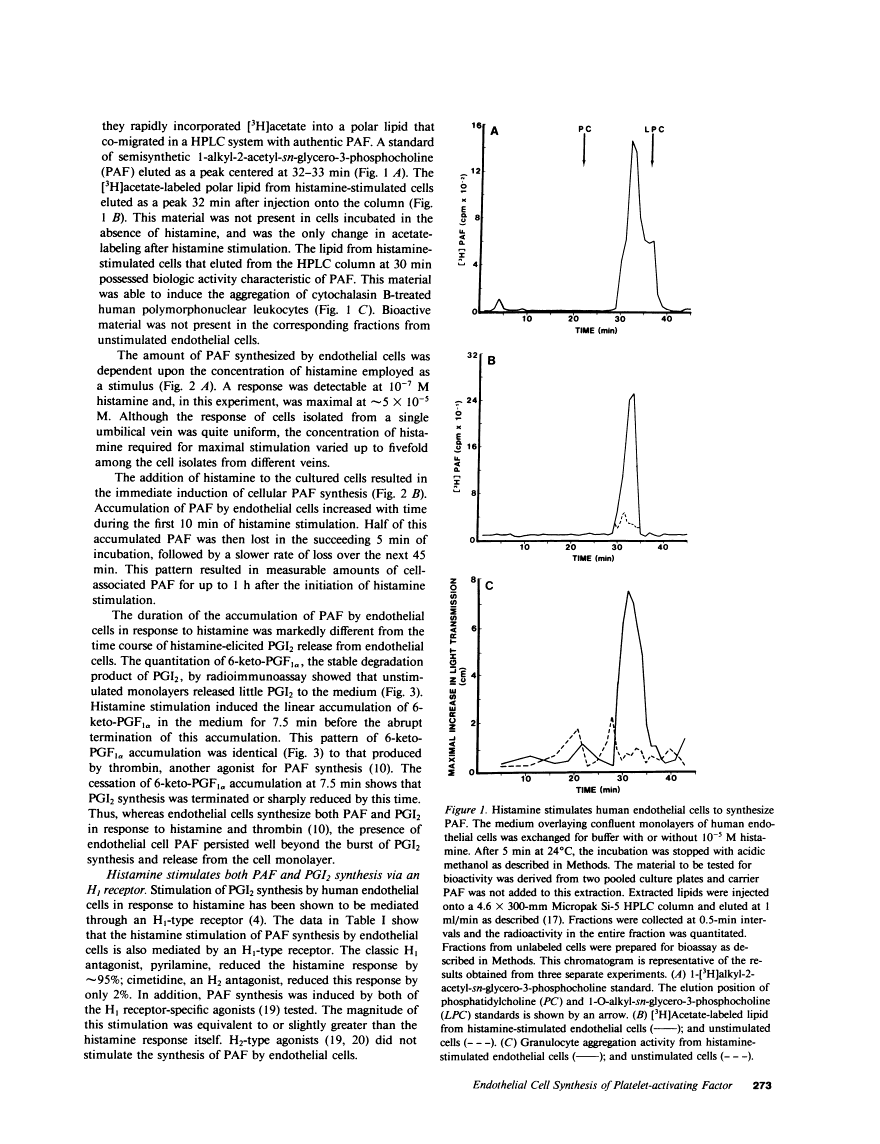
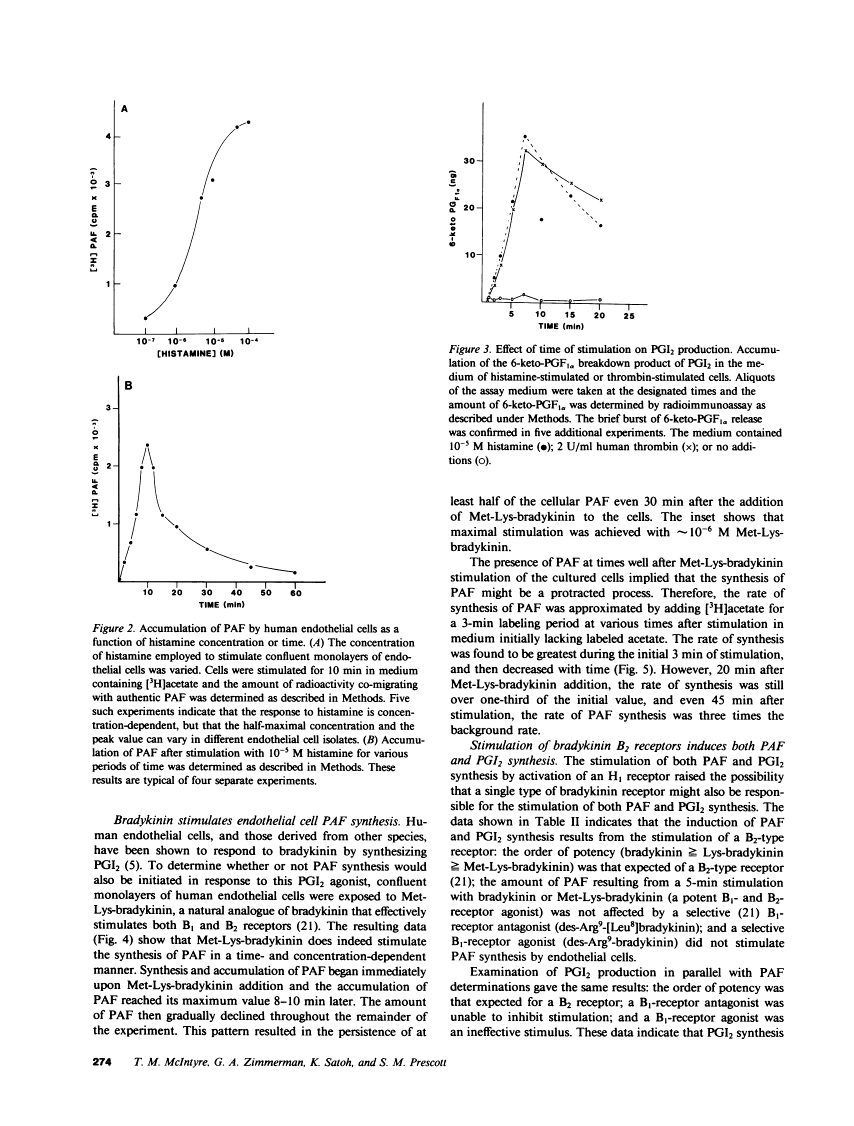
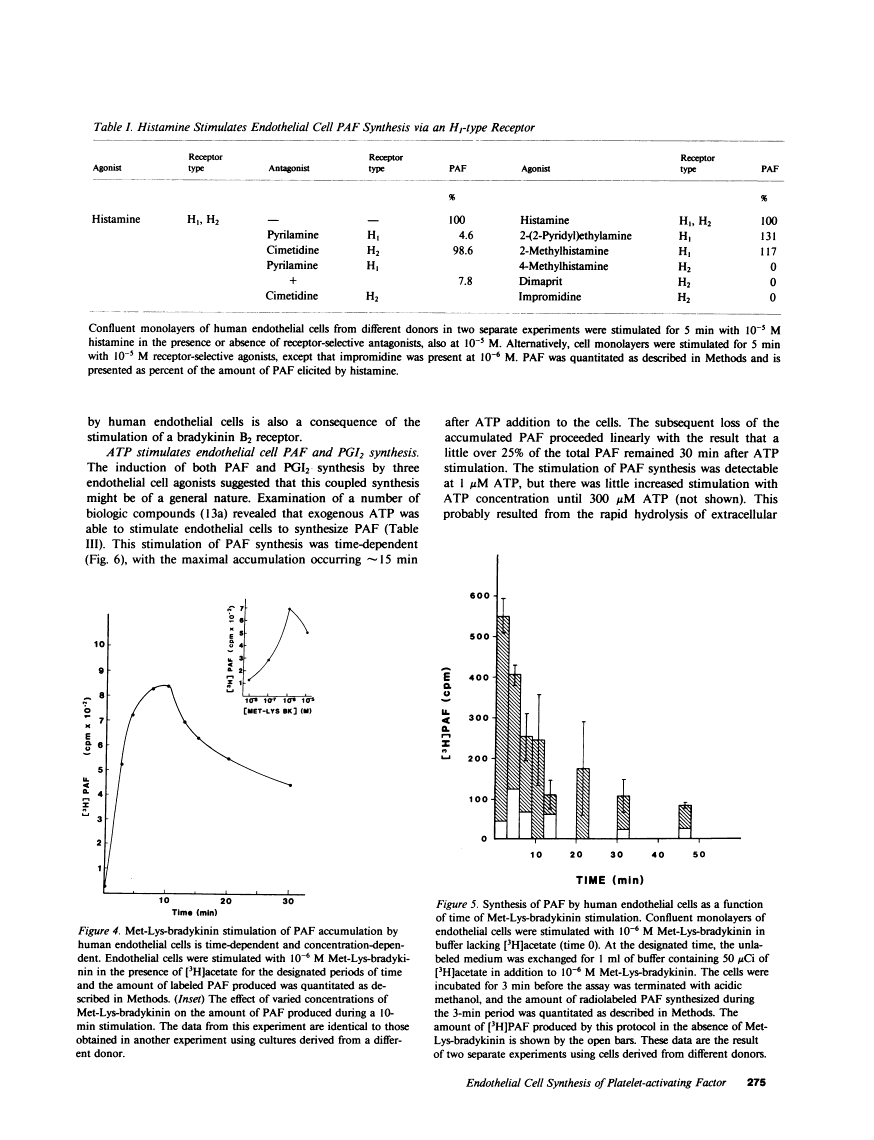
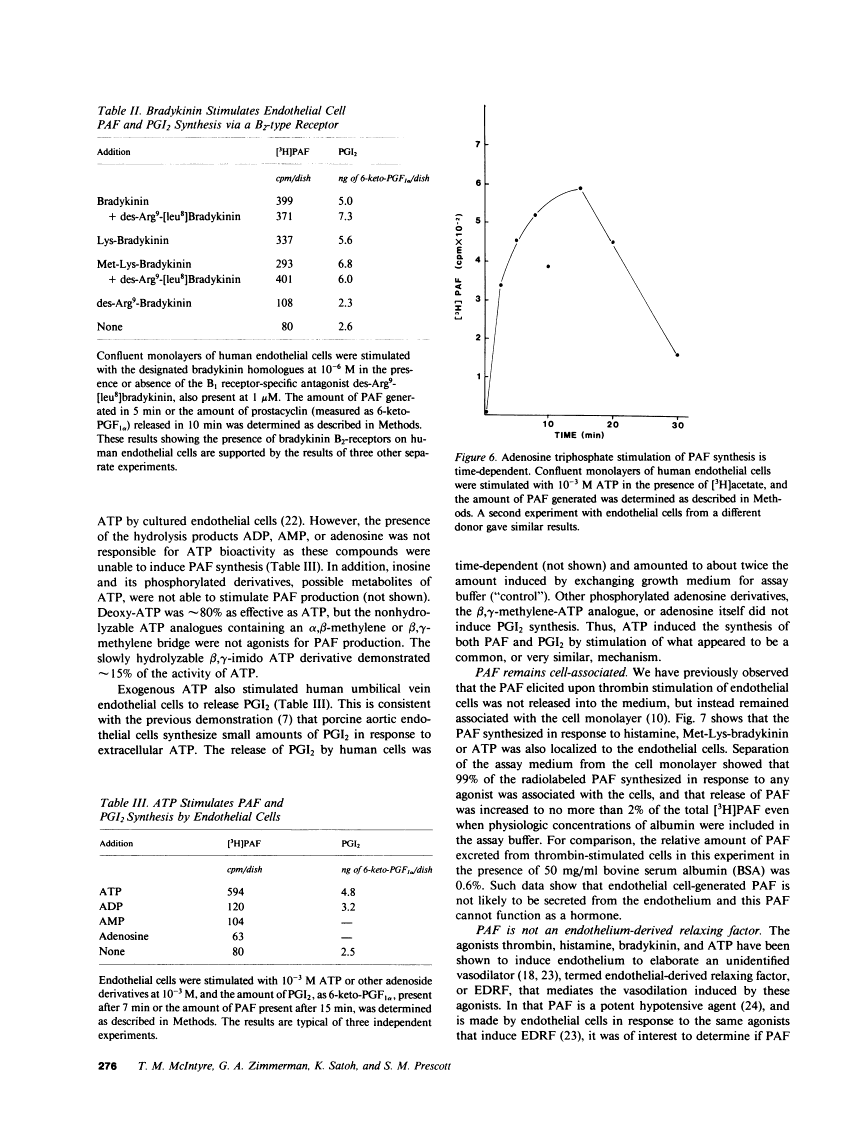
Abstract
Free full text

Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate.
Abstract
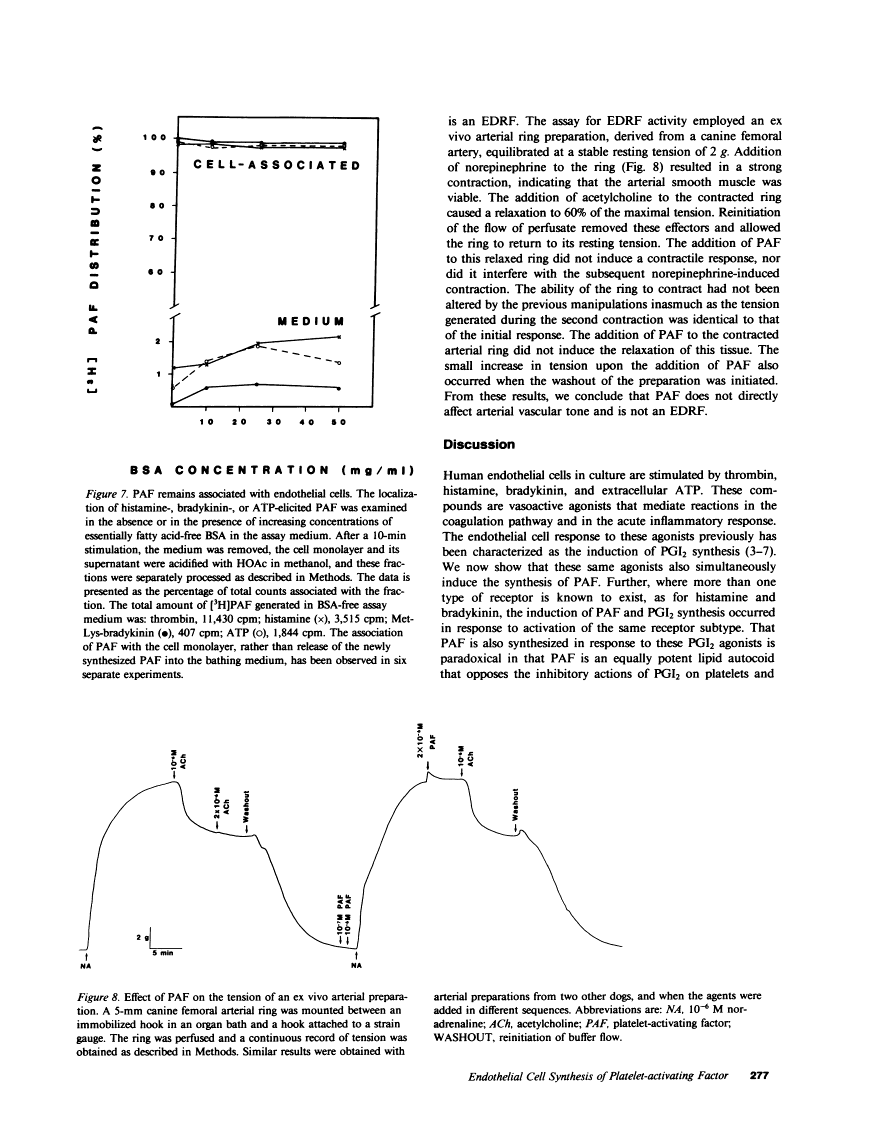
Cultured human endothelial cells synthesize prostacyclin (PGI2), a potent inhibitor of platelet function, when stimulated with histamine, bradykinin, or ATP. Paradoxically, we report that these agonists also induced the rapid and sustained synthesis of platelet-activating factor (PAF) by endothelial cells. In fact, the synthesis of this potent activator of platelets and neutrophils was induced by stimulation of the same receptor subtype that induced PGI2 synthesis: stimulation of a histamine H1 or a bradykinin B2 receptor induced both PAF and PGI2 synthesis. However, two physiologically important differences exist between the production of PAF and PGI2 by endothelial cells. The synthesis of PGI2 proceeded for only 7.5 min before the abrupt termination of synthesis, whereas the synthesis of PAF was clearly detectable even 45 min after stimulation. Although maximal accumulation of PAF occurred after 10-15 min of stimulation, the prolonged synthesis resulted in the presence of PAF for up to 1 h after stimulation. Secondly, whereas PGI2 was released from the cell monolayer, PAF remained cell-associated without significant release to the external medium. Endothelial cell-generated PAF, therefore, does not function as a hormone. The prolonged association of this potent activator of platelets and neutrophils with endothelial cells may mediate some of the inflammatory properties of histamine and bradykinin. It may also be a factor in the formation of a thrombogenic vascular surface, an event suggested to play a primary role in the pathogenesis of thrombosis and atherosclerosis.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Moncada S, Herman AG, Higgs EA, Vane JR. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. [Abstract] [Google Scholar]
- Weksler BB, Marcus AJ, Jaffe EA. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. [Europe PMC free article] [Abstract] [Google Scholar]
- Weksler BB, Ley CW, Jaffe EA. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. [Europe PMC free article] [Abstract] [Google Scholar]
- Baenziger NL, Force LE, Becherer PR. Histamine stimulate prostacyclin synthesis in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1435–1440. [Abstract] [Google Scholar]
- Hong SL. Effect of bradykinin and thrombin on prostacyclin synthesis in endothelial cells from calf and pig aorta and human umbilical cord vein. Thromb Res. 1980 Jun 15;18(6):787–795. [Abstract] [Google Scholar]
- Cramer EB, Pologe L, Pawlowski NA, Cohn ZA, Scott WA. Leukotriene C promotes prostacyclin synthesis by human endothelial cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4109–4113. [Europe PMC free article] [Abstract] [Google Scholar]
- Pearson JD, Slakey LL, Gordon JL. Stimulation of prostaglandin production through purinoceptors on cultured porcine endothelial cells. Biochem J. 1983 Jul 15;214(1):273–276. [Europe PMC free article] [Abstract] [Google Scholar]
- Czervionke RL, Hoak JC, Fry GL. Effect of aspirin on thrombin-induced adherence of platelets to cultured cells from the blood vessel wall. J Clin Invest. 1978 Oct;62(4):847–856. [Europe PMC free article] [Abstract] [Google Scholar]
- Barnhart MI, Chen ST. Vessel wall models for studying interaction capabilities with blood platelets. Semin Thromb Hemost. 1978 Fall;5(2):112–155. [Abstract] [Google Scholar]
- Prescott SM, Zimmerman GA, McIntyre TM. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3534–3538. [Europe PMC free article] [Abstract] [Google Scholar]
- Camussi G, Aglietta M, Malavasi F, Tetta C, Piacibello W, Sanavio F, Bussolino F. The release of platelet-activating factor from human endothelial cells in culture. J Immunol. 1983 Nov;131(5):2397–2403. [Abstract] [Google Scholar]
- O'Flaherty JT, Wykle RL, Miller CH, Lewis JC, Waite M, Bass DA, McCall CE, DeChatelet LR. 1-O-Alkyl-sn-glyceryl-3-phosphorylcholines: a novel class of neutrophil stimulants. Am J Pathol. 1981 Apr;103(1):70–78. [Europe PMC free article] [Abstract] [Google Scholar]
- BLIGH EG, DYER WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. [Abstract] [Google Scholar]
- Mueller HW, O'Flaherty JT, Wykle RL. Biosynthesis of platelet activating factor in rabbit polymorphonuclear neutrophils. J Biol Chem. 1983 May 25;258(10):6213–6218. [Abstract] [Google Scholar]
- Caramelo C, Fernández-Gallardo S, Marín-Cao D, Iñarrea P, Santos JC, López-Novoa JM, Sanchez Crespo M. Presence of platelet-activating factor in blood from humans and experimental animals. Its absence in anephric individuals. Biochem Biophys Res Commun. 1984 May 16;120(3):789–796. [Abstract] [Google Scholar]
- Blank ML, Snyder F. Improved high-performance liquid chromatographic method for isolation of platelet-activating factor from other phospholipids. J Chromatogr. 1983 Apr 8;273(2):415–420. [Abstract] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. [Abstract] [Google Scholar]
- Durant GJ, Duncan WA, Ganellin CR, Parsons ME, Blakemore RC, Rasmussen AC. Impromidine (SK&F 92676) is a very potent and specific agonist for histamine H2 receptors. Nature. 1978 Nov 23;276(5686):403–405. [Abstract] [Google Scholar]
- Regoli D, Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [Abstract] [Google Scholar]
- Pearson JD, Gordon JL. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. [Abstract] [Google Scholar]
- Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. [Abstract] [Google Scholar]
- Blank ML, Snyder F, Byers LW, Brooks B, Muirhead EE. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1194–1200. [Abstract] [Google Scholar]
- Shaw JO, Pinckard RN, Ferrigni KS, McManus LM, Hanahan DJ. Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycerol-3-phosphorylcholine (platelet activating factor). J Immunol. 1981 Sep;127(3):1250–1255. [Abstract] [Google Scholar]
- Brotherton AF, Hoak JC. Prostacyclin biosynthesis in cultured vascular endothelium is limited by deactivation of cyclooxygenase. J Clin Invest. 1983 Oct;72(4):1255–1261. [Europe PMC free article] [Abstract] [Google Scholar]
- Egan RW, Paxton J, Kuehl FA., Jr Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem. 1976 Dec 10;251(23):7329–7335. [Abstract] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. [Abstract] [Google Scholar]
- Van Coevorden A, Boeynaems JM. Physiological concentrations of ADP stimulate the release of prostacyclin from bovine aortic endothelial cells. Prostaglandins. 1984 Apr;27(4):615–626. [Abstract] [Google Scholar]
- Kenzora JL, Pérez JE, Bergmann SR, Lange LG. Effects of acetyl glyceryl ether of phosphorylcholine (platelet activating factor) on ventricular preload, afterload, and contractility in dogs. J Clin Invest. 1984 Oct;74(4):1193–1203. [Europe PMC free article] [Abstract] [Google Scholar]
- Cabot MC, Blank ML, Welsh CJ, Horan MJ, Cress EA, Snyder F. Metabolism of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine by cell cultures. Life Sci. 1982 Dec 20;31(25):2891–2898. [Abstract] [Google Scholar]
- Ross R, Glomset JA. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. [Abstract] [Google Scholar]
- Jacob HS, Craddock PR, Hammerschmidt DE, Moldow CF. Complement-induced granulocyte aggregation: an unsuspected mechanism of disease. N Engl J Med. 1980 Apr 3;302(14):789–794. [Abstract] [Google Scholar]
- King GL, Buchwald S. Characterization and partial purification of an endothelial cell growth factor from human platelets. J Clin Invest. 1984 Feb;73(2):392–396. [Europe PMC free article] [Abstract] [Google Scholar]
- Gimbrone MA, Jr, Aster RH, Cotran RS, Corkery J, Jandl JH, Folkman J. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature. 1969 Apr 5;222(5188):33–36. [Abstract] [Google Scholar]
- Kitchens CS, Weiss L. Ultrastructural changes of endothelium associated with thrombocytopenia. Blood. 1975 Oct;46(4):567–578. [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci111957
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/111957/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/126118935
Article citations
Experimental Models of Traumatic Injuries: Do They Capture the Coagulopathy and Underlying Endotheliopathy Induced by Human Trauma?
Int J Mol Sci, 24(13):11174, 06 Jul 2023
Cited by: 1 article | PMID: 37446351 | PMCID: PMC10343021
Review Free full text in Europe PMC
Classes of Lipid Mediators and Their Effects on Vascular Inflammation in Atherosclerosis.
Int J Mol Sci, 24(2):1637, 13 Jan 2023
Cited by: 3 articles | PMID: 36675152 | PMCID: PMC9863938
Review Free full text in Europe PMC
Vascular Endothelial Cells: Heterogeneity and Targeting Approaches.
Cells, 10(10):2712, 10 Oct 2021
Cited by: 60 articles | PMID: 34685692 | PMCID: PMC8534745
Review Free full text in Europe PMC
Bioactive Ether Lipids: Primordial Modulators of Cellular Signaling.
Metabolites, 11(1):41, 08 Jan 2021
Cited by: 16 articles | PMID: 33430006 | PMCID: PMC7827237
Review Free full text in Europe PMC
A biotinylated peptide, BP21, as a novel potent anti-anaphylactic agent targeting platelet-activating factor.
J Pept Sci, 23(9):727-735, 19 Jun 2017
Cited by: 0 articles | PMID: 28627122
Go to all (236) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Endothelium from diverse vascular sources synthesizes platelet-activating factor.
Arteriosclerosis, 8(3):321-331, 01 May 1988
Cited by: 33 articles | PMID: 3130833
Human corneal epithelial cell functional responses to inflammatory agents and their antagonists.
Invest Ophthalmol Vis Sci, 39(13):2562-2571, 01 Dec 1998
Cited by: 23 articles | PMID: 9856766
IL-1 and related cytokines enhance thrombin-stimulated PGI2 production in cultured endothelial cells without affecting thrombin-stimulated von Willebrand factor secretion or platelet-activating factor biosynthesis.
J Immunol, 142(11):3993-3999, 01 Jun 1989
Cited by: 46 articles | PMID: 2497185
Voltage-gated and receptor-mediated ionic currents in the membrane of endothelial cells.
J Mol Cell Cardiol, 21 Suppl 1:103-108, 01 Feb 1989
Cited by: 12 articles | PMID: 2471836
Review