclovir on days 7–22 following transplantation. Mice were sacrificed 4 weeks after the termination of ganciclovir treatment and brains were analyzed. There was no tumor formation, but the grafts had developed into a tissue integrated into the mouse brain. We observed no difference between PBS and ganciclovir treatment (a) Cresyl violet coloration of brain coronal section at the level of the striatum. Transplants were positive for beta 3-tubulin-immunoreactivity (B upper panel), and for TH-immunoreactivity (b lower panel). (c) In the graft many cells were PCNA-immunoreactive, but fewer were Ki67-immunoreactive. (d) BrdU-labeled cells were detected in the proliferating peri-ventricular zone (right image) but not in the graft (left image). (e) Size of graft under different experimental conditions in the absence (grey histogram) or presence (black histogram) of ganciclovir. Histograms on the left and middle show transplants after injection of pluripotent stem cells (PSC) treated for 2 weeks with ganciclovir 5 days (early treatment) or 30 days after cell transplantation (late treatment). Histograms on the right show transplants after injection of NPC treated with ganciclovir, 5 days after transplantation. Error bars = mean ± SEM, n = 3 to 5 in each group of mice, ns = not significant, * P = 0.0286 in Mann–Whitney test. Abbreviation: lv, lateral ventricle. Scale bar: a A = 2mm, a B= 700 µm, b A = 700 µm, b B = 140 µm, c = 350 µm, d = 110 µm.
We next investigated markers of cellular proliferation by staining of the transplants with antibodies against proliferating cell nuclear antigen (PCNA) and Ki67 (Figure 8c). Surprisingly, many of the transplanted cells were PCNA positive, while Ki67 staining of the transplants was low to absent. Ganciclovir treatment did not alter this pattern (Figure 8c, left panels, control and right panels, ganciclovir treatment). To verify whether the PCNA-positive, Ki67-negative cells were proliferating cells, we performed BrdU experiments (see Materials and Methods). In region of the transplant, no BrdU (5 bromo-2’-deoxyuridine)-labeled cells were observed (Figure 8D, left panel). As an internal positive control, we used the periventricular region, which was clearly BrdU-positive (Figure 8d, right panel). This demonstrates that the absence of Ki67 correctly indicates postmitotic cells, while PCNA expression may persist even after proliferation arrest.
To compare tumor formation and response to ganciclovir under our different experimental conditions, we quantified graft surfaces (Figure 8e). When PSC were transplanted, large tumors (graft surface ~10–15 mm2) develop, which was completely prevented by early ganciclovir treatment, while late ganciclovir treatment did not have any effect on tumor size. Finally, upon transplantation of NPC (derived from 2–3 weeks old neurospheres), small nontumoral transplants (graft surface ~2–3
mm2) develop, which was completely prevented by early ganciclovir treatment, while late ganciclovir treatment did not have any effect on tumor size. Finally, upon transplantation of NPC (derived from 2–3 weeks old neurospheres), small nontumoral transplants (graft surface ~2–3 mm2) were observed. The size of these transplants was not affected by ganciclovir.
mm2) were observed. The size of these transplants was not affected by ganciclovir.
Discussion
In this study, we have developed a novel tool that allows in vivo to remove proliferating, potentially tumorigenic cells from neural transplants. The system is based on the expression of HSV-TK under the control of the cell cycle-dependent Ki67 promoter. It will be particularly useful for the transplantation of pluripotent stem cell-derived neurons. Indeed, HSV-TK expressing cells can be eliminated by treatment of patients with the clinically used, CNS (Central Nervous System)-permeant drug ganciclovir. As the Ki67 promoter will be inactive in mature neurons, treatment with ganciclovir will only eliminate proliferating precursors, but preserve the integrity of differentiated postmitotic neurons. Our system provides a switch-off in suicide gene activity when cells progress from precursors to mature postmitotic neurons and we will refer to it as “safety switch technology”.
We chose HSV-TK as a suicide gene because the clinically used compound ganciclovir has a well-documented penetration into the central nervous system and efficiently eliminates HSV-TK expressing cells. Ganciclovir is clinically used as a treatment for viruses of the herpes family, in particular cytomegalovirus CMV. Interestingly, the drug used for the treatment of Herpes simplex virus, acyclovir was—at relevant concentrations—not efficient to induce suicide gene activity. The reason for this difference between the two nucleotide analogues seems to be a more efficient phosphorylation of ganciclovir as compared to acyclovir.18,19 The following alternatives for HSV-TK as an in-vivo suicide gene exist:
(i) Inducible caspase-9, which can be activated by the small molecule AP1903, would be an interesting alternative to HSV-TK as a suicide gene. We have not used this system because the penetration of AP1903 into the central nervous system has not been documented and there is only limited clinical experience with the compound.20,21
(ii) Cytosine deaminase can be used as a suicide gene in combination with 5-fluorocytine as prodrug. To our knowledge 5-fluorocytine has not been used in patients and hence has a limited translational potential. Also, cytosine deaminase has a poor binding affinity towards 5-FC compared to the natural substrate cytosine, unless a mutated form of the enzyme is used.22
(iii) Selective elimination of PSC by an oleate synthesis inhibitor discovered in a high-throughput screen23; however these compounds have so far been given only pretransplantation and most likely act only on cells in the pluripotency stage and not on proliferating neuroprecursors.
(iv) A recent study has suggested that irradiation of transplanted cells could be an alternative to a suicide gene technology to achieve elimination of proliferating cells. Given the potential toxicity of CNS irradiation, we do not think that this approach is the most promising for future application in patients.24
Our construct was designed to avoid expression of HSV-TK in postmitotic neurons, while rendering proliferative cells (i.e., PSC and proliferating neuroprecursors) sensitive to ganciclovir. Given the mechanisms of action of ganciclovir, namely strain-breaks in nascent DNA molecules, one might wonder whether it is important to avoid expression of HSV-TK in mature, nondividing neurons. The answer is yes, for two reasons:
In postmitotic neurons, renewal of mitochondria requires mitochondrial DNA synthesis. Thus, ganciclovir (in the presence of TK), will damage nascent mitochondrial DNA and thereby lead to cell death through apoptosis25,26
Ubiquitous expression of the viral HSV-TK protein has the potential to lead to immune rejection27 of transplanted neurons.
In initial experiments, we attempted to transplant postmitotic neurons, however, cell survival was poor. In contrast, when NPC, derived from 2–3-week-old neurospheres, were transplanted, cell survival was observed. We were worried because fractions of these NPC still express Ki67 (Figure 2a). Interestingly, despite this Ki67 expression in the injected cell material, no tumor was observed, and there was no difference in implant size between ganciclovir- and control-treated animals. Thus, upon transplantation, these NPC rapidly differentiate into postmitotic neurons. However, translation to human therapy requires a much higher number of cells, and thus implicitly will lead to a higher tumor risk. For example, for Parkinson’s therapy, the following number of neurons will need to be transplanted for different species: mice 105; rats 4 ×
× 105; rhesus monkeys 6
105; rhesus monkeys 6 ×
× 106 and by extrapolation 3
106 and by extrapolation 3 ×
× 107 in humans. Thus, approximately 300 times more cells need to be transplanted into humans, as compared to mice, which will lead to a proportional (i.e., 300-fold) increase in poorly differentiated, tumorigenic cells. Note that a priori there is no difference between embryonic and induced pluripotent stem cells with respect to tumor risk.
107 in humans. Thus, approximately 300 times more cells need to be transplanted into humans, as compared to mice, which will lead to a proportional (i.e., 300-fold) increase in poorly differentiated, tumorigenic cells. Note that a priori there is no difference between embryonic and induced pluripotent stem cells with respect to tumor risk.
As expected, transplantation of undifferentiated pluripotent stem cells led to tumor formation. This tumor formation was entirely abolished by early (e.g., 5 days post-transplantation) ganciclovir treatment of transplanted animals. However, when ganciclovir treatment was initiated at a later time point (30 days post-transplantation), the treatment had strictly no effect on tumor size. This is in line with several previous observations that established TK-expressing tumors do not respond to ganciclovir treatment. Several reasons might account for these observations:
(i) In some studies, TK was expressed under the control of pluripotency-associated promoters (e.g., Oct4), which are downregulated much faster than cell proliferation antigens. Thus, these tumors might consist of proliferating cells that have had lost the Oct4 promoter activity and therefore did not respond to ganciclovir.10,12
(ii) One study15 suggested that a cryptic splice site in the HSV-TK sequence leads to the formation of a nonfunctional TK, which could account for ganciclovir resistance. This was not the case in our experiments, as we used a HSV-TK sequence devoid of these splice sites (Figure 7).
(iii) Another study suggested that upon ganciclovir treatment of HSV-TK-expressing tumors, there is a selection of HSV-TK point mutations and this mechanism could account for ganciclovir resistance.16 We have experimentally verified this possibility and indeed observed several HSV-TK clones with point mutations. Interestingly, these point mutations were located in a strategically relevant area of the TK enzyme (e.g., between the catalytic site and the nucleotide binding site). In contrast, none of the clones of the region coding for the HSV-TK C-terminus showed point mutations. Thus, it is possible that—in the presence of a large tumor mass—ganciclovir selects certain point mutations. However, we do not think that this is the main mechanism of resistance. Indeed, the direct sequencing of PCR products without subcloning showed 100% identity with the plasmid sequence without any ambiguous nucleotides in the sequence.
(iv) We therefore think that the most likely explanation for the resistance of established tumors to ganciclovir treatment is tumor size. In order to be effective against solid tumors, drugs must penetrate to the inside of the tumor and reach concentrations sufficient to exert a therapeutic effect.28,29 In our studies, we observed a certain extent of tumor vascularization (Figure 6b,E). However, there is evidence that a poor organization of blood vessels suffices to render tumors resistant to chemotherapy.28,29
The big advantage of our safety switch technology is the potential to induce suicide gene activity systematically in all patients after transplantation. However in order to allow dedicated neural precursors to differentiate into postmitotic neurons, a time window between transplantation and initiation of ganciclovir treatment needs to be respected. We chose a time window of 5 days based on in-vitro experiments showing the arrest of proliferation of differentiating NPCs within this time (Figure 2c). Experimental in vivo verification confirmed the choice of this time window, as it allowed establishment of mature, ganciclovir resistant neurons when dedicated neural precursors were transplanted (Figure 8), while it allowed a complete elimination of tumor-forming PSC.
We describe here a “safety switch technology”, which allows elimination of proliferating cells shortly after transplantation of PSC-derived neuronal precursors. The essential elements behind this system are the control of HSV-TK expression by the Ki67 promoter, which allows selectively, through treatment with the HSV-TK substrate ganciclovir, killing of proliferating cells, while preserving the integrity of mature neurons. Treatment needs to be installed early (~5 days post-transplantation) because of treatment resistance of established tumors. We think that the safety switch technology should be systematically applied in human neural cell transplantation to avoid risk of tumor formation. It is likely that our technology might also be useful for the transplantation of other cells where final transplant will be postmitotic cells (e.g., cardiomyocytes, islet cells, etc.).
Materials and Methods
Culture of undifferentiated pluripotent stem cells
Human ES cell line HS415 (used from passage 17 to 30, kindly provided by Outi Hovatta, Karolinska Institute, Stockholm, Sweden) was cultured onto extracellular matrix (Matrigel, dilution 1/100, Invitrogen, Carlsbad, CA, USA) in a feeder-free culture medium (Nutristem, Biological Industries, Cromwell, CT). Medium was changed one another day to maintain pluripotency. Cells were passaged with enzymatic procedure (Accutase; Invitrogen) and replated with Rho-associated protein kinase (ROCK) inhibitor (10 μmol/l Y-27632;Axon medchem, Groningen, Netherlands) during 24 hours before removal.
Lentiviral vector construction, cell transduction, and selection
Construction of plasmids and lentiviral vectors (Figure 1a): the final lentivector plasmid was generated by a LR Clonase II (Invitrogen)-mediated recombination of a pENTR plasmid containing the Ki67 promoter (pENTR-L4-Ki67-L1R), a pENTR plasmid containing the fusion gene TK/Sh, corresponding to the thymidine kinase (TK) gene from Herpes simplex virus type 1 (HSV1) and the Sh ble gene conferring zeocin resistance, and a pCLX-R4-DEST-R2 lentivector destination cassette.
Lentiviral vector production and titration: lentiviral: vector stocks were generated using transient transfection of HEK 293T cells with the specific lentivector transfer plasmid, the psPAX2 plasmid encoding gag/pol and the pCAG-VSVG envelope plasmid. Lentivector titer was performed using transduction of HT-1080 cells followed by flow cytometry quantification of Green Fluorescent Protein (GFP)-positive cells 5 days after infection. Cell culture: HEK 293T and HT-1080 cells were cultured in high-glucose Dulbecco’s modified eagle medium (Sigma, Saint-Louis, MO) supplemented with 10% fetal calf serum, 1% Penicillin, 1% Streptomycin, and 1% l-glutamine. Transduction of HS415 cell line: 1 up to 5 copies of the lentiviral vector were introduced. HS415 cells were cultured for 5 days before zeocin selection at 2 µmol/l (corresponding to ~200 µg/ml, a dose response curve has been made before on wild-type HS415 line with different zeocin concentration) during 10 days.
Generation of neurospheres containing dopaminergic neuron precursors
NPC was generated as neurospheres and contain dopaminergic precursors (for details, see our previous paper30). Briefly, neural midbrain orientation is performed during the first week followed by 2 additional weeks of maturation to obtain 2- or 3-week-old NPC (Figures 1b and and3a3a).
Generation of mature neurons from NPC in two-dimensional culture (2D)
For differentiation in 2D, 2–3-week-old neurospheres were dissociated with Accutase (Invitrogen) and replated on polyornithine -(15 μg/ml; Sigma) and laminin- (2 μg/cm2; R&D System) coated cover slips (diameter = 0.8 cm) in 24-well plates at 200,000 cells/cm2 in maturation medium (neurobasal+b27 supplement + growth factor + compound E, Invitrogen), details in our previous paper30 for 1 week before analysis by immunofluorescence staining (Figure 1b)
cm) in 24-well plates at 200,000 cells/cm2 in maturation medium (neurobasal+b27 supplement + growth factor + compound E, Invitrogen), details in our previous paper30 for 1 week before analysis by immunofluorescence staining (Figure 1b)
Immunofluorescence staining of fixed cells from 2D culture
After 1 week of culture, 2D cultures on cover slips were fixed in 4% paraformalgehyde (PFA) for 10 minutes at room temperature, washed and processed for conventional immunocytochemistry. Primary antibodies were incubated in phosphate buffer saline (PBS) + 0.1% triton ×100 at 4 °C o/n, and were against TH for tyrosin hydroxylase (rabbit polyclonal 1/500, Merck Millipore, Darmstadt, Germany), Ki67 (mouse 1/100, Chemicon, Merck Millipore, Damstadt, Germany), HSV-TK (mouse monoclonal 1/100, Gentaur, Kampenhout, Belgium). Detection of primary antibodies was performed using appropriate species-specific Alexa 488- or Alexa 555-labeled secondary antibodies, 1 hour at room temperature in the dark. Controls included examination of the cell or tissue autofluorescence and omission of the first antibody. Cell nuclei were stained with 4’-6-diamidino-2-phenylindole (DAPI). Cell cultures were mounted in Fluorosave (Calbiochem, Merck Millipore) and observed with an Axioscop 2 plus microscope equipped with appropriate filters, Axiocam color camera, and Axiovision software (Leitz).
Flow cytometry
Undifferentiated ES cells were enzymatically detached as single cells from matrix (Accutase, Invitrogen), washed with PBS before a 10 min fixation step in 4% PFA at room temperature. One or 2–3-week-old neurospheres were dissociated as single cells (Accutase, Invitrogen). To detect intracellular antigens, cells were permeabilized in 1× perm/wash buffer (BD bioscience, Franklin, NJ) for10 minutes, before 30 minutes incubation in the dark at room temperature with different antibodies (PE Nanog and PerCp-Cy 5.5 Oct ¾, Life technology, rabbit FITC-Ki67, Abcam, Cambridge, UK). After washing, cells were immediately run or stored at 4 °C for 24 hours maximum. For each sample run, 10,000 events were recorded and analyzed. Flow cytometry acquisition was performed using FacsCanto I equipment with 488 and 633 lasers (BD bioscience) and data analysis by Flowjo software.
min fixation step in 4% PFA at room temperature. One or 2–3-week-old neurospheres were dissociated as single cells (Accutase, Invitrogen). To detect intracellular antigens, cells were permeabilized in 1× perm/wash buffer (BD bioscience, Franklin, NJ) for10 minutes, before 30 minutes incubation in the dark at room temperature with different antibodies (PE Nanog and PerCp-Cy 5.5 Oct ¾, Life technology, rabbit FITC-Ki67, Abcam, Cambridge, UK). After washing, cells were immediately run or stored at 4 °C for 24 hours maximum. For each sample run, 10,000 events were recorded and analyzed. Flow cytometry acquisition was performed using FacsCanto I equipment with 488 and 633 lasers (BD bioscience) and data analysis by Flowjo software.
Cell proliferation measurement by calcein assay
Cells were plated onto a 96 wells precoated with matrigel for PSC (1/100, invitrogen) or polyornithine/laminine for NPC and cultured in PSC medium (Nutristem) or maturation medium. Respectively, 1,000 cells for PSC, 5,000 cells for 1 week NPC and 30,000 cells for 2–3-week NPC. Ganciclovir (Cymevene diluted in PBS, Roche, Bâle, Switzerland) was added the day after cell plating. Calcein (2 µmol/l in PBS) was added onto cells for 1 hour before microplate reading and at different time course of ganciclovir treatment (24 to 96 hours). Cell survival was measured by calcein integration (wavelength excitation at 495 nm, acquisition data at 515
nm, acquisition data at 515 nm, on microplate reader, flexstation 3, Molecular device, Sunnyvale, CA).
nm, on microplate reader, flexstation 3, Molecular device, Sunnyvale, CA).
Stereotaxic engraftment and ganciclovir treatment
Experiments on animal are compliant to local ethical committee guidelines. Undifferentiated PSC (20,000 cells/μl) and dissociated neurospheres, 2- or 3-week-old NPC (100,000 cells/μl) were injected in the striatum of anesthetized mice (isoflurane) using a 27-G Hamilton syringe. Injection coordinates for striatum were: bregma = 0.6 mm, mediolateral = 2
mm, mediolateral = 2 mm, dorsoventral = 3
mm, dorsoventral = 3 mm. In each case, we used n = 6 animals, Mice were euthanized 1 month after ganciclovir treatment termination (40
mm. In each case, we used n = 6 animals, Mice were euthanized 1 month after ganciclovir treatment termination (40 mg/kg, 5 consecutive days a week during 2 weeks).
mg/kg, 5 consecutive days a week during 2 weeks).
Morphological and phenotypic analysis of injected surviving cells
Anesthetized mice were fixed by intracardiac perfusion of 4% PFA in PBS. Paraffin embedded brains were sectioned (coronal sections 10 µm thick) and processed either for cresyl violet staining aiming morphological assessment of the graft or for immunohistochemistry. Primary antibody was incubated at 4 °C O/N in agitation in PBS + 0.3% Triton X. Detection was performed using Alexa 488 or 555-labeled secondary antibody (room temperature for 1 hour). The following primary antibodies were used: nestin (rabbit polyclonal anti-human 1/400, Chemicon), β3-tubulin (mouse monoclonal from Sigma or rabbit polyclonal 1/2000, Covance, Biolegend, Sandiego, CA), PCNA (mouse monoclonal 1/100, Dako, Agilent Technologies, Santa Clara, CA), Tyrosin Hydroxylase (TH, rabbit polyclonal 1/500, Millipore), Iba 1, for ionizing calcium-binding adaptor molecule 1, specifically expressed in microglia (polyclonal rabbit 1/500, Wako Laboratory Chemicals, Cape Charles, VA), HSV-TK (mouse monoclonal1/100, Gentaur), Ki67 (monoclonal rabbit 1/100, Abcam, or mouse, 1/100, Chemicon). CD31 for endothelial cells (1/40, Dako), Oct 3/4 (1/1000, Chemicon). Controls included examination of the cell or tissue auto-fluorescence and omission of the first antibody. Cell nuclei were stained with DAPI. Sections were mounted in Fluorosave (Calbiochem) or Eukitt (Kindler GmbH, Germany) and observed under microscope (see above)
Brdu labeling
BrdU (100 mg/kg, Millipore) was injected i.p. twice daily for 3 consecutive days. Mice were sacrificed 5 days later and perfused, through the heart, with PBS and 4% PFA in PBS. Brain and small intestin were embedded in paraffin and 10
mg/kg, Millipore) was injected i.p. twice daily for 3 consecutive days. Mice were sacrificed 5 days later and perfused, through the heart, with PBS and 4% PFA in PBS. Brain and small intestin were embedded in paraffin and 10 mm thick coronal section were processed for BrdU immunohistochemical detection using a BrdU immunohistochemistry kit (Chemicon, Cat N° 2760), small intestine sections from the manufacturer serving as control.
mm thick coronal section were processed for BrdU immunohistochemical detection using a BrdU immunohistochemistry kit (Chemicon, Cat N° 2760), small intestine sections from the manufacturer serving as control.
Thymidine kinase sequencing
Total RNA was extracted and purified according the manufacturers’ instructions (high pure FFPE RNA micro kit, Roche) from PFA-fixed, paraffin-embedded tissue delimited to tumor area induced by PSC transplantation and late ganciclovir treatment (Figure 7). Typically, RNA isolated from formalin-fixed tissue is fragmented and the bulk of RNA obtained is around 200 bases in length. After DNase treatment, CDNA synthesis is performed from RNA pool extracted (Takara Bio USA, Mountain View, CA). Thymidine kinase gene expressed inside the tumor was amplified by using a set of different overlapping primers along the thymidine sequence (1162 bp). PCR reaction is performed with a proofreading taq polymerase (Q5 high fidelity, NEB) during 35 cycles with 30” at 95 °C, 30”at 58 °C and 1 minute at 72 °C. Primers for fragment 1 are for forward F 5′-GAG CGGTGGTTCGACAAGTGG-3′ and reverse R 5′-CCTCAGCAGGGTTGGCATC-3′, fragment 2, F 5′-GATGCCAACCCTGCTGAGG-3′ and R 5′-GTCCAGCCTGTGCTGGGTG-3′, fragment 3, F 5′-CACCCAGCACAGGCTGGAC-3′ and R 5′-CAGGGCCACAAAAGCCAGCAC-3′, fragment 4, F 5′-GTGCTGGCTTTTGTGGCCCTG-3′ and R 5′-CCAGAGAGCTGTCCCCAGTC-3′, fragment 5, F 5′-GTCCCCTGCTGGATGCAGAG-3′ and R 5′-CTC TGC ATCCAGCAGGGGAC-3′. Fragment 1 to 5 were directly sequenced or subcloned into topo TA cloning kit (Invitrogen) before performing sequencing of the different bacterial clones (Microsynth, AG, Balgach, Switzerland).
bp). PCR reaction is performed with a proofreading taq polymerase (Q5 high fidelity, NEB) during 35 cycles with 30” at 95 °C, 30”at 58 °C and 1 minute at 72 °C. Primers for fragment 1 are for forward F 5′-GAG CGGTGGTTCGACAAGTGG-3′ and reverse R 5′-CCTCAGCAGGGTTGGCATC-3′, fragment 2, F 5′-GATGCCAACCCTGCTGAGG-3′ and R 5′-GTCCAGCCTGTGCTGGGTG-3′, fragment 3, F 5′-CACCCAGCACAGGCTGGAC-3′ and R 5′-CAGGGCCACAAAAGCCAGCAC-3′, fragment 4, F 5′-GTGCTGGCTTTTGTGGCCCTG-3′ and R 5′-CCAGAGAGCTGTCCCCAGTC-3′, fragment 5, F 5′-GTCCCCTGCTGGATGCAGAG-3′ and R 5′-CTC TGC ATCCAGCAGGGGAC-3′. Fragment 1 to 5 were directly sequenced or subcloned into topo TA cloning kit (Invitrogen) before performing sequencing of the different bacterial clones (Microsynth, AG, Balgach, Switzerland).
Author Contributions
V.T. conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing. O.C.: Conception and design lentiviral vector, data analysis, and interpretation. E.G.: Technical assistance for transplantation. A.Z.: Conception and designed the plasmid containing the fragment of Ki67 promoter. D.C.: Design and performed in vitro test for ganciclovir sensitivity. P.S.: Provided technical support for lentiviral construction. M.D.D.: Conception and design mice transplantation and immunohistochemistry analysis, manuscript writing. K.H.K.: Conception and design, financial support, administrative support, data analysis and interpretation, final approval of manuscript.
Acknowledgments
This work was supported by Clayton foundation for research (Houston, Texas, USA), Carigest SA, (Geneva, Switzerland), Hospital cantonal of Geneva (HUG), department of medicine genetic and laboratory. We would like to thank Liza Ho and Catherine Metral (HUG) for their technical assistance (RNA extraction of PFA-fixed, paraffin-embedded tissue). We declare that we have no conflict of interest.
Notes
The last two authors contributed equally to this work.
References
- Tabar, V and Studer, L (2014). Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet 15: 82–92. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee, AS, Tang, C, Rao, MS, Weissman, IL and Wu, JC (2013). Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med 19: 998–1004. [Europe PMC free article] [Abstract] [Google Scholar]
- Roy, NS, Cleren, C, Singh, SK, Yang, L, Beal, MF and Goldman, SA (2006). Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med 12: 1259–1268. [Abstract] [Google Scholar]
- Amariglio, N, Hirshberg, A, Scheithauer, BW, Cohen, Y, Loewenthal, R, Trakhtenbrot, L et al. (2009). Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 6: e1000029. [Abstract] [Google Scholar]
- Fillat, C, Carrió, M, Cascante, A and Sangro, B (2003). Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase gene/Ganciclovir system: fifteen years of application. Curr Gene Ther 3: 13–26. [Abstract] [Google Scholar]
- Jones, BS, Lamb, LS, Goldman, F and Di Stasi, A (2014). Improving the safety of cell therapy products by suicide gene transfer. Front Pharmacol 5: 254. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu, K, Zhu, F, Du, B, Gao, F, Cheng, H and Pan, X (2008). Prophylaxis of graft-versus-host disease by lentiviral-mediated expression of herpes simplex virus-thymidine kinase and ganciclovir treatment. Transplant Proc 40: 2665–2669. [Abstract] [Google Scholar]
- Mastaglio, S, Stanghellini, MT, Bordignon, C, Bondanza, A, Ciceri, F and Bonini, C (2010). Progress and prospects: graft-versus-host disease. Gene Ther 17: 1309–1317. [Abstract] [Google Scholar]
- Contassot, E, Ferrand, C, Angonin, R, Cohen, JL, de Carvalho Bittencourt, M, Lorchel, F et al. (2000). Ganciclovir-sensitive acute graft-versus-host disease in mice receiving herpes simplex virus-thymidine kinase-expressing donor T cells in a bone marrow transplantation setting. Transplantation 69: 503–508. [Abstract] [Google Scholar]
- Hara, A, Aoki, H, Taguchi, A, Niwa, M, Yamada, Y, Kunisada, T et al. (2008). Neuron-like differentiation and selective ablation of undifferentiated embryonic stem cells containing suicide gene with Oct-4 promoter. Stem Cells Dev 17: 619–627. [Abstract] [Google Scholar]
- Menzel, O, Birraux, J, Wildhaber, BE, Jond, C, Lasne, F, Habre, W et al. (2009). Biosafety in ex vivo gene therapy and conditional ablation of lentivirally transduced hepatocytes in nonhuman primates. Mol Ther 17: 1754–1760. [Europe PMC free article] [Abstract] [Google Scholar]
- Lim, TT, Geisen, C, Hesse, M, Fleischmann, BK, Zimmermann, K and Pfeifer, A (2013). Lentiviral vector mediated thymidine kinase expression in pluripotent stem cells enables removal of tumorigenic cells. PLoS One 8: e70543. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen, F, Cai, B, Gao, Y, Yuan, X, Cheng, F, Wang, T et al. (2013). Suicide gene-mediated ablation of tumor-initiating mouse pluripotent stem cells. Biomaterials 34: 1701–1711. [Abstract] [Google Scholar]
- Zambon, AC (2010). Use of the Ki67 promoter to label cell cycle entry in living cells. Cytometry A 77: 564–570. [Europe PMC free article] [Abstract] [Google Scholar]
- Chalmers, D, Ferrand, C, Apperley, JF, Melo, JV, Ebeling, S, Newton, I et al. (2001). Elimination of the truncated message from the herpes simplex virus thymidine kinase suicide gene. Mol Ther 4: 146–148. [Abstract] [Google Scholar]
- Kotini, AG, de Stanchina, E, Themeli, M, Sadelain, M and Papapetrou, EP (2016). Escape mutations, ganciclovir resistance, and teratoma formation in human iPSCs expressing an HSVtk suicide gene. Mol Ther Nucleic Acids 5: e284. [Europe PMC free article] [Abstract] [Google Scholar]
- Andrei, G, Balzarini, J, Fiten, P, De Clercq, E, Opdenakker, G and Snoeck, R (2005). Characterization of herpes simplex virus type 1 thymidine kinase mutants selected under a single round of high-dose brivudin. J Virol 79: 5863–5869. [Europe PMC free article] [Abstract] [Google Scholar]
- Zimmermann, A, Michel, D, Pavić, I, Hampl, W, Lüske, A, Neyts, J et al. (1997). Phosphorylation of aciclovir, ganciclovir, penciclovir and S2242 by the cytomegalovirus UL97 protein: a quantitative analysis using recombinant vaccinia viruses. Antiviral Res 36: 35–42. [Abstract] [Google Scholar]
- Kokoris, MS and Black, ME (2002). Characterization of herpes simplex virus type 1 thymidine kinase mutants engineered for improved ganciclovir or acyclovir activity. Protein Sci 11: 2267–2272. [Europe PMC free article] [Abstract] [Google Scholar]
- Di Stasi, A, Tey, SK, Dotti, G, Fujita, Y, Kennedy-Nasser, A, Martinez, C et al. (2011). Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 365: 1673–1683. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu, C, Hong, SG, Winkler, T, Spencer, DM, Jares, A, Ichwan, B et al. (2014). Development of an inducible caspase-9 safety switch for pluripotent stem cell-based therapies. Mol Ther Methods Clin Dev 1: 14053. [Europe PMC free article] [Abstract] [Google Scholar]
- Raza, A, Kohila, V and Ghosh, SS (2015). Redesigned Escherichia coli cytosine deaminase: a new facet of suicide gene therapy. J Gene Med 17: 132–139. [Abstract] [Google Scholar]
- Ben-David, U, Gan, QF, Golan-Lev, T, Arora, P, Yanuka, O, Oren, YS et al. (2013). Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell 12: 167–179. [Abstract] [Google Scholar]
- Katsukawa, M, Nakajima, Y, Fukumoto, A, Doi, D and Takahashi, J (2016). Fail-safe therapy by gamma-ray irradiation against tumor formation by human-induced pluripotent stem cell-derived neural progenitors. Stem Cells Dev 25: 815–825. [Abstract] [Google Scholar]
- Beltinger, C, Fulda, S, Kammertoens, T, Uckert, W and Debatin, KM (2000). Mitochondrial amplification of death signals determines thymidine kinase/ganciclovir-triggered activation of apoptosis. Cancer Res 60: 3212–3217. [Abstract] [Google Scholar]
- Laberge, RM, Adler, D, DeMaria, M, Mechtouf, N, Teachenor, R, Cardin, GB et al. (2013). Mitochondrial DNA damage induces apoptosis in senescent cells. Cell Death Dis 4: e727. [Europe PMC free article] [Abstract] [Google Scholar]
- Traversari, C, Marktel, S, Magnani, Z, Mangia, P, Russo, V, Ciceri, F et al. (2007). The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood 109: 4708–4715. [Abstract] [Google Scholar]
- Minchinton, AI and Tannock, IF (2006). Drug penetration in solid tumours. Nat Rev Cancer 6: 583–592. [Abstract] [Google Scholar]
- Curnis, F, Sacchi, A and Corti, A (2002). Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest 110: 475–482. [Europe PMC free article] [Abstract] [Google Scholar]
- Tieng, V, Stoppini, L, Villy, S, Fathi, M, Dubois-Dauphin, M and Krause, KH (2014). Engineering of midbrain organoids containing long-lived dopaminergic neurons. Stem Cells Dev 23: 1535–1547. [Abstract] [Google Scholar]
Articles from Molecular Therapy. Methods & Clinical Development are provided here courtesy of American Society of Gene & Cell Therapy
Full text links
Read article at publisher's site: https://doi.org/10.1038/mtm.2016.69
Read article for free, from open access legal sources, via Unpaywall:
https://www.cell.com/article/S2329050116301966/pdf
Citations & impact
Impact metrics
Article citations
Directional induction of neural stem cells, a new therapy for neurodegenerative diseases and ischemic stroke.
Cell Death Discov, 9(1):215, 01 Jul 2023
Cited by: 8 articles | PMID: 37393356 | PMCID: PMC10314944
Review Free full text in Europe PMC
Neural Progenitor Cells Expressing Herpes Simplex Virus-Thymidine Kinase for Ablation Have Differential Chemosensitivity to Brivudine and Ganciclovir.
Front Cell Neurosci, 15:638021, 06 Dec 2021
Cited by: 0 articles | PMID: 34938162 | PMCID: PMC8685296
Optimization of Thymidine Kinase-Based Safety Switch for Neural Cell Therapy.
Cells, 11(3):502, 31 Jan 2022
Cited by: 1 article | PMID: 35159311 | PMCID: PMC8834506
Human stem cells harboring a suicide gene improve the safety and standardisation of neural transplants in Parkinsonian rats.
Nat Commun, 12(1):3275, 27 May 2021
Cited by: 15 articles | PMID: 34045451 | PMCID: PMC8160354
Identification of Autophagy-Related Genes as Targets for Senescence Induction Using a Customizable CRISPR-Based Suicide Switch Screen.
Mol Cancer Res, 19(10):1613-1621, 22 Jun 2021
Cited by: 5 articles | PMID: 34158393 | PMCID: PMC7611779
Go to all (12) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Genes & Proteins
- (1 citation) UniProt - Q9QNF7
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Optimization of Thymidine Kinase-Based Safety Switch for Neural Cell Therapy.
Cells, 11(3):502, 31 Jan 2022
Cited by: 1 article | PMID: 35159311 | PMCID: PMC8834506
Herpes simplex virus thymidine kinase/ganciclovir-mediated killing of tumor cell induces tumor-specific cytotoxic T cells in mice.
Cancer Gene Ther, 4(2):91-96, 01 Mar 1997
Cited by: 35 articles | PMID: 9080117
Increased Cytotoxicity of Herpes Simplex Virus Thymidine Kinase Expression in Human Induced Pluripotent Stem Cells.
Int J Mol Sci, 20(4):E810, 14 Feb 2019
Cited by: 10 articles | PMID: 30769780 | PMCID: PMC6413063
Neuron-like differentiation and selective ablation of undifferentiated embryonic stem cells containing suicide gene with Oct-4 promoter.
Stem Cells Dev, 17(4):619-627, 01 Aug 2008
Cited by: 27 articles | PMID: 18393636





