Abstract
Free full text

Using neuroimaging to individualize TMS treatment for depression: Toward a new paradigm for imaging-guided intervention
Abstract
The standard clinical technique for using repetitive transcranial magnetic stimulation (rTMS) for major depressive disorder (MDD) is associated with limited efficacy to date. Such limited efficacy may be due to reliance on scalp-based targeting rather than state-of-the-science methods which incorporate fMRI-guided neuronavigation based on a specific model of neurocircuit dysfunction. In this review, we examine such a specific model drawn from regulatory focus theory, which postulates two brain/behavior systems, the promotion and prevention systems, underlying goal pursuit. Individual differences in these systems have been shown to predict vulnerability to MDD as well as to comorbid generalized anxiety disorder (GAD). Activation of an individual’s promotion or prevention goals via priming leads to motivational and affective responses modulated by the individual’s appraisal of their progress in attaining the goal. In addition, priming promotion vs. prevention goals induces discriminable patterns of brain activation that are sensitive to the effects of depression and anxiety: MDD is associated with promotion system failure, anhedonic/dysphoric symptoms, and hypoactivation in specific regions in left prefrontal cortex, whereas GAD is associated with prevention system failure, hypervigilant/agitated symptoms, and hyperactivation in right prefrontal cortex (PFC). These left and right PFC locations can be directly targeted in an individualized manner for TMS. Additionally, this individually targeted rTMS can be integrated with cognitive interventions designed to activate the neural circuitry associated with promotion vs. prevention, thus allowing the neuroplasticity induced by the rTMS to benefit the systems likely to be involved in remediating depression. Targeted engagement of cortical systems involved in emotion regulation using individualized fMRI guidance may help increase the efficacy of rTMS in depression.
1. Current status of rTMS treatment for unipolar depression
Repetitive transcranial magnetic stimulation (rTMS) was approved by the FDA in 2008 for treatment-resistant unipolar major depression. However, despite its increasing use, typical effect sizes of rTMS treatment have been modest (Berlim et al., 2014; Lefaucheur et al., 2014), and both methodological and conceptual challenges remain regarding how to optimize its efficacy (Downar and Daskalakis, 2013; Daskalakis et al., 2008). This review considers two such challenges: specifically, targeting and context of stimulation. Rather than being targeted on specific brain regions functionally linked to depression on an individualized basis, rTMS is presently targeted by finding scalp locations which in general overlie brain regions which have been linked anatomically to depression in group-based analyses. We propose that refining rTMS via a systematic model of the functional neurocircuitry underlying depression, applying such a model to personalize the site of stimulation, and combining that stimulation with focused cognitive techniques targeting the brain circuits of interest is likely to improve its efficacy.
rTMS was first shown to be efficacious for the treatment of depression in the mid-1990s (George et al., 1995; and replicated: Pascual-Leone et al., 1996a, 1996b). Stimulation was applied at 20 Hz to left dorsolateral prefrontal cortex (DLPFC). This anatomical location was targeted because left prefrontal regions had shown decreased activation with depression in imaging studies, because patients with left prefrontal strokes were at increased risk for developing depression, and because left unilateral electroconvulsive therapy (ECT) was more effective than right (George et al., 1995). High frequency rTMS was chosen because it has the general property, at least when given at or above motor threshold, of increasing cortical excitability. In addition, it was postulated that repeated administration of high frequency stimulation would counteract the left prefrontal hypoactivation found with depressed patients (Kimbrell et al., 1999).
Given the initial success of the George et al. (1995) study, a number of similarly designed clinical trials followed in which their treatment paradigm was generally followed (George et al., 2010), with some exceptions, for example, using low frequency stimulation over right prefrontal cortex (e.g., Klein et al., 1999). This process of treatment development culminated in a successful industry-sponsored trial (O’Reardon et al., 2007), leading to FDA approval at the following parameters: 4 s trains of 10 Hz rTMS (26 s inter-train interval) to left PFC at 120% motor threshold intensity for 3000 pulses daily for 6 weeks. While a number of meta-analytic studies have concluded that rTMS has a significant anti-depressant effect in comparison to sham stimulation (e.g., Schutter, 2009; Slotema et al., 2010), the typical effect sizes have been modest. For example, the response and remission rates in the O’Reardon et al. (2007) trial were 25% and 16%. These rates are relatively disappointing, given typical remission rates of 65–75% using ECT (Sackeim et al., 2008). More recent studies have generally found modest response and remission rates as well: a recent meta-analysis of 29 studies (1371 patients) reported similar average rates (e.g., 29% average response rate) across studies (Berlim et al., 2014). Thus, while rTMS is clearly a promising treatment for unipolar depression, there remains significant work to be done in order to maximize its clinical utility.
2. Challenges in targeting of TMS for treating depression
One possible reason for the limited clinical response rates associated with TMS to date is non-optimized targeting (Downar and Daskalakis, 2013). The original method for determining the coil position used by George et al. (1995) was to find the site over motor cortex that evoked a maximal finger twitch, and then moving the coil to a point 5 cm anterior, with the 5 cm based on an estimation from the Talairach Atlas. This targeting system was built into the device used in the clinical trials leading up to FDA approval, and became part of the standard TMS treatment protocol for depression. In retrospect, the choice of this targeting method may not have been optimal, as it ignored variability due to head size, which is taken into account in neuroimaging methods such as the International 10/20 System for EEG. Indeed, it has been demonstrated using structural MRI that the original 5 cm rule in general often resulted in coil positions well short of DLPFC (Fitzgerald et al., 2009a), and that using structural MRIs to position the TMS coil over DLPFC resulted in response and remission rates of 42% and 30% respectively, compared with 18% and 11% using the 5 cm rule (Fitzgerald et al., 2009b).
Through the use of brain imaging, TMS targeting has begun to be refined from scalp-based methods to the use of neuronavigational systems which permit the targeting of individual cortical locations with potentially millimeter accuracy (Sparing et al., 2010). The combined use of MRI and neuronavigation allows a further step in efficacy of targeting TMS coils: moving from anatomical positioning to positioning based on functional imaging. In this case, sites of activation found in a single individual’s fMRI can be overlaid on his or her structural MRI, and targeted directly. Such neuronavigational approaches are, in some cases, translated from basic neuroscience research and represent an important frontier in the application of TMS to treatment of psychiatric disorders.
Sack et al. (2009) provided a demonstration of the dramatic increase in the efficacy of TMS in modulating cortical function as one proceeds from scalp-based systems through neuronavigation using structural MRIs, group fMRI, and individual fMRI. Previously, TMS applied to parietal cortex during a Stroop-like task caused changes in task performance (Kadosh et al., 2007). In Sack et al. (2009), TMS was targeted to parietal cortex by the different methods in four different groups of subjects, using a scalp-based system (10/20 coordinate P4), anatomical imagery (individual structural MRI), group-based functional imagery (a group-averaged site based on Talaraich coordinates), and individual functional imagery (peak parietal activation in individual fMRI images recorded during task performance). Based on the task performance of each group, a power analysis was used to estimate the number of subjects needed to achieve a TMS effect on task performance at a p < 0.05 significance level. It was found that only five subjects were needed to observe a statistically significant behavioral effect of TMS on the task when individual fMRIs were used for targeting, while double that number were required to see the same effect using structural MRIs or group fMRIs, and a total of 47 subjects were needed when the 10/20 system was used. The dramatic differences in the effects on statistical power in this experiment were solely due to differences in targeting strategy, specifically the availability of individual-level fMRI data from a relevant task.
As has now been demonstrated repeatedly in a variety of experimental contexts, imaging-guided TMS can target and engage specific functional brain networks with high resolution and with the highly desirable ability to take into account individual differences in location. We propose that a further refinement in targeting can be included to generate long-lasting changes in these specifically-engaged networks by adding a dynamic element: that is, by activating the network of interest (e.g., by having the subject perform a task requiring neural processing within the network) simultaneously with TMS stimulation.
The well-developed paradigm of paired-associate stimulation (PAS) provides an example of this principle in its simplest form. In standard PAS, co-activation of sensorimotor cortex with afferent stimulation of the median nerve in the wrist precisely timed to arrive as a TMS pulse is delivered to motor cortex has been shown to significantly enhance cortical response in subsequent testing (Ziemann et al., 2008). Similarly, it has been suggested that increasing cortical plasticity in targeted networks with TMS while simultaneously activating them with tasks involving processing specific to those networks could induce enhanced effects. Such a synergistic impact of TMS plus a behavioral task could increase network-specific plasticity via a Hebbian-like synaptic mechanism that follows the functional principle “fire together, wire together” (Ragert et al., 2003; Thickbroom, 2007).
Such an enhancement effect has been demonstrated, for example, in the use of multiple sessions of simultaneous rTMS and working memory task performance to remediate working memory deficits in sleep-deprived individuals, where memory performance showed continued enhancement a full day after the last rTMS session (Luber et al., 2013). In this study, 5 Hz rTMS was applied while subjects performed a working memory task during four sessions over the course of 48 hours of sleep deprivation. Twenty-seven subjects (13 active TMS, 14 sham) completed the protocol. Another 21 (10 active TMS, 11 sham) non-sleep deprived subjects participated as controls. At the end of the sleep deprivation period, the sleep-deprived subjects receiving sham rTMS exhibited degraded performance in the working memory task, with slowed RT and lapsing (i.e., non-responses in task trials) at a rate of 6.4 per block of trials. In contrast, those receiving active rTMS performed similarly to the non-sleep deprived controls, exhibiting a similar speeding of RT attributed to practice, and a reduced lapsing rate of 1.7. Importantly, the sleep deprived group receiving active TMS showed rTMS-induced facilitation of DMS performance a full 18 hours after the last rTMS session, long after the acute action of rTMS at the local site of stimulation wore off. In the pre- and post-sleep deprivation contrasts of fMRI recorded during working memory performance, multivariate covariance modeling revealed that the Active TMS sleep deprived group (but not the Sham group) had a significant increase in fMRI-derived activity in a cortical area directly beneath where the TMS coil had been positioned. In affecting neural circuitry involved in WM to ameliorate the impact of SD, this study thus united the ideas of using multiple sessions to create a cumulative effect with the method of simultaneous task and TMS activation of cortical neurons to generate Hebbian-like effects. Although it should be noted that the Luber et al. (2013) study used only four sessions of the combined rTMS/WM intervention, nonetheless it represents a proof-of-concept which helps to justify further exploration of the therapeutic potential of TMS in combination with targeted cognitive interventions.
These advances in TMS targeting and network engagement have the potential to significantly enhance the efficacy of TMS treatment of depression. However, to date little progress has been made to incorporate them into standard clinical practice. One reason for this delay, no doubt, is that there has been no consensus as to what specific brain circuitry ideally should be engaged to treat depression (indeed, in large measure because of the likelihood of individual differences in the functioning of relevant neural networks as well as the influence of successive episodes per se). Moreover, according to one recent review, the standard target for depression, DLPFC, may only have a peripheral role in mood regulation, and a number of other prefrontal regions were nominated as better possible targets for TMS (Downar and Daskalakis, 2013). A second related issue is that the exact location of cortical functional areas can vary greatly between individuals, making it difficult (in the absence of activating and imaging the circuit on an individual basis) to know where to target optimally for a particular individual even if the ideal network(s) for targeting were to be identified.
Our research group has taken a theory-based approach to these challenges. In the next section, we summarize the conceptual model (drawn from the behavioral literature on self-regulation of goal pursuit) we are using to provide a template for individualized assessment and targeting of TMS for depression. The model also provides opportunities to combine individually-targeted TMS (intended to target self-regulatory neural circuits hypothesized to be relevant to the individual’s emotion regulation) with cognitive psychotherapeutic interventions which simultaneously engage the circuits of interest and which provide the patient with a set of practical skills for more effective goal pursuit.
3. Targeting brain/behavior systems for self-regulation
One potential response to the challenge of optimizing rTMS targeting is to focus on the processes associated with regulation of emotion. Many of the psychological functions that are impaired in depression, including self-evaluation, self-focus, affect regulation, and reward sensitivity, are linked to neural systems which instantiate basic social-cognitive processes and enable interpersonal goal pursuit. For example, the cortical midline structures model (Northoff and Bermpohl, 2004), which includes the orbital and medial PFC, the anterior cingulate (particularly the supragenual region), the dorsomedial prefrontal cortex (DMPFC), the precuneus, and the posterior cingulate, has been applied to conceptualizing information processing abnormalities in depression. Grimm et al. (2009) observed BOLD signal hypointensities in DMPFC, anterior cingulate, and ventral striatum in depressed individuals compared with matched controls while engaging in a self-evaluation task in the context of emotionally arousing stimuli. Alcaro et al. (2010) observed similar resting state abnormalities in depression and hypothesized that cortical/subcortical functional connectivity underlying self-referential information processing was disrupted in depression. While many of these structures are outside of the direct reach of TMS, their role in well-established functional (and even structural) networks suggests that one promising strategy would be to target loci within a circuit of interest which is reliably accessible to TMS. Below we describe two roughly parallel neural systems involved in self-regulation (and shown to be associated with depression when self-regulation is unsuccessful) which include cortical regions accessible to TMS.
One of the defining characteristics of human behavior is that it is organized by an individual’s personal goals, which are mental representations of desired end-states. Adults characteristically strive to attain personal goals and standards – to become the particular kind of person that they see as desirable (“ideal”) or as obligatory (“ought”). Within social psychology, the term self-regulation refers to the processes by which individuals control their behavior in the service of personal goal pursuit (Hoyle and Gallagher, 2015). In this ongoing process, people continuously compare their actual behaviors with their representations of the kind of person they are striving to become. In turn, these comparisons have significant repercussions for the individual’s emotional and motivational state and ultimately for both well-being and social adaptation (Carver and Scheier, 1998).
Austin and Vancouver (1996) identified approach and avoidance goals as among the most important classes of goals. The literatures on approach and avoidance attest to the centrality of these dimensions for understanding goal-directed behavior. Those literatures are dominated by the behavioral activation and inhibition systems model, postulating brain/behavior systems that underlie temperament-based, spatiotemporal approach and avoidance as well as dispositional positive and negative affectivity (Watson et al., 1999). Both the behavioral activation system (BAS) and behavioral inhibition system (BIS) are hypothesized to regulate proximal goal-directed behaviors in response to evolutionarily significant cues for reward, in the case of BAS, or threat, in the case of BIS (Carver and Scheier, 1998).
Other influential theories take a social cognition perspective on the regulation of approach and avoidance, emphasizing abstract, higher-order goals that often are cross-situational and typically integrated within the individual’s sense of self. Higgins (1997) proposed a theory of regulatory focus that postulates two cognitive/motivational systems for attainment of desired outcomes promotion (“making something good happen”, typically in reference to ideals) and prevention (“keeping something bad from happening”, typically in reference to oughts). In contrast to BAS and BIS, which operate as “bottom-up” temperament-based systems in response to cues for spatiotemporal approach or avoidance (Depue and Collins, 1999; Watson et al., 1999), the promotion and prevention systems are “top-down” learning-based systems for strategic approach and avoidance via activation of generalized personal goals or concerns (Strauman et al., 2013). Each system is activated by the interpersonal context but also manifests trait-like properties across situations. Individual differences in regulatory focus are stable over time and predict which goals will be more likely to be used to guide behavior, as well as the strategies and means for pursuing them (e.g., Strauman, 1996). The motivational, behavioral, and affective consequences of individual differences in regulatory focus are well established (Higgins, 2006).
Individuals vary both in the characteristic ways they construe their goals and their chosen strategies to pursue them. As a consequence of variation in life experiences, a person might acquire increased value or personal relevance for one type of goal. A strong value placed on prevention goals will result in goal pursuit strategies that involve keeping bad things from happening – for example, by avoiding pitfalls and negative outcomes in the service of ultimate goal attainment. In addition, the same desired end-state can be represented in different ways by prevention-oriented versus promotion-oriented individuals. The same personal goal – such as being successful, friendly, loving, or intelligent – could be represented as an ideal/aspiration (a promotion goal) or as an obligation/responsibility (a prevention goal). There are now more than 200 published articles supporting the distinction between promotion and prevention in behavioral domains as varied as decision making, attitudes, social perception, and political preferences (e.g., Boldero and Higgins, 2011).
Strauman (2017) proposed a self-regulatory model of depression postulating that dysregulation within the promotion system can ultimately lead to MDD. According to this model, individuals who are impaired in their ability to effectively pursue promotion goals (i.e. are unable to “make good things happen”) are at heightened risk for the development of a unipolar mood disorder. Promotion system dysregulation can develop in different ways: through chronic promotion goal pursuit failure, through individual differences in socialization based strength of orientation toward promotion goals (i.e. low orientation toward this type of goal), and/or by perseveration on unattainable promotion goals (Trew, 2011). This self-regulation model of depression involves four postulates: (1) Depression results from cumulative or catastrophic failure of the individual’s neurobiological and psychological capacity for successful goal pursuit; (2) an initial episode of depression is a functional disorder resulting from failure of self-regulation; (3) core symptoms of depression reflect dysregulation of approach/promotion (e.g., mood, appetite, anhedonia, energy, concentration, worthlessness, hopelessness, low self-esteem) or dysregulation of reciprocal inhibition between approach/promotion and avoidance/prevention (e.g., sleep disturbance, guilt, agitation/anxiety, HPA axis dysfunction); and (4) as episodes of depression accumulate, neural and psychological self-regulatory mechanisms may be permanently altered.
Other investigators also have linked deficits in goal pursuit to depression, often focusing on the inability to disengage from unattainable goals as a precursor to symptomatology. Karoly (1999) proposed using goal pursuit as a “final common analytic pathway” in the investigation of both normative and psychopathological behavior – reminiscent of the final common pathway model developed by Akiskal and McKinney 1973) to conceptualize the heterogeneity of possible causes for depression. According to Karoly (1999), all social behavior can ultimately be evaluated in terms of its relation to goal pursuit, and proposed that both acute self-discrepancies and an inability to disengage from goals which cannot be attained ultimately leads to depressive symptoms such as negative affect and feelings of worthlessness. Pyszczynski and Greenberg (1987) proposed a similar model, suggesting that depression arises when an individual becomes “stuck” in a self-regulatory process for which there is no actual way to reduce the discrepancy between their actual and ideal self (i.e. when they are unable to disengage from an unachievable goal). Research by Wrosch and colleagues (Wrosch and Miller, 2009; Wrosch et al., 2003) provides empirical support for both these models. Wrosch et al. observed that people who are better able to disengage from pursuing unattainable goals – that is, to effectively self-regulate their behavior and affective states – are less vulnerable to depressive symptoms. However, their overarching message with regard to depression is similar to that proposed by regulatory focus theory: dysregulated goal pursuit, be it through repeated promotion goal failure, low orientation toward promotion goals, and/or impaired goal disengagement, can significantly contribute to the onset and maintenance of depression.
4. Neuroimaging of mechanisms underlying self-regulation in depression
Several recent studies have identified cortical regions associated with activation of promotion and prevention goals, including prefrontal (Amodio and Frith, 2006) and midline cortical (Northoff and Bermpohl, 2004) structures, and have shown that individual differences in promotion/prevention orientation (Scholer et al., 2010) predict the extent of activation in those regions following idiographic goal priming. Further, neural correlates of individual differences in promotion and prevention orientation have been identified, demonstrating that the promotion and prevention systems are associated with distinct neural activation patterns (e.g., Amodio et al., 2004; Cunningham et al., 2005; Touryan et al., 2007). Eddington et al. (2007) used incidental semantic priming to examine patterns of cortical activation associated with promotion and prevention goals via fMRI. They found that an area of left orbital prefrontal cortex (OPFC) was activated during idiographic promotion goal priming, and that the magnitude of activation in this left OPFC region was correlated significantly with a self-report measure of individual differences in strength of orientation to promotion goals. The observed activation in response to promotion goal priming was statistically and functionally independent of the particular tasks in which participants were engaged while in the scanner, suggesting a neural “signature” for promotion goal activation that was detectable even when individuals are not intentionally or explicitly engaged in personal goal pursuit. The locus of activation was found in a region of OPFC that has been postulated to play a critical role in integrating outcomes across separate cognitive operations in pursuit of abstract, higher-order goals, as well as in modulating emotional and motivational responses to goal-relevant stimuli (Miller and Cohen, 2001; Ramnani and Owen, 2004).
Eddington et al. (2009) examined the neural correlates of promotion and prevention goal priming in a sample of unmedicated adult patients meeting DSM-IV criteria for depression, with or without comorbid GAD, as well as an age- and gender-matched control sample of adults with no psychiatric history. Using the procedure from their 2007 study, Eddington and colleagues incidentally exposed participants to their own promotion and prevention goals during fMRI scanning. They hypothesized that depressed patients would show an attenuated left OPFC response to promotion priming compared to the controls. Consistent with this hypothesis, Eddington et al. observed a significant difference in activation between the depressed and non-depressed groups following promotion goal priming, in which the controls showed greater left OPFC activation following promotion priming than the depressed patients. In addition, they compared depressed patients with versus without comorbid GAD and observed a region in right OPFC that was uniquely activated following prevention priming, but only for the patients with comorbid anxiety. These activation patterns in response to promotion or prevention goal priming were detected even though participants were not explicitly engaged in self-evaluation, providing evidence for neural activation “signatures” of abnormal goal processing associated with depression and anxiety.
Strauman et al. (2013) used a different personal goal priming procedure to further elucidate the neural correlates of the promotion and prevention systems. A rapid masked exposure technique was used in which participants were exposed subliminally to their own promotion and prevention goals. The task involved rapid masked presentation of words and non word letter strings. Some of the words presented were taken from subjects’ responses to an earlier Interview, but were presented so rapidly that subjects could not consciously perceive them. The task for the subject was to simply watch the continuous presentation of characters on the screen and to press a button box whenever a set of characters appeared in red (which controlled for visual attention during the stimulus presentation). In fMRI recorded during this task, distinct patterns of neural activation associated with promotion vs. prevention were observed. Promotion priming led to activation in left prefrontal and occipital regions as well as caudate and thalamus, whereas prevention priming was associated with activation in precuneus and posterior cingulate cortex as well as right PFC. Individual differences in dysphoric/anxious affect and regulatory focus, but not differences in BAS/BIS strength, were predictive of differential activation in response to personal goal priming. The regions activated in response to promotion and prevention goal priming broadly mapped onto the cortical midline network that has been shown to index processing of self-referential stimuli (Northoff and Bermpohl, 2004).
Fig. 1 summarizes data from a recent study by Detloff and Strauman (2016) using personalized promotion and prevention goal priming in clinically depressed vs. matched nondepressed control participants. The figure presents findings from an analysis conducted to determine whether any regions reliably characterized responses to promotion vs. prevention goal priming across the two participant groups and whether any regions distinguished between the depressed and control participants following promotion vs. prevention goal priming. There were two regions which discriminated between depressed and nondepressed participants following exposure to promotion goal priming (left BA 9/middle frontal gyrus and left BA 25/subgenual cortex respectively, both p < .01). There was one region (right BA 9/superior frontal gyrus) which discriminated between depressed and nondepressed participants following exposure to idiographic prevention goal priming (p < .05), and also discriminated between depressed participants with vs. without GAD (p < .05).
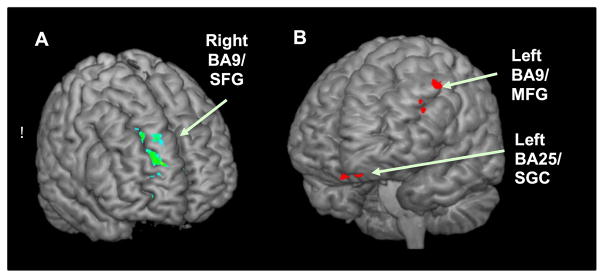
Results from a Group (Depressed, Control) X Priming Type (promotion, prevention priming) analysis. (A) The parametric map shows a right BA9/superior frontal gyrus region with significantly greater activation for prevention vs. promotion priming and greater activation in Depressed (DEP) vs. Control (CTL) participants. (B) The parametric map shows two regions (left BA9/middle frontal gyrus and left BA25/subgenual cortex) with significantly greater activation for promotion vs. prevention priming and greater activation in CTL vs. DEP participants.
These fMRI findings have significant implications for individualizing TMS for depression. First, they provide evidence that the promotion and prevention systems are reliably distinguishable at the neural level in addition to their distinct cognitive, motivational, and behavioral characteristics. Second, by use of idiographic goal priming techniques, we were able to observe that whereas the content of individuals’ personal goals was variable, the psychological and neural structures that instantiate them, which we hypothesize to constitute the promotion and prevention systems, were highly consistent. Third, just as behavioral and emotional responses to personal goal cues are correlated with individual differences in strength of promotion and prevention focus, so are BOLD signal responses to promotion and prevention goals related to measures of such individual differences. Overall, the neural correlates of self-regulation and personal goal pursuit, as revealed through fMRI, suggest potential target circuits in left and right PFC that are accessible to TMS, are sensitive to depression, and which can be located on an individual basis using idiographic goal priming tasks. The hypoactivation of left PFC and hyperactivation of right PFC in depression corresponds to the need for high-frequency rTMS to up-regulate cortical excitability on the left and low frequency rTMS to down-regulate excitability on the right for efficacious treatment of depression. More speculatively, these fMRI studies suggest that the effectiveness of TMS as a treatment for depression, may be due in part to its impact on the BA9 regions involved with emotional regulation circuits such as the promotion and prevention systems.
The imaging research examining the neural substrate underlying promotion and prevention systems thus provides a depression-sensitive network to engage with brain stimulation and an ability to target it on an individual basis. Given the functional targeting formulation discussed earlier in the context of remediation of working memory deficits, we propose a novel integrative multimodal treatment for MDD consisting of a self-regulation theory-based protocol for individualized optimization of rTMS site of stimulation plus a concurrent behavioral intervention targeting the same dysfunctional neural circuitry, in the hopes of a Hebbian-like synergistic interaction between the plasticity induced by the TMS and the neurons activated in response to the behavioral intervention. As the behavioral intervention, we suggest self-system therapy (SST: Strauman et al., 2006). SST is a brief structured therapy for depression that is similar to cognitive therapy (CT) but focuses primarily on self-evaluation as opposed to CT’s primary focus on cognitive distortions, and has been shown to have comparable efficacy as CT. The self-regulation-based procedures of SST were designed to activate the same self-regulation neural circuitry as that targeted using the fMRI guidance (Eddington et al., 2015).
According to regulatory focus theory, the high degree of comorbidity between mood and anxiety disorders can be conceptualized in terms of the combination of a hypoactive promotion system and a hyperactive prevention system (Klenk et al., 2011). And in fact, recent experimental data from behavioral research in two analog samples showed that interventions specifically targeting up-regulation of the promotion system discriminantly led to an increase in positive affectivity, whereas interventions targeting down-regulation of the prevention system were discriminantly associated with decreased anxiety and negative affectivity (Strauman et al., 2015). Given these findings, along with previous correlational data linking promotion failure with dysphoria/anhedonia and prevention failure with anxiety/vigilance (Jones et al., 2013), the potential efficacy of combined TMS/SST across a range of internalizing disorders deserves further study. Our model implies that individuals with prominent anxiety symptoms might benefit from low-frequency rTMS to reduce excitability within promotion-system cortical circuitry in right PFC. This additional TMS/cognitive intervention combination could likewise be targeted, and provided with individually-relevant content, using the same idiographic goal priming fMRI task that provides the targeting location for left PFC high-frequency rTMS. Additional clinical testing could determine whether both promotion-engaging and prevention-dampening interventions could be delivered efficaciously within a single session, or whether a better clinical outcome is obtained by focusing particular sessions (or series of sessions) on up-regulating the promotion system vs. down-regulating the prevention system respectively.
On an exploratory note, a number of the potential links between self-regulation of personal goal pursuit and neurocognitive mechanisms of emotion regulation have yet to be examined in detail (Hoyle and Gallagher, 2015; McRae et al., 2011). Traditionally within the adult behavioral literature, the term self-regulation has been used to denote regulation and evaluation of goal pursuit, whereas the term emotion regulation has denoted efforts to manage affective responses to interpersonal feedback (which includes, but is not limited to, situations involving goal pursuit). Nonetheless, there is reason to expect that the neural correlates of promotion and prevention goal pursuit have predictable functional interactions with neural mechanisms for affect regulation – for example, in the patterns of dynamic variability often observed across amygdala, anterior cingulate cortex, and dorsolateral prefrontal cortex (e.g., Hariri et al., 2002).
5. Conclusions
The standard clinical technique by which rTMS is used for the treatment of depression has been associated with limited efficacy to date. In this review, we began with the assertion that such limited efficacy may be due to reliance on nomothetic, scalp-based targeting rather than methods which incorporate fMRI-guided neuronavigation based on a specific model of neurocircuit dysfunction. We presented such a model of depression as a disorder of self-regulation which postulates two brain/behavior systems, the promotion and prevention systems, underlying goal pursuit. We summarized research indicating that activation of an individual’s promotion or prevention goals via priming leads to motivational and affective responses modulated by the individual’s appraisal of their progress in attaining the goal. We also noted that priming promotion vs. prevention goals induces discriminable patterns of brain activation that are sensitive to the effects of depression and anxiety. Our model suggests that these left and right PFC locations could be directly targeted in an individualized manner using rTMS. Additionally, this individually targeted rTMS can be integrated with cognitive interventions designed to activate the neural circuitry associated with promotion vs. prevention, thus allowing the neuroplasticity induced by the rTMS to benefit the systems most likely involved in remediating depression. Overall, our intent has been to provide conceptual and empirical support for the assertion that targeting TMS by fully engaging emotional regulation circuitry through both individualized fMRI-based coil positioning and simultaneous activation of that circuitry with theory-based cognitive interventions is a sounder and more rational approach to treating depression with non-invasive brain stimulation than standard targeting using scalp measurements and TMS alone. Of course, blinded sham controlled randomized clinical trials will be needed to fully test this possibility. More generally, we hope to have provided an example of the use of noninvasive brain stimulation to help translate a sophisticated line of basic imaging research into an innovative means of improving therapeutic efficacy.
Acknowledgments
Research reported in this article was made possible by grants MH052281, MH067447, MH039429, and DA031579 from the National Institutes of Health, and was supported by NIH grants (in part) by the Intramural Research Program of the NIMH.
References
- Akiskal HS, McKinney WT. Depressive disorders: toward a unified hypothesis. Science. 1973;182(4107):20–29. [Abstract] [Google Scholar]
- Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G. Is subcortical–cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neurosci Biobehav Rev. 2010;34(4):592–605. [Abstract] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. [Abstract] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG, Curtin JJ, Hartley SL, Covert AE. Neural signals for the detection of unintentional race bias. Psychol Sci. 2004;15(2):88–93. [Abstract] [Google Scholar]
- Austin JT, Vancouver JB. Goal constructs in psychology: structure, process, and content. Psychol Bull. 1996;120:338–375. [Google Scholar]
- Berlim MT, Van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(02):225–239. [Abstract] [Google Scholar]
- Boldero JM, Higgins ET. Regulatory focus and political decision making: when people favor reform over the status quo. Political Psychol. 2011;32(3):399–418. [Google Scholar]
- Carver CS, Scheier MF. On the self-regulation of behavior. Cambridge University Press; New York: 1998. [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cogn Affect Behav Neurosci. 2005;5(2):202–211. [Abstract] [Google Scholar]
- Daskalakis ZJ, Levinson AJ, Fitzgerald PB. Repetitive transcranial magnetic stimulation for major depressive disorder: a review. Can J Psychiatry. 2008;53(9):555. [Abstract] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22:491–569. [Abstract] [Google Scholar]
- Detloff AM, Strauman TJ. Individual differences in the neural response to personal goal pursuit success and failure. 2016 Manuscript in Preparation. [Google Scholar]
- Downar J, Daskalakis ZJ. New targets for rTMS in depression: a review of convergent evidence. Brain Stimul. 2013;6(3):231–240. [Abstract] [Google Scholar]
- Eddington KM, Dolcos F, Cabeza R, Krishnan KRR, Strauman TJ. Neural correlates of promotion and prevention goal activation: an fMRI study using an idiographic approach. J Cogn Neurosci. 2007;19:1152–1162. [Abstract] [Google Scholar]
- Eddington KM, Dolcos F, McLean AN, Cabeza R, Krishnan KRR, Strauman TJ. Neural correlates of idiographic goal priming in depression: goal-specific dysfunctions in the orbitofrontal cortex. Social Cogn Affect Neurosci. 2009;4:238–246. [Europe PMC free article] [Abstract] [Google Scholar]
- Eddington KM, Silvia PJ, Foxworth TE, Hoet A, Kwapil TR. Motivational deficits differentially predict improvement in a randomized trial of self-system therapy for depression. J CONSULT CLIN PSYCH. 2015;83(3):602–616. [Europe PMC free article] [Abstract] [Google Scholar]
- Fitzgerald PB, Maller JJ, Hoy KE, Thomson R, Daskalakis ZJ. Exploring the optimal site for the localization of dorsolateral prefrontal cortex in brain stimulation experiments. Brain Stimul. 2009a;2(4):234–237. [Abstract] [Google Scholar]
- Fitzgerald PB, Hoy K, McQueen S, Maller JJ, Herring S, Segrave R, Daskalakis ZJ. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009b;34(5):1255–1262. [Abstract] [Google Scholar]
- George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Holtzheimer PE. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch General Psychiatry. 2010;67(5):507–516. [Abstract] [Google Scholar]
- George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, Post RM. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853–1856. [Abstract] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, Northoff G. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Human Brain Mapp. 2009;30:2617–2627. [Europe PMC free article] [Abstract] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17:317–323. [Abstract] [Google Scholar]
- Higgins ET. Value from hedonic experience and engagement. Psychol Rev. 2006;113:439–460. [Abstract] [Google Scholar]
- Higgins ET. Beyond pleasure and pain. Am Psychol. 1997;52(12):1280. [Abstract] [Google Scholar]
- Hoyle RH, Gallagher P. The interplay of personality and self-regulation. In: Mikulincer M, Shaver PR, Cooper ML, Larsen RJ, Mikulincer M, Shaver PR, Larsen RJ, editors. APA Handbook of Personality and Social Psychology, Personality Processes and Individual Differences 4. American Psychological Association; Washington, DC, US: 2015. pp. 189–207. http://dx.doi.org/10.1037/14343-009. [Google Scholar]
- Jones NP, Papadakis AA, Orr CA, Strauman TJ. Cognitive processes in response to goal failure: a study of ruminative thought and its affective consequences. J Social Clin Psychol. 2013;32:482–503. [Europe PMC free article] [Abstract] [Google Scholar]
- Kadosh RC, Kadosh KC, Schuhmann T, Kaas A, Goebel R, Henik A, Sack AT. Virtual dyscalculia induced by parietal-lobe TMS impairs automatic magnitude processing. Curr Biol. 2007;17(8):689–693. [Abstract] [Google Scholar]
- Karoly P. A goal systems–self-regulatory perspective on personality, psychopathology, and change. Rev General Psychol. 1999;3(4):264–291. [Google Scholar]
- Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM, Speer AM. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry. 1999;46(12):1603–1613. [Abstract] [Google Scholar]
- Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, Feinsod M. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch General Psychiatry. 1999;56(4):315–320. [Abstract] [Google Scholar]
- Klenk MM, Strauman TJ, Higgins ET. Regulatory focus and anxiety: a self-regulatory model of GAD-depression comorbidity. Personal Individ Differ. 2011;50:935–943. [Europe PMC free article] [Abstract] [Google Scholar]
- Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Devanne H. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125(11):2150–2206. [Abstract] [Google Scholar]
- Luber B, Steffener J, et al. Extended remediation of sleep deprivation-induced working memory deficits using fMRI-guided repetitive transcranial magnetic stimulation. Sleep. 2013;36:857–871. [Europe PMC free article] [Abstract] [Google Scholar]
- McRae K, Ochsner KN, Gross JJ. The reason in passion: a social cognitive neuroscience approach to emotion regulation. In: Vohs KD, Baumeister RF, Vohs KD, Baumeister RF, editors. Handbook of Self-regulation: Research, Theory, and Applications. 2. Guilford Press; New York, NY, US: 2011. pp. 186–203. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. [Abstract] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. [Abstract] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, Demitrack MA. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–1216. [Abstract] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardó F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996a;348(9022):233–237. [Abstract] [Google Scholar]
- Pascual-Leone A, Catala MD, Pascual APL. Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology. 1996b;46(2):499–502. [Abstract] [Google Scholar]
- Pyszczynski T, Greenberg J. Self-regulatory preservation and the depressive self-focusing style: a self-awareness theory of reactive depression. Psychol Bull. 1987;102:122–138. [Abstract] [Google Scholar]
- Ragert P, Dinse HR, Pleger B, Wilimzig C, Frombach E, Schwenkreis P, Tegenthoff M. Combination of 5 Hz repetitive transcranial magnetic stimulation (rTMS) and tactile coactivation boosts tactile discrimination in humans. Neurosci Lett. 2003;348(2):105–108. [Abstract] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into functions from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. [Abstract] [Google Scholar]
- Sack AT, Kadosh RC, Schuhmann T, Moerel M, Walsh V, Goebel R. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J Cogn Neurosci. 2009;21(2):207–221. [Abstract] [Google Scholar]
- Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N, Devanand DP. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71–83. [Europe PMC free article] [Abstract] [Google Scholar]
- Scholer AA, Zou X, Fujita K, Stroessner SJ, Higgins ET. When risk-seeking becomes a motivational necessity. J Personal Social Psychol. 2010;99:215–231. [Abstract] [Google Scholar]
- Schutter DJLG. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychol Med. 2009;39(01):65–75. [Abstract] [Google Scholar]
- Slotema CW, Blom JD, Hoek HW, Sommer IE. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71(7):1–478. [Abstract] [Google Scholar]
- Sparing R, Hesse MD, Fink GR. Neuronavigation for transcranial magnetic stimulation (TMS): where we are and where we are going. Cortex. 2010;46:118–120. [Abstract] [Google Scholar]
- Strauman TJ. Stability within the self: a longitudinal study of the structural implications of self-discrepancy theory. J Personal Social Psychol. 1996;71(6):1142. [Abstract] [Google Scholar]
- Strauman TJ, Vieth AZ, Merrill KA, Kolden GG, Woods TE, Klein MH, Papadakis AA, Schneider KL, Kwapil L. Self-system therapy as an intervention for self-regulatory dysfunction in depression: a randomized comparison with cognitive therapy. J Consult Clin Psych. 2006;74(2):367–376. [Abstract] [Google Scholar]
- Strauman TJ, Detloff AM, Sestokas R, Smith DV, Goetz EL, Rivera C, Kwapil L. What shall I be, what must I be: neural correlates of personal goal activation. Front Integr Neurosci. 2013 http://dx.doi.org/10.3389/fnint.2012.00123. [Europe PMC free article] [Abstract]
- Strauman TJ, Socolar Y, Kwapil L, Cornwell JFM, Franks B, Sehnert S, Higgins ET. Micro interventions targeting regulatory focus and fit selectively reduce dysphoric and anxious mood. Behav Res Ther. 2015;72:18–29. [Europe PMC free article] [Abstract] [Google Scholar]
- Strauman TJ. Self-Regulation and Psychopathology: Toward an Integrative Translational Research Paradigm. Ann Rev Clin Psych. 2017:13. http://dx.doi.org/10.1146/annurev-clinpsy-032816-045012. [Abstract]
- Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180:583–593. [Abstract] [Google Scholar]
- Touryan SR, Johnson MK, Mitchell KJ, Farb N, Cunningham WA, Raye CL. The influence of self-regulatory focus on encoding for, and memory for, emotional words. Social Neurosci. 2007;2:14–27. [Abstract] [Google Scholar]
- Trew JL. Exploring the roles of approach and avoidance in depression: an integrative model. Clin Psychol Rev. 2011;31(7):1156–1168. [Abstract] [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: structural findings, evolutionary considerations, and psychobiological evidence. J Personal Social Psychol. 1999;76:820–838. [Google Scholar]
- Wrosch C, Miller GE. Depressive symptoms can be useful: self-regulatory and emotional benefits of dysphoric mood in adolescence. J Personal Social Psychol. 2009;96(6):1181–1190. [Abstract] [Google Scholar]
- Wrosch C, Scheier MF, Miller GE, Schulz R, Carver CS. Adaptive self-regulation of unattainable goals: goal disengagement, Goal reengagement, and subjective well-being. Personasl Social Psychol Bull. 2003;29(12):1494–1508. [Abstract] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, Rothwell JC. Consensus: motor cortex plasticity protocols. Brain Stimul. 2008;1(3):164–182. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.neuroimage.2016.12.083
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc5344760?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.neuroimage.2016.12.083
Article citations
Towards a neurodevelopmental model of bipolar disorder: a critical review of trait- and state-related functional neuroimaging in adolescents and young adults.
Mol Psychiatry, 27 Sep 2024
Cited by: 0 articles | PMID: 39333385
Review
Target engagement of the subgenual anterior cingulate cortex with transcranial temporal interference stimulation in major depressive disorder: a protocol for a randomized sham-controlled trial.
Front Neurosci, 18:1390250, 29 Aug 2024
Cited by: 0 articles | PMID: 39268031 | PMCID: PMC11390435
Efficacy of personalized rTMS to enhance upper limb function in subacute stroke patients: a protocol for a multi-center, randomized controlled study.
Front Neurol, 15:1427142, 03 Jul 2024
Cited by: 0 articles | PMID: 39022726 | PMCID: PMC11253596
The Use of Transcranial Magnetic Stimulation in Attention Optimization Research: A Review from Basic Theory to Findings in Attention-Deficit/Hyperactivity Disorder and Depression.
Life (Basel), 14(3):329, 29 Feb 2024
Cited by: 0 articles | PMID: 38541654 | PMCID: PMC10970838
Review Free full text in Europe PMC
Personalized, parcel-guided rTMS for the treatment of major depressive disorder: Safety and proof of concept.
Brain Behav, 13(11):e3268, 05 Oct 2023
Cited by: 3 articles | PMID: 37798655 | PMCID: PMC10636393
Review Free full text in Europe PMC
Go to all (41) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Reprint of ''Using neuroimaging to individualize TMS treatment for depression: Toward a new paradigm for imaging-guided intervention''.
Neuroimage, 151:65-71, 01 May 2017
Cited by: 4 articles | PMID: 28476213 | PMCID: PMC10072336
Repetitive transcranial magnetic stimulation for treatment of major depressive disorder with comorbid generalized anxiety disorder.
Ann Clin Psychiatry, 27(3):192-196, 01 Aug 2015
Cited by: 23 articles | PMID: 26247218
A multivariate neuroimaging biomarker of individual outcome to transcranial magnetic stimulation in depression.
Hum Brain Mapp, 40(16):4618-4629, 22 Jul 2019
Cited by: 27 articles | PMID: 31332903 | PMCID: PMC6865758
How Does Repetitive Transcranial Magnetic Stimulation Influence the Brain in Depressive Disorders?: A Review of Neuroimaging Magnetic Resonance Imaging Studies.
J ECT, 34(2):79-86, 01 Jun 2018
Cited by: 10 articles | PMID: 29324522
Review
Funding
Funders who supported this work.
Intramural NIH HHS (1)
Grant ID: Z99 MH999999
NIDA NIH HHS (1)
Grant ID: R01 DA031579
NIMH NIH HHS (2)
Grant ID: R24 MH067447
Grant ID: R01 MH039429





