Abstract
Free full text

Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial
Abstract
Purpose
Cabozantinib is an oral potent inhibitor of vascular endothelial growth factor receptor 2, MET, and AXL and is a standard second-line therapy for metastatic renal cell carcinoma (mRCC). This randomized phase II multicenter trial evaluated cabozantinib compared with sunitinib as first-line therapy in patients with mRCC.
Patients and Methods
Eligible patients had untreated clear cell mRCC and Eastern Cooperative Oncology Group performance status of 0 to 2 and were intermediate or poor risk per International Metastatic Renal Cell Carcinoma Database Consortium criteria. Patients were randomly assigned at a one-to-one ratio to cabozantinib (60 mg once per day) or sunitinib (50 mg once per day; 4 weeks on, 2 weeks off). Progression-free survival (PFS) was the primary end point. Objective response rate (ORR), overall survival, and safety were secondary end points.
Results
From July 2013 to April 2015, 157 patients were randomly assigned (cabozantinib, n = 79; sunitinib, n = 78). Compared with sunitinib, cabozantinib treatment significantly increased median PFS (8.2 v 5.6 months) and was associated with a 34% reduction in rate of progression or death (adjusted hazard ratio, 0.66; 95% CI, 0.46 to 0.95; one-sided P = .012). ORR was 33% (95% CI, 23% to 44%) for cabozantinib versus 12% (95% CI, 5.4% to 21%) for sunitinib. All-causality grade 3 or 4 adverse events were 67% for cabozantinib and 68% for sunitinib and included diarrhea (cabozantinib, 10% v sunitinib, 11%), fatigue (6% v 15%), hypertension (28% v 22%), palmar-plantar erythrodysesthesia (8% v 4%), and hematologic adverse events (3% v 22%).
Conclusion
Cabozantinib demonstrated a significant clinical benefit in PFS and ORR over standard-of-care sunitinib as first-line therapy in patients with intermediate- or poor-risk mRCC.
INTRODUCTION
Metastatic clear cell renal cell carcinoma (RCC) remains largely incurable. However, prognosis of patients with metastatic disease varies widely depending on well-characterized risk factors. The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC)1 and the Memorial Sloan Kettering Cancer Center (MSKCC)2 have developed prognostic criteria to classify patients with metastatic RCC (mRCC) into risk categories on the basis of pretreatment characteristics. Poor- and intermediate-risk groups have inferior clinical outcomes in terms of overall survival (OS), progression-free survival (PFS), and response to antiangiogenic agents when compared with favorable-risk patients.
Antiangiogenic agents that target the vascular endothelial growth factor (VEGF) and its receptors are standard treatments based on improved clinical outcomes in randomized phase III trials.3,4 Sunitinib is a common first-line therapy for patients with mRCC and serves as the control arm of several ongoing randomized phase III trials in untreated patients with advanced disease.5,6 Median PFS in patients with advanced RCC ranges from 8 to 11 months for first-line sunitinib or pazopanib for the entire patient population as reported in clinical trials,7-9 but it has been estimated to be 5.6 months for first-line targeted therapy (primarily VEGF-targeted therapies) when the population is restricted to intermediate- or poor-risk patients on the basis of data from the IMDC.10
Most patients treated with a VEGF-targeted agent ultimately develop resistance as evidenced by disease progression. Like VEGF, both MET and AXL are upregulated in von Hippel-Lindau–deficient RCC cells as a result of the control of their expression by hypoxia-inducible factors.11-14 High expression of MET or AXL is associated with poor prognosis15,16 and resistance to VEGF receptor (VEGFR) inhibitors in preclinical models of several cancers, including RCC.17,18 Given the known oncogenic potential of MET and AXL and their upregulation along with VEGF as part of the underlying pathobiology of RCC, targeting these two oncoproteins in addition to VEGFRs may provide additional anticancer effects in patients with RCC over more selective VEGFR-inhibition strategies.
Cabozantinib, an oral small-molecule inhibitor of tyrosine kinases, including VEGFRs, MET, and AXL,19 was recently approved for the treatment of patients with RCC who have received prior antiangiogenic therapy on the basis of a phase III trial (METEOR; Metastatic Renal Cell Carcinoma Phase III Study Evaluating Cabozantinib Vs Everolimus) showing an improvement in PFS, objective response rate (ORR), and OS compared with everolimus.20,21 Here, we report the results of Alliance for Clinical Trials in Oncology study A031203, a randomized, open-label phase II trial comparing cabozantinib with standard-of-care sunitinib in IMDC intermediate- and poor-risk patients with advanced RCC in the first-line setting.
PATIENTS AND METHODS
Patients
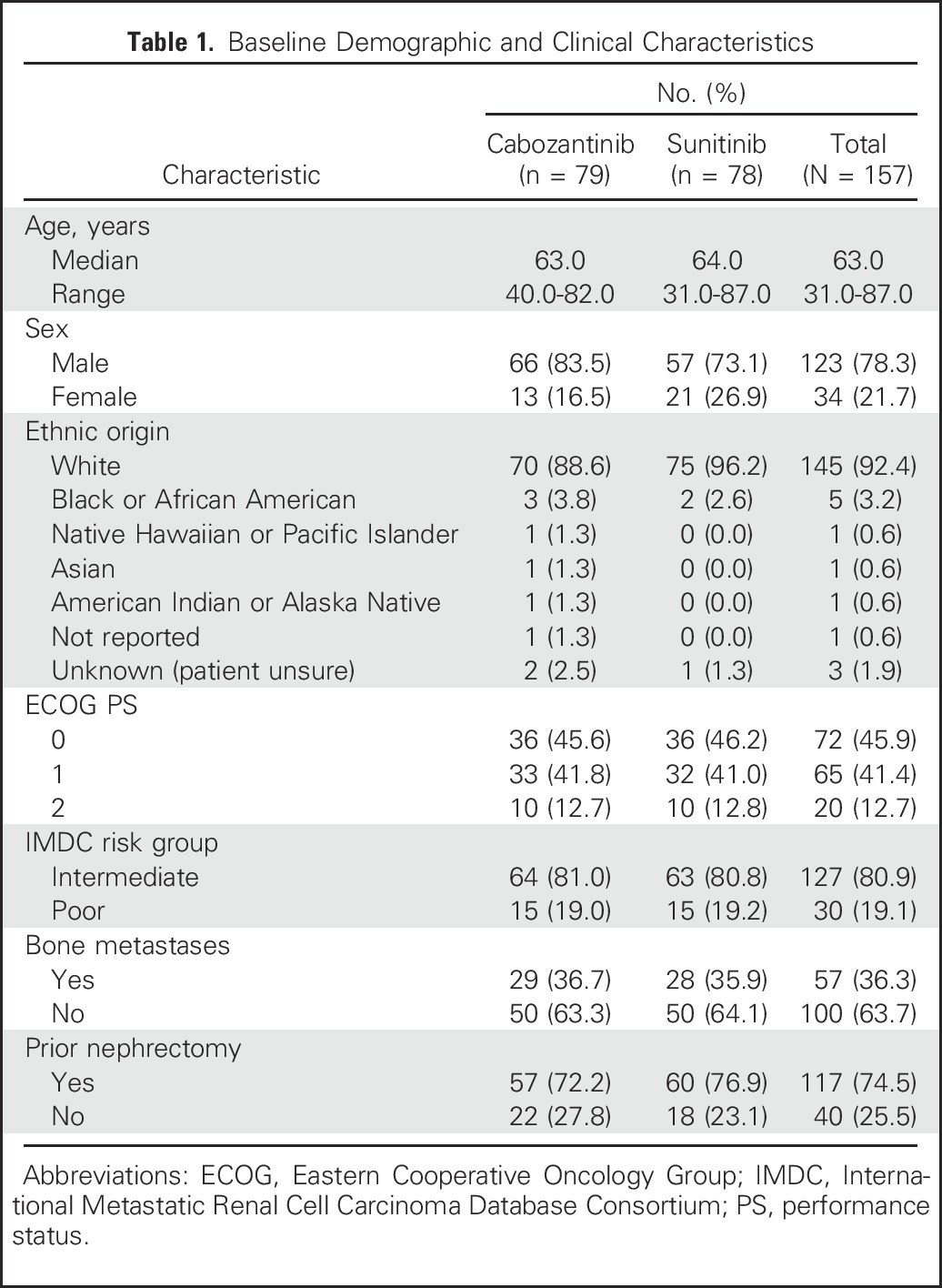
Eligible patients were 18 years of age or older with advanced RCC or mRCC (not amenable to curative surgery or radiotherapy) with a clear cell component and measurable disease. Patients must have been classified as intermediate or poor risk by IMDC criteria1 and must not have received prior systemic treatment. Patients with known brain metastases who were adequately treated and stable for 3 months were eligible. Eligible patients also had to have Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2 and adequate end-organ and marrow function with no uncontrolled significant illness.
Treatment Assignment
Patients were randomly assigned at a one-to-one allocation ratio to receive either cabozantinib or sunitinib. Random assignment was stratified by IMDC risk category (intermediate or poor) and presence of bone metastases (yes or no) using the dynamic allocation method.
Cabozantinib was provided by Exelixis (South San Francisco, CA) and administered orally once per day at a dose of 60 mg. Sunitinib was available as part of standard of care and administered orally once per day at a dose of 50 mg for 4 weeks, followed by a 2-week break. A treatment cycle was defined as 6 weeks in both study groups. Adverse events were managed with treatment interruptions and dose reductions. Cabozantinib dose reductions were to 40 and 20 mg, and sunitinib dose reductions were to 37.5 and 25 mg. Treatment was continued until disease progression, intolerance to therapy, or withdrawal of consent for treatment. Crossover between treatment arms was not prescribed by the protocol.
End Points and Assessments
The primary end point was duration of PFS, defined as the interval between the dates of random assignment and first documentation of disease progression (investigator assessed) or death resulting from any cause. Secondary end points were OS, ORR, and safety. OS was defined from the date of random assignment to the date of death resulting from any cause. Tumor response and progression were assessed in all patients by computed tomography or magnetic resonance imaging scans using RECIST (version 1.1)22 at screening and every 12 weeks (two treatment cycles) after random assignment until progression. Classification as a complete or partial response for calculation of ORR required confirmation at greater than 4 weeks after the first identified response. Routine safety evaluations were performed and adverse event severity grades were assessed by the investigator using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).23
Study Oversight
The protocol was approved by the institutional review board or ethics committee at each center, and the study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Each participant signed an institutional review board–approved, protocol-specific informed consent form in accordance with federal and institutional guidelines. Safety was monitored by an independent data monitoring committee on a regular basis. Data were collected by the Alliance for Clinical Trials in Oncology, reviewed by the study chair (T.K.C.), and analyzed in collaboration with the authors. The authors vouch for the accuracy and completeness of the data and the fidelity of the study to the protocol. The first draft of the manuscript was written by the first author, with all authors contributing to subsequent drafts. All authors agreed to submit the manuscript for publication. The study protocol is available at the journal Web site.
Study Design and Data Analysis
The study was designed to evaluate whether cabozantinib increased PFS compared with sunitinib in the target population. The planned sample size to evaluate PFS (the primary end point) was 140 randomly assigned patients. The null hypothesis was that the hazard ratio (HR) of progression of the two treatment arms would be 1.0; the alternative hypothesis was that the HR would be 0.67, favoring the experimental arm (cabozantinib) over the control arm (sunitinib). With 123 events (progressions or deaths), the log-rank statistic had 85% power to detect an HR of 0.67 for PFS, assuming a one-sided type I error of 0.12 (equivalent to an increase in median PFS from 8 months in the sunitinib arm to 12 months in the cabozantinib arm). The one-sided test corresponded to the one- sided study hypothesis. The following assumptions were made to achieve the target of 123 PFS events: an accrual rate of 5.8 patients per month over a 24-month enrollment period, 20 months of follow-up after study closure for the PFS end point, and an exponential distribution of PFS. Allowing for a 7% ineligibility rate, the total sample size was 150 patients.
A futility analysis was conducted for the PFS end point. The final analysis was performed when 123 PFS events had been observed, and the data base for the primary end point was locked on April 11, 2016. Analysis of OS was performed with a data base lock of September 15, 2016. The primary analysis of the PFS end point was based on a one-sided stratified log-rank test for treatment effect, adjusting for the stratification factors. In addition, the proportional hazards model was used to perform exploratory analyses to assess the importance of the treatment effect in predicting PFS in subgroup analyses. The Kaplan-Meier method was used to estimate OS and PFS distributions by treatment arm. An intent-to-treat approach was used for the analyses of all clinical outcomes except safety, where patients who received at least one dose of study drug were included.
All analyses were performed using SAS (SAS Institute, Cary, NC) and R software. The study was designed by the Alliance for Clinical Trials in Oncology, endorsed by the ECOG–American College of Radiology Imaging Network Group, and approved by the Cancer Therapy Evaluation Program of the National Cancer Institute. The Alliance Statistics and Data Center performed registration, data collection, and statistical analyses.
RESULTS
Patients
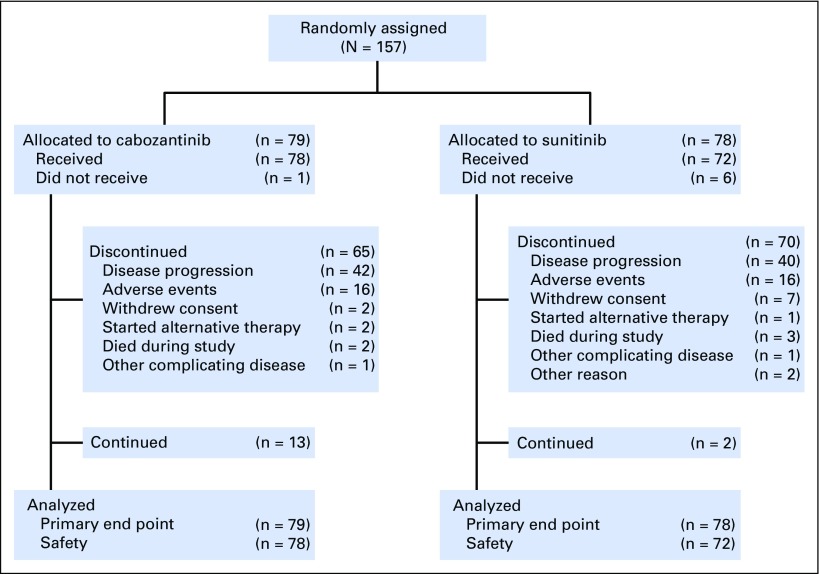
From July 9, 2013, to April 6, 2015, 157 patients were randomly assigned to receive cabozantinib (n = 79) or sunitinib (n = 78). Overall, the treatment groups were balanced with respect to baseline demographic and disease characteristics (Table 1). Eighty-one percent of enrolled patients were classified as IMDC intermediate risk and 19% as poor risk, and 36% of patients had bone metastases.
Table 1.
Baseline Demographic and Clinical Characteristics
As of the data cutoff date for the primary end point of PFS of April 11, 2016, 13 patients treated with cabozantinib and two treated with sunitinib were continuing to receive study treatment. The most common reason for discontinuing treatment was radiographic disease progression for both treatment groups (Fig 1).
Efficacy
The analysis of the primary end point of PFS was conducted after the required 123 events had occurred. Median PFS was 8.2 months (95% CI, 6.2 to 8.8 months) with cabozantinib and 5.6 months (95% CI, 3.4 to 8.1 months) with sunitinib. Cabozantinib reduced the rate of disease progression or death by 34% compared with sunitinib (adjusted HR for progression or death, 0.66, 95%, CI 0.46 to 0.95; one-sided P = .012; Fig 2). Subgroup analyses by stratification factors (IMDC risk groups and presence or absence of bone metastases) consistently favored cabozantinib (Appendix Fig A1, online only).
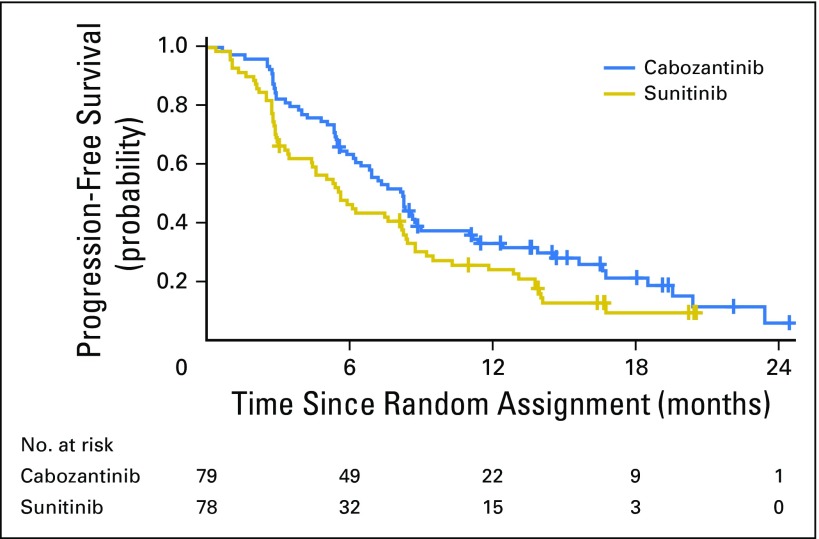
Kaplan-Meier plot of progression-free survival through April 11, 2016. Disease progression was assessed per investigator. All randomly assigned patients were included in the analysis.
Cabozantinib was associated with a significant improvement in ORR, as assessed by investigator review. Complete or partial responses were confirmed in 26 patients (33%; 95% CI, 23% to 44%) in the cabozantinib group compared with 9 patients (12%; 95% CI, 5.4% to 21%) in the sunitinib group (Table 2). A best response of stable disease occurred in 36 patients (46%) with cabozantinib versus 33 patients (42%) with sunitinib, and progressive disease as best response occurred in 14 patients (18%) with cabozantinib versus 20 patients (26%) with sunitinib. Any reduction in target lesions was observed for 87% of the cabozantinib group and 44% of the sunitinib group (Fig 3).
Table 2.
Tumor Response

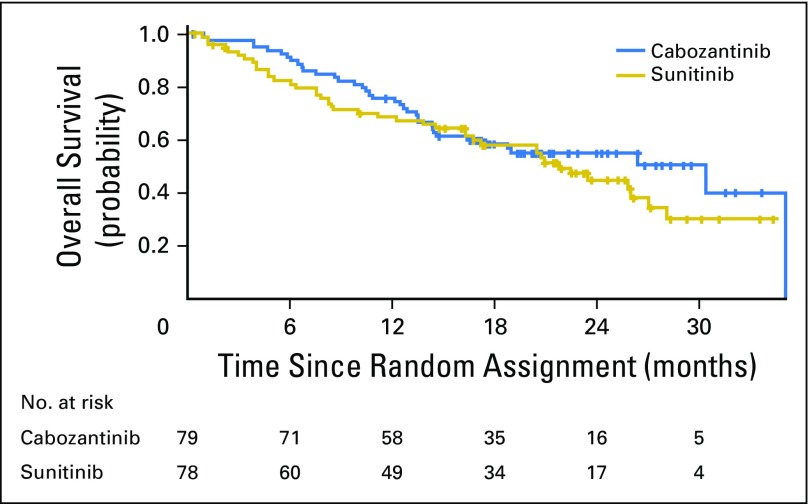
As of September 15, 2016, the median follow-up of surviving patients was 21.4 months. Overall, 37 deaths had occurred in the cabozantinib arm and 41 in the sunitinib arm. Median OS with cabozantinib was 30.3 months (95% CI, 14.6 to 35.0 months) versus 21.8 months (95% CI, 16.3 to 27.0 months) with sunitinib (adjusted HR, 0.80; 95% CI, 0.50 to 1.26; Fig 4). Subsequent anticancer therapy was received by 52% of patients in the cabozantinib group and 58% of patients in the sunitinib group and included systemic therapies (cabozantinib, 47% v sunitinib, 60%), radiotherapy (8% v 18%), and surgery (6% v 4%; Appendix Table A1, online only).
Safety
The safety population consisted of 78 patients treated with cabozantinib and 72 treated with sunitinib. Median number of 6-week treatment cycles was five (range, zero to 19) among patients who received cabozantinib and two (range, zero to 17) among patients who received sunitinib, corresponding to 6.9 months (range, 0 to 26.2 months) and 2.8 months (range, 0 to 23.5 months), respectively. Dose reductions occurred in 36 patients (46%) treated with cabozantinib and 25 patients (35%) treated with sunitinib. The rate of treatment discontinuation because of adverse events was 20% (n = 16) and 21% (n = 16) in the cabozantinib and sunitinib groups, respectively.
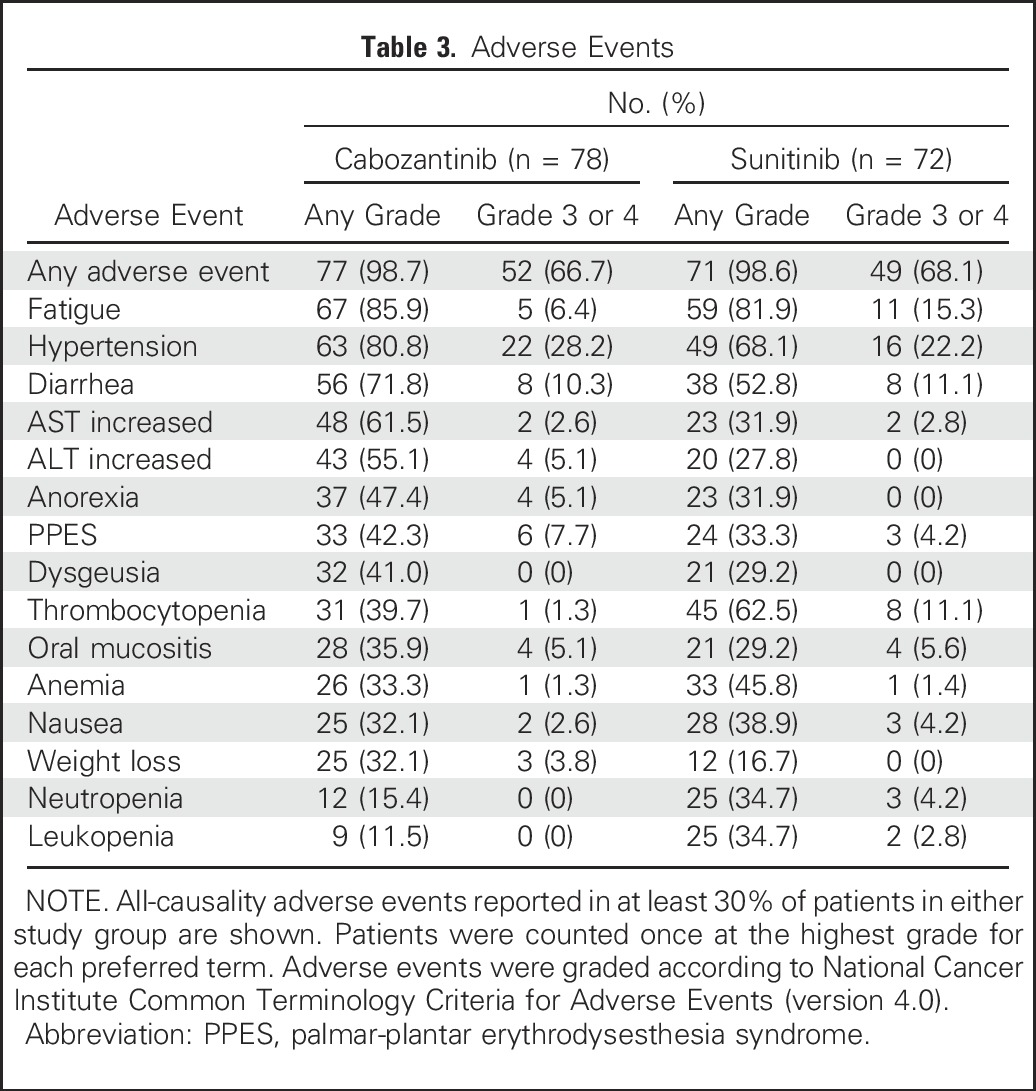
The incidence of adverse events (any grade) regardless of causality was 99% with cabozantinib and 99% with sunitinib, and the incidence of grade 3 or 4 adverse events was 67% with cabozantinib and 68% with sunitinib (Table 3). The most common grade 3 or 4 adverse events with cabozantinib were hypertension (28%), diarrhea (10%), palmar-plantar erythrodysesthesia (8%), and fatigue (6%); with sunitinib, they were hypertension (22%), fatigue (15%), diarrhea (11%), and thrombocytopenia (11%). Grade 5 adverse events occurred in four patients (5%) in the cabozantinib group and five patients (7%) in the sunitinib group. Treatment-related grade 5 events occurred in three patients in the cabozantinib group (acute kidney injury, sepsis, and jejunal perforation) and three patients in the sunitinib group (sepsis, respiratory failure, and vascular disorders).
Table 3.
Adverse Events
DISCUSSION
Cabozantinib improved PFS and response rate compared with sunitinib in this randomized phase II trial of IMDC intermediate- and poor-risk patients with RCC who were previously untreated with systemic agents. The efficacy of cabozantinib was notable and clinically meaningful, with an observed median PFS of 8.2 months compared with 5.6 months with sunitinib and an HR of 0.66, corresponding to a 34% reduction in the rate of disease progression or death. Objective tumor responses were higher with cabozantinib (33%) compared with sunitinib (12%). Preliminary data on OS showed a 20% decrease in the rate of death with cabozantinib.
The study was not designed to test for differences in OS, a secondary end point of the trial. Further follow-up will provide more mature OS results. Approximately half of all patients received subsequent anticancer therapy, with a similar percentage receiving therapy in both treatment groups. Therefore, subsequent therapy would not be expected to confound the OS results.
The safety profiles of cabozantinib and sunitinib in this trial were consistent with prior experience in this patient population.8,20,21 Common adverse events with cabozantinib included fatigue, hypertension, diarrhea, abnormal liver function tests, anorexia, and palmar-plantar erythrodysesthesia syndrome. These adverse effects have also been observed with other VEGFR tyrosine kinase inhibitors in patients with RCC.24 Adverse events observed with sunitinib were similar to those with cabozantinib overall, but with lower rates of palmar-plantar erythrodysesthesia syndrome, weight loss, and anorexia and higher rates of hematologic toxicities such as neutropenia and thrombocytopenia. Dose reductions were common with both agents, reflecting a strategy to titrate the agents to individual tolerability. The rate of discontinuation of study treatment because of adverse events was also similar in both arms. High-grade adverse events irrespective of causality were similar in both arms and consistent with prior reports for cabozantinib and sunitinib.
Sunitinib was used as the comparator because it is a standard first-line treatment.3,4 Pazopanib, another VEGFR-targeted therapy that was found to be noninferior to sunitinib in the COMPARZ (Comparing the Efficacy Safety and Tolerability of Pazopanib Versus Sunitinib) trial,7 could have been an alternative choice as a comparator. We focused on IMDC intermediate- and poor-risk groups because these groups would capture 70% to 80% of all patients with advanced disease who are the most in need of systemic therapy and disease control, whereas the favorable-risk group includes many patients with relatively indolent, lower-volume disease.10,25
In addition to including poor- and intermediate-risk groups, our patient population had a high rate of bone metastases, a known negative prognostic factor in RCC. As shown in a recent large French study, patients with RCC with bone metastases had a reduced benefit from sunitinib, even when adjusting for known prognostic factors in advanced RCC.26 Similar findings were seen in a study from the IMDC as well as in an analysis using pooled data from six prospective clinical trials.27,28 In contrast, encouraging clinical activity with cabozantinib in patients with RCC with bone metastases was observed in the phase III METEOR trial,21 where a marked improvement in PFS and OS in patients with bone metastases was observed with cabozantinib compared with everolimus. In our study, we conducted subgroup analyses in patients with bone metastases versus without bone metastases (as well as poor v intermediate risk) and observed a PFS benefit with cabozantinib in all subgroups of patients, consistent with the overall results. However, these analyses were limited by the small number of patients in each subgroup.
Median PFS and the ORR estimated for sunitinib in our study, 5.6 months and 18%, respectively, were lower than those reported in previous trials that included patients of all risk groups, including favorable risk.7,8 For example, median PFS per investigator was 10.2 months and ORR per investigator was 29% with sunitinib in the COMPARZ trial.7 The lower values for sunitinib in our study are consistent with the less favorable prognosis of our study population. In our study, no patients were favorable risk, 13% had an ECOG PS of 2, and 36% had bone metastases. In comparison, the sunitinib group in the COMPARZ trial consisted of 25% favorable-risk patients, none had an ECOG PS of 2, and only 15% of patients had bone metastases. Consistent with our results, an analysis from the IMDC including only intermediate- and poor-risk patients estimated a median PFS of 5.6 months for patients with RCC treated with first-line targeted therapy.10
The superiority of cabozantinib over sunitinib may reflect the target profile of cabozantinib, which includes MET and AXL in addition to VEGFR. Further investigation of biomarkers may help to clearly define the roles of these targets in the clinical activity of cabozantinib. Analysis of MET expression is ongoing; however, the role of MET tumor expression was investigated in the METEOR trial and was not found to be predictive of the clinical activity of cabozantinib over everolimus.21 Future studies should investigate additional blood and tissue biomarkers that might indicate response with cabozantinib in patients with RCC. Furthermore, given the recent demonstration of improved OS with cabozantinib21 or nivolumab29 compared with everolimus in two phase III studies in second-line RCC and the immunomodulatory effects of cabozantinib in the tumor microenvironment,30 evaluation of the combination of cabozantinib with immune checkpoint inhibition is ongoing (ClinicalTrials.gov identifier NCT02496208).31
Our study results show for the first time to our knowledge an agent that demonstrates clinical superiority over sunitinib, an established standard of care for more than 10 years. Nevertheless, our study has some limitations. The study did not include favorable-risk patients, and at this time, extrapolation of our findings to the favorable-risk population is not possible. However, there is no clinical or biologic rationale to support cabozantinib being inferior to sunitinib in that subgroup. Second, this study did not collect quality-of-life data. With relatively similar toxicity profiles, quality of life might be expected to be comparable between the two agents, although the sunitinib schedule could be favored because it includes a 2-week off-therapy break. Finally, central imaging review was not performed in our open-label study; however, results on the basis of central image review and investigator assessments led to similar efficacy conclusions in the phase III METEOR trial comparing cabozantinib with everolimus.21
In conclusion, cabozantinib demonstrated significant improvements in PFS and ORR relative to sunitinib in the initial treatment of patients with intermediate- or poor-risk clear cell mRCC. Therefore, cabozantinib represents a potential new treatment option for patients with previously untreated mRCC.
ACKNOWLEDGMENT
We thank the patients, their families, the investigators and site staff, the Alliance for Clinical Trials in Oncology, Exelixis, the Cancer Therapy Evaluation Program (John Wright), and the study teams participating in this trial.
Appendix
Table A1.
Subsequent Anticancer Therapy

Fig A1.
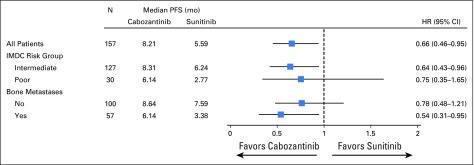
Forest plots of progression-free survival through April 11, 2016. All randomly assigned patients were included in the analyses. Hazard ratios (HRs) are unadjusted with the exception of that for the overall population, where stratification factors for random assignment were used. IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; PFS, progression-free survival.
Footnotes
Written on behalf of the CABOSUN investigators.
Processed as a Rapid Communication manuscript.
Clinical trial information: NCT01835158.
Supported by Grants No. U10CA180821 and U10CA180882 from the National Institutes of Health and by Exelixis, which provided cabozantinib.
See accompanying Editorial on page 577
AUTHOR CONTRIBUTIONS
Conception and design: Toni K. Choueiri, Susan Halabi, Eric J. Small, Daniel J. George, Michael J. Morris
Financial support: All authors
Administrative support: Toni K. Choueiri, Ben L. Sanford, Olwen Hahn, Michael J. Morris
Provision of study materials or patients: Toni K. Choueiri, Olwen Hahn, M. Dror Michaelson, Meghara K. Walsh, Darren R. Feldman, Thomas Olencki, Joel Picus, Shaker Dakhil, Daniel J. George
Collection and assembly of data: Toni K. Choueiri, Susan Halabi, Ben L. Sanford, M. Dror Michaelson, Meghara K. Walsh
Data analysis and interpretation: Toni K. Choueiri, Susan Halabi, Ben L. Sanford, Olwen Hahn, M. Dror Michaelson, Darren R. Feldman, Thomas Olencki, Joel Picus, Eric J. Small, Shaker Dakhil, Daniel J. George, Michael J. Morris
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Toni K. Choueiri
Honoraria: National Comprehensive Cancer Network, UpToDate
Consulting or Advisory Role: Pfizer, Bayer HealthCare Pharmaceuticals, Novartis, Merck, Bristol-Myers Squibb, Genentech, Eisai, Prometheus, Foundation Medicine, Cerulean Pharma, AstraZeneca, Peloton Therapeutics, Exelixis
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), TRACON Pharma (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Genentech (Inst), Celldex (Inst), Takeda Pharmaceuticals (Inst)
Susan Halabi
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals, Genentech
Travel, Accommodations, Expenses: Bayer HealthCare Pharmaceuticals
Ben L. Sanford
No relationship to disclose
Olwen Hahn
Honoraria: Cardinal Health (I), Via Oncology
Travel, Accommodations, Expenses: Cardinal Health (I)
M. Dror Michaelson
Employment: Jounce Therapeutics (I)
Consulting or Advisory Role: Novartis, Medivation, Pfizer, Exelixis, Eisai
Research Funding: Pfizer (Inst), Eisai (Inst), Argos Therapeutics (Inst), Millennium Pharmaceuticals (Inst), Novartis (Inst), TRACON Pharma (Inst)
Meghara K. Walsh
No relationship to disclose
Darren R. Feldman
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals, Gilead Sciences (I), Seattle Genetics
Research Funding: Novartis
Thomas Olencki
Research Funding: Bristol-Myers Squibb (Inst), Pfizer (Inst), TRACON Pharma (Inst)
Joel Picus
Consulting or Advisory Role: Novo Nordisk
Research Funding: Novartis, Bioclin, Altor BioScience, Agensys, Oncogenex, Mirati Therapeutics, Astex Pharmaceuticals
Eric J. Small
Stock or Other Ownership: Fortis Therapeutics, Harpoon Therapeutics
Consulting or Advisory Role: Gilead Sciences, Dendreon
Shaker Dakhil
No relationship to disclose
Daniel J. George
Honoraria: Dendreon, Novartis, Sanofi
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals, Dendreon, Exelixis, Medivation, Novartis, Pfizer, BIND Biosciences, Sanofi, GlaxoSmithKline, Astellas Pharma, Progenics, Innocrin Pharma, Genentech, Clovis Oncology, Merck, Bristol-Myers Squibb
Speakers’ Bureau: Dendreon, Novartis, BiPar Sciences, Sanofi
Research Funding: Exelixis, Genentech, Janssen Oncology, Novartis, Pfizer, Progenics, Astellas Pharma, Bristol-Myers Squibb, GlaxoSmithKline, Millennium Pharmaceuticals, Innocrin Pharma
Travel, Accommodations, Expenses: Astellas Pharma, Bayer HealthCare Pharmaceuticals, Dendreon, Pfizer, Sanofi, Progenics, Bristol-Myers Squibb, Merck, BIND, Medivation
Michael J. Morris
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals, Millennium Pharmaceuticals, Progenics
Research Funding: Bayer HealthCare Pharmaceuticals (Inst), Sanofi (Inst), Janssen Oncology (Inst), Endocyte (Inst)
Travel, Accommodations, Expenses: Fujifilm
REFERENCES
Articles from Journal of Clinical Oncology are provided here courtesy of American Society of Clinical Oncology
Full text links
Read article at publisher's site: https://doi.org/10.1200/jco.2016.70.7398
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5455807
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1200/jco.2016.70.7398
Article citations
Autoimmune complications of tyrosine kinase inhibitors in cancer therapy: Clinical insights, mechanisms, and future perspectives.
Medicine (Baltimore), 103(40):e39928, 01 Oct 2024
Cited by: 0 articles | PMID: 39465760 | PMCID: PMC11460853
Review Free full text in Europe PMC
The efficacy of second-line tyrosine kinase inhibitor for patients with metastatic non-clear cell renal cell carcinoma following first-line immune-oncology combination therapy.
World J Urol, 42(1):536, 26 Sep 2024
Cited by: 0 articles | PMID: 39325218
The Role of Surgery in Metastatic Renal Cell Carcinoma in 2024.
Clin Med Insights Oncol, 18:11795549241272447, 05 Sep 2024
Cited by: 0 articles | PMID: 39247714 | PMCID: PMC11378247
Outcomes of first-line treatment and their association with pretreatment neutrophil-to-lymphocyte ratio in patients with advanced renal cell carcinoma: Insights from a tertiary care institute in Pakistan.
Ecancermedicalscience, 18:1753, 03 Sep 2024
Cited by: 0 articles | PMID: 39430088 | PMCID: PMC11489116
Cabozantinib Plus Nivolumab in Adult Patients with Advanced or Metastatic Renal Cell Carcinoma: A Retrospective, Non-Interventional Study in a Real-World Cohort/GUARDIANS Project.
Cancers (Basel), 16(17):2998, 28 Aug 2024
Cited by: 0 articles | PMID: 39272856 | PMCID: PMC11393955
Go to all (334) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT01835158
- (1 citation) ClinicalTrials.gov - NCT02496208
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update.
Eur J Cancer, 94:115-125, 20 Mar 2018
Cited by: 142 articles | PMID: 29550566 | PMCID: PMC6057479
Quality-adjusted survival with first-line cabozantinib or sunitinib for advanced renal cell carcinoma in the CABOSUN randomized clinical trial (Alliance).
Cancer, 126(24):5311-5318, 06 Oct 2020
Cited by: 8 articles | PMID: 33022096 | PMCID: PMC7756547
Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma.
J Clin Oncol, 27(25):4068-4075, 03 Aug 2009
Cited by: 136 articles | PMID: 19652072
Overview of Current and Future First-Line Systemic Therapy for Metastatic Clear Cell Renal Cell Carcinoma.
Curr Treat Options Oncol, 19(1):6, 24 Jan 2018
Cited by: 27 articles | PMID: 29368125
Review
Funding
Funders who supported this work.
NCI NIH HHS (5)
Grant ID: U10 CA180833
Grant ID: U10 CA180867
Grant ID: U10 CA180882
Grant ID: P30 CA008748
Grant ID: U10 CA180821