Abstract
Free full text
Live imaging of X chromosome inactivation and reactivation dynamics
Abstract
The epigenetic phenomenon called X chromosome inactivation plays critical roles in female development in eutherian mammals, and has attracted attention in the fields of developmental biology and regenerative biology in efforts to understand the pluripotency of stem cells. X chromosome inactivation is routinely studied after cell fixation, but live imaging is increasingly being required to improve our understanding of the dynamics and kinetics of X chromosome inactivation and reactivation processes. Here, we describe our live imaging method to monitor the epigenetic status of X chromosomes using a gene knock‐in mouse strain named “Momiji” and give an overview of the application of this strain as a resource for biological and stem cell research.
Abstract
X chromosome inactivation (XCI) is commonly studied in cells after fixation, but live imaging is increasingly being required to improve our understanding of the kinetics of XCI and X chromosome reactivation (XCR). Recently, we developed a live‐imaging method using a transgenic mouse strain named “Momiji” to monitor XCI and XCR. In this review, we focus on the detection methods of XCI, comparing conventional methods with those using our Momiji mice, and discuss several applications of this strain as a resource for studies in developmental biology, stem cell research, and other fields.
Introduction
In eutherian mammals, female individuals have two X chromosomes while males have only one. For the autosomes, the number of each chromosome is under strict surveillance, and an abnormal number of chromosomes termed “aneuploidy” causes embryonic death or genetic disorders such as Down syndrome (Siegel & Amon 2012). However, the situation in the X chromosome is different from that of autosomes. In female eutherian mammals, one of the two X chromosomes is epigenetically inactivated to compensate for the potential genetic imbalance between genders (Lyon 1961). This dosage compensation is called X chromosome inactivation (XCI). Using the mouse as a model, it has been proved that the disruption of XCI leads to activation of two X chromosomes resulting in embryonic death (Marahrens et al. 1997). Thus, XCI is essential for the normal growth and development of embryos.
X chromosome inactivation is considered to be a common epigenetic phenomenon observed in eutherian mammals (Graves 2015). Among them, the mouse is the most favored model and has been well characterized. Which of the two X chromosomes is inactivated during development? The XCI pattern changes dynamically depending on the developmental stages and tissues of embryos. At preimplantation stages, the paternal X chromosome is preferentially inactivated and referred to as imprinted XCI. After implantation, this imprinted XCI is maintained in extraembryonic tissues, but lost in the epiblast (Epi) cells, which give rise to the embryo proper. Reactivation of the inactivated X chromosome occurs in the inner cell mass (ICM) cells in blastocysts; two X chromosomes become activated, which is referred to as X chromosome reactivation (XCR). Shortly after XCR, when embryogenesis proceeds, either the maternal or the paternal X chromosomes is selected randomly and inactivated in every cell of the whole embryo, which is called random XCI. Therefore, there are three different epigenetic patterns of XCI in vivo: i.e., imprinted XCI, XCR, and random XCI. Technical improvements that could enable us to distinguish these different patterns easily is a high priority in developmental biology research.
Besides basic research in developmental biology, XCI has attracted keen attention in stem cell research and regenerative biology. Pluripotent stem cells (PSCs) used in regenerative biology have been classified into two states; naïve and primed (Nichols & Smith 2009). Naïve PSCs can contribute to blastocyst chimeras and reflect an undifferentiated ground state of pluripotency. Primed PSCs possess very little capacity to contribute to chimeras, and are developmentally and functionally distinct from naïve PSCs. In the mouse, embryonic stem cells (ESCs) established from preimplantation embryos represent the naïve state, while epiblast stem cells (EpiSCs) from the epiblast of postimplantation embryos represent the primed state. In both types of PSCs, core pluripotent marker genes such as Oct3/4, Sox2, and Nanog, are expressed; therefore, these markers are not useful for distinguishing naïve from primed states. However, there is a major difference between the two types of PSCs, namely the activities of the two X chromosomes. ESCs have two active X chromosomes (XaXa) representing the XCR state, while EpiSCs have one epigenetically inactivated X chromosome (XiXa) corresponding to XCI. These epigenetic differences could be used as markers to distinguish the two different states of PSCs. So far, the differences between XCR and XCI have been analyzed using fixed cells. Therefore, it is impossible to study the characteristics of such cells further. The lack of a live imaging method to monitor XCI and XCR has been a major obstacle in performing stem cell research using living PSCs.
We have developed a live imaging method using a gene knock‐in mouse strain named “Momiji” to monitor XCI and XCR (Kobayashi et al. 2016). This review focuses on methods for detecting XCI by comparing conventional methods with our Momiji mice. We discuss several applications of this mouse strain as a resource in developmental biology, stem cell research, and other fields. For detailed explanations of the XCI mechanism and the relationship between XCR and pluripotency of stem cells, please see recent reviews (Ohhata & Wutz 2013; Payer & Lee 2014; Galupa & Heard 2015; Pasque & Plath 2015).
Research using X‐linked GFP transgenic mice
X chromosome inactivation has been studied for more than 50 years and various methods have been proposed. Table 1 summarizes the methods used. Almost all the commonly used methods require fixation of cells (or tissues) for the analysis. The method using X‐linked green fluorescent protein (GFP)‐expressing transgenic mice (XGFP mice) is an exception that can detect XCI in living cells and is now used as one of the most popular methods in detecting XCI in living cells noninvasively.
Table 1
Summary of conventional methods or detecting X chromosome inactivation (XCI)
| Detection methods | Pretreatment of samples | Note | References |
|---|---|---|---|
| Observation of Barr bodies | Fixation | Kanda's method, which renders the Xi dark staining | Rastan et al. (1980) |
| Replication timing | Fixation | Xi shows late replication within the S phase of the cell cycle | Takagi et al. (1982) |
| Measurement of enzymatic activity | Cell extraction | Activities of X‐linked enzymes (Hprt, Pgk1) | McMahon & Monk (1983),Monk & Harper (1979) |
| RNA fluorescent in situ hybridization (FISH) | Fixation | FISH detection of X‐linked gene expression, Cot‐1 exclusion from Xi, Xist expression as a marker of Xi | Huynh & Lee (2003), Mak et al. (2004), Okamoto et al. (2004) |
| Allele‐specific expression analysis | RNA extraction | Allele‐specific expression analysis using DNA polymorphisms detected by RT–PCR or RNA sequencing | Marks et al. (2015), Sugimoto & Abe (2007) |
| Antibodies | Fixation | Immunostaining using H3K27me3‐ or Eed‐specific antibodies to detect Xi; DNA polymerase II‐specific antibody to detect its exclusion from Xi | Okamoto et al. (2004) |
| Semi‐invasive; antigen‐binding fragments (Fabs) injection or loading into live cells | Fluorescently labeled H3K27me3‐specific Fabs are used to detect Xi | Hayashi‐Takanaka et al. (2011) | |
| Transgenic ESCs | Noninvasive | Ezh2‐Venus transgenic ESCs to detect Xi, MS2 tagged Xist RNA transgenic ESCs to detect Xi | Guyochin et al. (2014), Ng et al. (2011) |
| Transgenic mice | Fixation | HMG‐lacZ transgene inserted into the X chromosome to detect its activity | Tan et al. (1993) |
| Noninvasive | CAG‐eGFP transgene inserted into the X chromosome to detect its activity | Hadjantonakis et al. (2001),Takagi et al. (2002) | |
| Noninvasive | microH2A‐eGFP transgene inserted into autosomes to detect Xi | Soma et al. (2013) | |
| Noninvasive | CAG‐GFP and CAG‐tomato transgenes inserted into X chromosomes to detect them | Wu et al. (2014) |
Cot‐1, interspersed repetitive elements; eGFP, enhanced green fluorescent protein; ESC, embryonic stem cell; Ezh2, enhancer of zeste homologue 2; HMG‐lacZ, 3‐hydroxy‐3‐methylglutarylCoA reductase (HMG) promoter driving the Escherichia coli beta‐galactosidase (lacZ) gene; Hprt, hypoxanthine phosphoribosyltransferase; microH2A, variant of the core histone H2A; MS2, RNA‐binding protein derived from phage; Pgk1, phosphoglycerate kinase 1; RT–PCR, reverse transcription polymerase chain reaction; Xi, inactive X chromosome.
Originally, GFP‐expressing transgenic mice – so‐called “green mice” – were generated by Okabe's group and were the first mammals engineered to produce this fluorescent protein as a marker (Ikawa et al. 1995; Okabe et al. 1997). Using standard plasmid injection techniques, many transgenic lines were produced and then analyzed with fluorescence in situ hybridization (FISH) to obtain GFP‐tagged chromosomes of many kinds (Nakanishi et al. 2002). Among them, several transgenic lines had the GFP reporter cassette integrated in the X chromosome (XGFP). These strains were used for sexing preimplantation stage embryos and analyzing XCI (Hadjantonakis et al. 1998, 2001; Takagi et al. 2002; Isotani et al. 2005; Kobayashi et al. 2006, 2010, 2013). These mice are very useful for XCI research because the inactive X chromosome can easily be distinguished from the active X chromosome by just observing GFP fluorescence and no pretreatment of the samples is needed. However, there are restrictions in using these mice for some research purposes: they can only be used to monitor the activity of one X chromosome. In other words, one cannot detect XCR in developing embryos as well as in naïve PSCs and this approach cannot be used to distinguish the paternal from the maternal X chromosome during random XCI.
Live imaging of Momiji mice to detect XCI and XCR
The conventional XGFP transgenic mice have restrictions in terms of some XCI research purposes; therefore, we generated the so‐called Momiji strain (named after the leaves of the Japanese maple, which are tinged with red, green and yellow in autumn) by knocking in reporter genes for green and red fluorescent proteins (enhanced [e]GFP and mCherry, respectively) into each one of the X chromosomes (Fig. 1A). The previously reported XGFP mice had multiple copies of the CAG‐GFP reporter gene produced by random integration, but the precise integration sites were not clear. However, the integration site of a reporter is important if we are to monitor XCI precisely because some X‐linked genes are known to escape this fate. Furthermore, a recent study has proposed that spreading of silencing along the X chromosome depends on the three‐dimensional conformation of the X chromosome during the establishment of XCI in vitro (reviewed in Finestra & Gribnau 2017). This suggests that the position of any reporter gene integration site can affect the monitoring of XCI initiation. In Momiji mice, to monitor XCI more precisely, and assess the positional effects of the reporter gene, single copy transgenes were targeted to loci where X inactivation is known to occur – Hprt and Pgk1– and two different color reporter cassettes were inserted into the same loci of both X chromosomes (Fig. 1A). These two housekeeping gene loci were ideal for the insertion site because they are thought to be expressed ubiquitously throughout all developmental stages. We used the stronger CAG promoter to drive the reporter genes instead of the weaker endogenous Hprt or Pgk1 promoters. In addition, previous studies showed that the CAG promoter inserted in the X chromosome was subject to XCI and was completely inactivated at postimplantation stages (Hadjantonakis et al. 2001; Takagi et al. 2002) confirming the susceptibility of this promoter for monitoring XCI. In the Momiji system, the cells subject to XCI show either red or green fluorescence. By contrast, cells in the XCR state appear yellow, because two X chromosomes are active resulting in expression of both eGFP and mCherry (Fig. 1B).
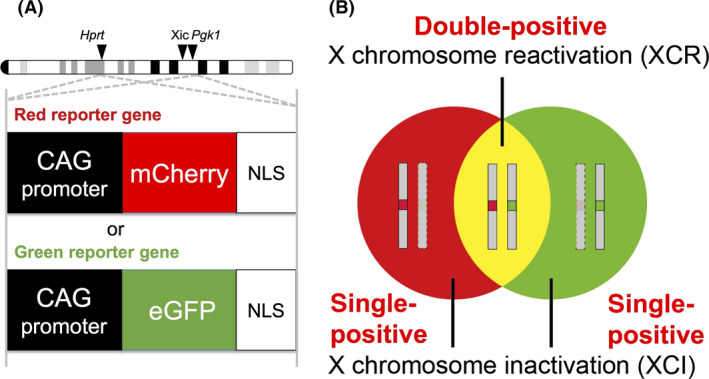
Momiji system for live‐cell imaging of the epigenetic status of X‐chromosomes. (A) Two different color reporter cassettes (red mCherry and green eGFP) were inserted into the X chromosome. Two X‐linked gene loci (Hprt and Pgk1) were used for the insertion of the reporter gene cassettes. NLS, nuclear localization signal; Xic, X‐inactivation center. (B) Representative patterns of X chromosome inactivation (XCI) and X chromosome reactivation (XCR) in Momiji mice. Cells undergoing XCI show either red or green florescent single‐positive cells; whereas the cells undergoing XCR show yellow fluorescent double‐positive cells. Modified from figure 1 of Kobayashi et al. (2016).
The Momiji mice allow us to detect imprinted XCI, XCR, and random XCI noninvasively (Fig. 2). Live‐cell imaging techniques also make it possible to detect changes in the XCI patterns at the single cell level. We describe our applications in the next two sections.

Dynamic changes in X chromosome inactivation status during early mouse development and in cultured cells. In addition to XCR, imprinted and random XCI were successfully detected in vivo as well as in vitro using Momiji mice as a resource. The timing of XCR in blastocysts seems to be slightly delayed or take a longer time to be completed than in the previous proposed model in which XCR occurs in the ICM of the early blastocyst. Xp, paternal X chromosome; Xm, maternal X chromosome; 2i/LIF, ES medium containing CHIR99021, PD0325901 and leukemia inhibitory factor; PGC, primordial germ cells; PE, primitive endoderm. Modified from figure 4 of Kobayashi et al. (2016).
The application of Momiji mice in developmental biology
Thus far, XCR has been reported to occur in the ICM of blastocysts before implantation at embryonic day (E) 4.5 (Mak et al. 2004; Okamoto et al. 2004). In these reports, XCR was analyzed by RNA FISH and immunostaining using several chromosome‐wide markers of inactive X chromosomes, such as Xist RNA coating, Eed protein enrichment, and histone H3 lysine 27 methylation (H3K27me) (Table 1). These markers disappeared gradually during ICM maturation. Another group (Williams et al. 2011) reported that XCR began in midstage blastocysts between E3.5 and E3.75 using RNA FISH analysis measuring X‐linked gene expression directly (Table 1). Taking advantage of our Momiji mice, we re‐analyzed the kinetics of XCR precisely during peri‐implantation stages at single‐cell levels. The results showed that the XCR state varied depending on individual blastomeres in the ICM of E3.5 midstage and in the epiblast of E4.5 late‐stage blastocysts. In our observations, some of the ICM cells showed double‐positive red and green signals, suggesting that XCR had begun at this stage. During E4.5 to E5.5 in peri‐ and postimplantation embryos, the numbers of double‐positive cells increased gradually. In E5.5 embryos, almost all Epi cells showed double‐positive signals, suggesting that XCR had been completed by this stage (Fig. 2).
Analysis of random XCI at the Hprt locus revealed that the double‐positive cells had disappeared by E6.5. Either red or green signals were detected in every Epi cell. These results indicated either the maternal or the paternal X chromosome had been inactivated randomly and XCI had been completed by this stage. In contrast, XCI in the Pgk1 locus was slightly delayed and had not been completed by E6.5. This delay was also observed using conventional methods using enzymatic activity assays for the Hprt and Pgk1 protein products (Table 1) (Monk & Harper 1979; McMahon & Monk 1983). This delay appears to reflect the positional effects of X‐linked genes in the establishment of XCI; we successfully detected the effect in vivo using Momiji mice.
Although our results were almost consistent with the previous reports using conventional XCI detection methods (Mak et al. 2004; Okamoto et al. 2004; Williams et al. 2011), the timing of XCR in blastocysts seemed to be slightly delayed or take longer to be completed compared with the previously proposed model in which XCR occurs in the ICM of the early blastocyst (Augui et al. 2011; Makhlouf & Rougeulle 2011; Fig. 2). Because XCR was apparent in ESCs derived from Momiji mice in terms of XCR, our results support the idea that ESCs are distinct from the early ICM but closely resemble preimplantation Epi cells at later stage (Boroviak et al. 2014; Plusa & Hadjantonakis 2014). Further analysis using single‐cell gene expression analysis would be helpful to clarify this point.
The application of Momiji mice to PSC research
X chromosome reactivation is thought to be closely coupled to the pluripotency of PSCs (Ohhata & Wutz 2013; Payer & Lee 2014; Pasque & Plath 2015). Although XCR is generally accepted as a marker to discriminate naïve from primed state PSCs, the commonly used XCR detection method requires fixation to detect the H3K27me3 immunostaining pattern (Table 1). Using Momiji mice as a resource, we have successfully distinguished XCR from XCI by observing the fluorescent color of the cells. This strain provides a simple method for distinguishing naïve from primed state PSCs based on XCR and for evaluating the quality of PSCs necessary for basic research in regenerative biology and medicine (Kobayashi et al. 2016; Fig. 3).
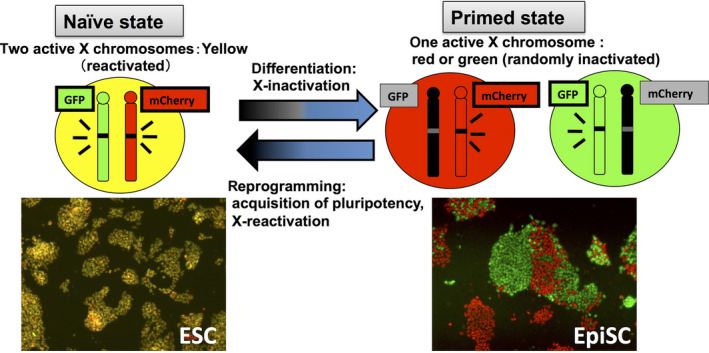
Detection of two phases of pluripotent stem cells using Momiji mice as a resource. Two different pluripotent stem cell lines – naïve state embryonic stem cells (ESCs) and primed state epiblast stem cells (EpiSCs) – can be distinguished by observing the fluorescent color of each cell. Naïve ESCs can be induced to differentiate into primed EpiSCs by exposure to activin A and basic fibroblast growth factor, whereas the reverse transition requires reprogramming factors, such as Klf4 or other small molecules. One can trace the epigenetic changes of XCI by observing the fluorescent colors during these transitions in both directions (differentiation and reprogramming). Lower images show representative colonies of ESCs and EpiSCs.
Future applications for Momiji mice: XCR in development
Because XCR also occurs in primordial germ cells (PGCs), Momiji mice are useful for monitoring its timing or kinetics. In mice, PGCs are first identified around E7.5 in the posterior epiblast. Then, PGCs start migrating to the genital ridges. So far, allelic expression analysis in single cells isolated from embryos, and X‐linked lacZ transgene analysis (Table 1) reported that the PGCs at E7.5 have already initiated random XCI (Tam et al. 1994; Sugimoto & Abe 2007). During this migration, PGCs proliferate and epigenetic modifications are reprogrammed on a genome‐wide scale, and inactivated X chromosomes become reactivated (reviewed in Hackett et al. 2012). Although XCR is thought to be associated with cell proliferation, migration, and interactions with other somatic tissues (Chuva De Sousa Lopes et al. 2008; Hackett et al. 2012), thorough spatiotemporal analysis is lacking and the precise mechanism of this process remains to be elucidated. Our Momiji mice would make it possible to analyze the process of XCR in PGC development spatiotemporally at the single cell level.
Future applications for Momiji mice: XCR and XCI in vitro
EpiSCs can be obtained from ESCs by exposure to activin A and basic fibroblast growth factor (Nichols & Smith 2009). This transition is thought to mimic authentic differentiation in vivo because one of the X chromosomes becomes inactivated. Consistent with this observation, during differentiation from ESCs to EpiSCs, PSCs derived from Momiji mice could be used to detect the establishment of random XCI in vitro. Whereas the reverse transition from EpiSCs to ESCs/induced PSCs requires reprograming factors such as Klf4 (Guo et al. 2009) or other small molecules (Murayama et al. 2015), and we also successfully detected this reprograming using PSCs from Momiji mice (S. Kobayashi et al. unpubl. data). Thus, this model can be used to trace changes in the X chromosome's epigenetic status in two directions in living cells in vitro (Fig. 3). Based on observation of the fluorescent color changes in each cell undergoing differentiation and/or reprogramming, the process of XCI establishment and/or reprogramming at a single cell level may be traced, the cells separated, and the detailed profiles of gene expression and epigenetic modification may be analyzed. In this way, single‐cell analysis allows us to analyze the molecular mechanisms of random XCI as well as reprograming more precisely than analyses using bulk cell populations. Furthermore, in human embryos, XCR is also observed in the ICM of blastocysts and could be used as a marker to distinguish naïve from primed PSCs in vitro (Sahakyan et al. 2017). The same strategy of live‐imaging of XCR could help in improving the culture conditions for human naïve PSCs. Establishment of primate Momiji‐type cells are currently underway. Fundamental analysis of genomic reprogramming would bring us closer to understanding the biology of naïve cells, in capturing this state stably, and help in the effective establishment of naïve human iPSCs from conventional primed ESCs.
Live‐imaging approach combined with cell and organ culture using Momiji mice
The need for fixation in conventional methods prevents us from following the fate of cells of interest in embryogenesis. However, using Momiji mice combined with embryo culture, we carried out time‐lapse imaging and successfully observed the initiation of imprinted XCI during cell division in early preimplantation embryos (Kobayashi et al. 2016). We also found that individual blastomeres showed not uniform but heterogeneous patterns in terms of XCI status, suggesting that imprinted XCI is incomplete during these stages.
Several attempts have been made to capture the process of postimplantation development, and oogenesis reconstituted using in vitro culture systems (Rivera‐Perez et al. 2010; Bedzhov et al. 2014; Hikabe et al. 2016). Random XCI occurs in postimplantation embryos, and XCR occurs in developing PGCs, which give rise to oocytes. In these processes, XCI and XCR need to be regulated spatially and temporally, along with cell division, cell cycle progression, and interactions with somatic cells. The live‐imaging approach using Momiji mice will enable us to follow the fate of differentiating cells and monitor the changes in XCI status in these cells. More precise observations in vitro will help us to understand the mechanisms of XCI and XCR in vivo.
Other research fields
Only a few cell types are known to show XCR during development, and XCR is thought to be rare in vivo (Heard & Disteche 2006). However, it can increase with aging and in cancerous cells (Wareham et al. 1987; Spatz et al. 2004; Chaligne et al. 2015) suggesting that XCR might not be limited to normal embryogenesis. Momiji mice might have advantages for exploring such cell types undergoing XCR. It is worth evaluating whether such cells can be found during development, aging, tumorigenesis, and dedifferentiation during tissue regeneration. It would also be interesting to investigate the common characteristics between such newly found cells undergoing XCR, especially in terms of the pluripotency of stem cells. Analysis of such cells could help in clarifying the mechanisms and biological significance of the reactivation of XCI in vivo.
Conclusions
Experimental systems using Momiji mice are powerful tools for visualizing epigenetic changes in XCI during development and disease as well as in pluripotent stem cells. These genomic knock‐in mice are available to the scientific community from RIKEN BRC upon request (BRC No. RBRC09532‐09537).
Acknowledgments
I thank all the collaborators, especially, Dr Fumitoshi Ishino at Tokyo Medical Dental University and Dr Masaru Okabe at Osaka University for helpful advice and encouragement, Drs Takashi Kohda, Hirosuke Shiura, and Yuki Kawasaki for critical reading and discussions. This work was supported by the Japan Science and Technology Agency, Precursory Research for Embryonic Science and Technology (PRESTO) and by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (23500492, 15H01468, 26430087, 17H05597, 17K07498) to S.K.
References
- Augui, S. , Nora, E. P. & Heard, E. 2011. Regulation of X‐chromosome inactivation by the X‐inactivation centre. Nat. Rev. Genet. 12, 429–442. [Abstract] [Google Scholar]
- Bedzhov, I. , Leung, C. Y. , Bialecka, M. & Zernicka‐Goetz, M. 2014. In vitro culture of mouse blastocysts beyond the implantation stages. Nat. Protoc. 9, 2732–2739. [Abstract] [Google Scholar]
- Boroviak, T. , Loos, R. , Bertone, P. , Smith, A. & Nichols, J. 2014. The ability of inner‐cell‐mass cells to self‐renew as embryonic stem cells is acquired following epiblast specification. Nat. Cell Biol. 16, 516–528. [Europe PMC free article] [Abstract] [Google Scholar]
- Chaligne, R. , Popova, T. , Mendoza‐Parra, M. A. , Saleem, M. A. , Gentien, D. , Ban, K. , Piolot, T. , Leroy, O. , Mariani, O. , Gronemeyer, H. , Vincent‐Salomon, A. , Stern, M. H. & Heard, E. 2015. The inactive X chromosome is epigenetically unstable and transcriptionally labile in breast cancer. Genome Res. 25, 488–503. [Europe PMC free article] [Abstract] [Google Scholar]
- Chuva De Sousa Lopes, S. M. , Hayashi, K. , Shovlin, T. C. , Mifsud, W. , Surani, M. A. & McLaren, A. 2008. X chromosome activity in mouse XX primordial germ cells. PLoS Genet. 4, e30. [Europe PMC free article] [Abstract] [Google Scholar]
- Finestra, T. R. & Gribnau, J. 2017. X chromosome inactivation: silencing, topology and reactivation. Curr. Opin. Cell Biol. 46, 54–61. [Abstract] [Google Scholar]
- Galupa, R. & Heard, E. 2015. X‐chromosome inactivation: new insights into cis and trans regulation. Curr. Opin. Genet. Dev. 31, 57–66. [Abstract] [Google Scholar]
- Graves, J. A. 2015. Weird mammals provide insights into the evolution of mammalian sex chromosomes and dosage compensation. J. Genet. 94, 567–574. [Abstract] [Google Scholar]
- Guo, G. , Yang, J. , Nichols, J. , Hall, J. S. , Eyres, I. , Mansfield, W. , Mansfield, W. & Smith, A. 2009. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063–1069. [Europe PMC free article] [Abstract] [Google Scholar]
- Guyochin, A. , Maenner, S. , Chu, E. T. , Hentati, A. , Attia, M. , Avner, P. & Clerc, P. 2014. Live cell imaging of the nascent inactive X chromosome during the early differentiation process of naive ES cells towards epiblast stem cells. PLoS ONE 9, e116109. [Europe PMC free article] [Abstract] [Google Scholar]
- Hackett, J. A. , Zylicz, J. J. & Surani, M. A. 2012. Parallel mechanisms of epigenetic reprogramming in the germline. Trends Genet. 28, 164–174. [Abstract] [Google Scholar]
- Hadjantonakis, A. K. , Gertsenstein, M. , Ikawa, M. , Okabe, M. & Nagy, A. 1998. Non‐invasive sexing of preimplantation stage mammalian embryos. Nat. Genet. 19, 220–222. [Abstract] [Google Scholar]
- Hadjantonakis, A. K. , Cox, L. L. , Tam, P. P. & Nagy, A. 2001. An X‐linked GFP transgene reveals unexpected paternal X‐chromosome activity in trophoblastic giant cells of the mouse placenta. Genesis 29, 133–140. [Abstract] [Google Scholar]
- Hayashi‐Takanaka, Y. , Yamagata, K. , Wakayama, T. , Stasevich, T. J. , Kainuma, T. , Tsurimoto, T. , Tachibana, M. , Shinkai, Y. , Kurumizaka, H. , Nozaki, N. & Kimura, H. 2011. Tracking epigenetic histone modifications in single cells using Fab‐based live endogenous modification labeling. Nucleic Acids Res. 39, 6475–6488. [Europe PMC free article] [Abstract] [Google Scholar]
- Heard, E. & Disteche, C. M. 2006. Dosage compensation in mammals: fine‐tuning the expression of the X chromosome. Genes Dev. 20, 1848–1867. [Abstract] [Google Scholar]
- Hikabe, O. , Hamazaki, N. , Nagamatsu, G. , Obata, Y. , Hirao, Y. , Hamada, N. , Shimamoto, S. , Imamura, T. , Nakashima, K. , Saitou, M. & Hayashi, K. 2016. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 539, 299–303. [Abstract] [Google Scholar]
- Huynh, K. D. & Lee, J. T. 2003. Inheritance of a pre‐inactivated paternal X chromosome in early mouse embryos. Nature 426, 857–862. [Abstract] [Google Scholar]
- Ikawa, M. , Kominami, K. , Yoshimura, Y. , Tanaka, K. , Nishimune, Y. & Okabe, M. 1995. A rapid and non‐invasive selection of transgenic embryos before implantation using green fluorescent protein (GFP). FEBS Lett. 375, 125–128. [Abstract] [Google Scholar]
- Isotani, A. , Nakanishi, T. , Kobayashi, S. , Lee, J. , Chuma, S. , Nakatsuji, N. , Ishino, F. & Okabe, M. 2005. Genomic imprinting of XX spermatogonia and XX oocytes recovered from XX↔XY chimeric testes. Proc. Natl Acad. Sci. USA 102, 4039–4044. [Europe PMC free article] [Abstract] [Google Scholar]
- Kobayashi, S. , Isotani, A. , Mise, N. , Yamamoto, M. , Fujihara, Y. , Kaseda, K. , Nakanishi, T. , Ikawa, M. , Hamada, H. , Abe, K. & Okabe, M. 2006. Comparison of gene expression in male and female mouse blastocysts revealed imprinting of the X‐linked gene, Rhox5/Pem, at preimplantation stages. Curr. Biol. 16, 166–172. [Abstract] [Google Scholar]
- Kobayashi, S. , Fujihara, Y. , Mise, N. , Kaseda, K. , Abe, K. , Ishino, F. & Okabe, M. 2010. The X‐linked imprinted gene family Fthl17 shows predominantly female expression following the two‐cell stage in mouse embryos. Nucleic Acids Res. 38, 3672–3681. [Europe PMC free article] [Abstract] [Google Scholar]
- Kobayashi, S. , Totoki, Y. , Soma, M. , Matsumoto, K. , Fujihara, Y. , Toyoda, A. , Sakaki, Y. , Okabe, M. & Ishino, F. 2013. Identification of an imprinted gene cluster in the X‐inactivation center. PLoS ONE 8, e71222. [Europe PMC free article] [Abstract] [Google Scholar]
- Kobayashi, S. , Hosoi, Y. , Shiura, H. , Yamagata, K. , Takahashi, S. , Fujihara, Y. , Kohda, T. , Okabe, M. & Ishino, F. 2016. Live imaging of X chromosome reactivation dynamics in early mouse development can discriminate naive from primed pluripotent stem cells. Development 143, 2958–2964. [Abstract] [Google Scholar]
- Lyon, M. F. 1961. Gene action in the X‐chromosome of the mouse (Mus musculus L.). Nature 190, 372–373. [Abstract] [Google Scholar]
- Mak, W. , Nesterova, T. B. , De Napoles, M. , Appanah, R. , Yamanaka, S. , Otte, A. P. & Brockdorff, N. 2004. Reactivation of the paternal X chromosome in early mouse embryos. Science 303, 666–669. [Abstract] [Google Scholar]
- Makhlouf, M. & Rougeulle, C. 2011. Linking X chromosome inactivation to pluripotency: necessity or fate?. Trends Mol. Med. 17, 329–336. [Abstract] [Google Scholar]
- Marahrens, Y. , Panning, B. , Dausman, J. , Strauss, W. & Jaenisch, R. 1997. Xist‐deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 11, 156–166. [Abstract] [Google Scholar]
- Marks, H. , Kerstens, H. H. , Barakat, T. S. , Splinter, E. , Dirks, R. A. , Van Mierlo, G. , Joshi, O. , Wang, S. Y. , Babak, T. , Albers, C. A. , Kalkan, T. , Smith, A. , Jouneau, A. , De Laat, W. , Gribnau, J. & Stunnenberg, H. G. 2015. Dynamics of gene silencing during X inactivation using allele‐specific RNA‐seq. Genome Biol. 16, 149. [Europe PMC free article] [Abstract] [Google Scholar]
- McMahon, A. & Monk, M. 1983. X‐chromosome activity in female mouse embryos heterozygous for Pgk‐1 and Searle's translocation, T(X;16) 16H. Genet. Res. 41, 69–83. [Abstract] [Google Scholar]
- Monk, M. & Harper, M. I. 1979. Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos. Nature 281, 311–313. [Abstract] [Google Scholar]
- Murayama, H. , Masaki, H. , Sato, H. , Hayama, T. , Yamaguchi, T. & Nakauchi, H. 2015. Successful reprogramming of epiblast stem cells by blocking nuclear localization of beta‐catenin. Stem Cell Reports 4, 103–113. [Europe PMC free article] [Abstract] [Google Scholar]
- Nakanishi, T. , Kuroiwa, A. , Yamada, S. , Isotani, A. , Yamashita, A. , Tairaka, A. , Hayashi, T. , Takagi, T. , Ikawa, M. , Matsuda, Y. & Okabe, M. 2002. FISH analysis of 142 EGFP transgene integration sites into the mouse genome. Genomics 80, 564–574. [Abstract] [Google Scholar]
- Ng, K. , Daigle, N. , Bancaud, A. , Ohhata, T. , Humphreys, P. , Walker, R. , Ellenberg, J. & Wutz, A. 2011. A system for imaging the regulatory noncoding Xist RNA in living mouse embryonic stem cells. Mol. Biol. Cell 22, 2634–2645. [Europe PMC free article] [Abstract] [Google Scholar]
- Nichols, J. & Smith, A. 2009. Naive and primed pluripotent states. Cell Stem Cell 4, 487–492. [Abstract] [Google Scholar]
- Ohhata, T. & Wutz, A. 2013. Reactivation of the inactive X chromosome in development and reprogramming. Cell. Mol. Life Sci. 70, 2443–2461. [Europe PMC free article] [Abstract] [Google Scholar]
- Okabe, M. , Ikawa, M. , Kominami, K. , Nakanishi, T. & Nishimune, Y. 1997. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407, 313–319. [Abstract] [Google Scholar]
- Okamoto, I. , Otte, A. P. , Allis, C. D. , Reinberg, D. & Heard, E. 2004. Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303, 644–649. [Abstract] [Google Scholar]
- Pasque, V. & Plath, K. 2015. X chromosome reactivation in reprogramming and in development. Curr. Opin. Cell Biol. 37, 75–83. [Europe PMC free article] [Abstract] [Google Scholar]
- Payer, B. & Lee, J. T. 2014. Coupling of X‐chromosome reactivation with the pluripotent stem cell state. RNA Biol. 11, 798–807. [Europe PMC free article] [Abstract] [Google Scholar]
- Plusa, B. & Hadjantonakis, A. K. 2014. Embryonic stem cell identity grounded in the embryo. Nat. Cell Biol. 16, 502–504. [Europe PMC free article] [Abstract] [Google Scholar]
- Rastan, S. , Kaufman, M. H. , Handyside, A. H. & Lyon, M. F. 1980. X‐chromosome inactivation in extra‐embryonic membranes of diploid parthenogenetic mouse embryos demonstrated by differential staining. Nature 288, 172–173. [Abstract] [Google Scholar]
- Rivera‐Perez, J. A. , Jones, V. & Tam, P. P. 2010. Culture of whole mouse embryos at early postimplantation to organogenesis stages: developmental staging and methods. Methods Enzymol. 476, 185–203. [Abstract] [Google Scholar]
- Sahakyan, A. , Kim, R. , Chronis, C. , Sabri, S. , Bonora, G. , Theunissen, T. W. , Kuoy, E. , Langerman, J. , Clark, A. T. , Jaenisch, R. & Plath, K. 2017. Human naive pluripotent stem cells model X chromosome dampening and X inactivation. Cell Stem Cell 20, 87–101. [Europe PMC free article] [Abstract] [Google Scholar]
- Siegel, J. J. & Amon, A. 2012. New insights into the troubles of aneuploidy. Annu. Rev. Cell Dev. Biol. 28, 189–214. [Europe PMC free article] [Abstract] [Google Scholar]
- Soma, A. , Sato, K. & Nakanishi, T. 2013. Visualization of inactive X chromosome in preimplantation embryos utilizing MacroH2A‐EGFP transgenic mouse. Genesis 51, 259–267. [Abstract] [Google Scholar]
- Spatz, A. , Borg, C. & Feunteun, J. 2004. X‐chromosome genetics and human cancer. Nat. Rev. Cancer 4, 617–629. [Abstract] [Google Scholar]
- Sugimoto, M. & Abe, K. 2007. X chromosome reactivation initiates in nascent primordial germ cells in mice. PLoS Genet. 3, e116. [Europe PMC free article] [Abstract] [Google Scholar]
- Takagi, N. , Sugawara, O. & Sasaki, M. 1982. Regional and temporal changes in the pattern of X‐chromosome replication during the early post‐implantation development of the female mouse. Chromosoma 85, 275–286. [Abstract] [Google Scholar]
- Takagi, N. , Sugimoto, M. , Yamaguchi, S. , Ito, M. , Tan, S. S. & Okabe, M. 2002. Nonrandom X chromosome inactivation in mouse embryos carrying Searle's T(X;16)16H translocation visualized using X‐linked LacZ and GFP transgenes. Cytogenet. Genome Res. 99, 52–58. [Abstract] [Google Scholar]
- Tam, P. P. , Zhou, S. X. & Tan, S. S. 1994. X‐chromosome activity of the mouse primordial germ cells revealed by the expression of an X‐linked lacZ transgene. Development 120, 2925–2932. [Abstract] [Google Scholar]
- Tan, S. S. , Williams, E. A. & Tam, P. P. 1993. X‐chromosome inactivation occurs at different times in different tissues of the post‐implantation mouse embryo. Nat. Genet. 3, 170–174. [Abstract] [Google Scholar]
- Wareham, K. A. , Lyon, M. F. , Glenister, P. H. & Williams, E. D. 1987. Age related reactivation of an X‐linked gene. Nature 327, 725–727. [Abstract] [Google Scholar]
- Williams, L. H. , Kalantry, S. , Starmer, J. & Magnuson, T. 2011. Transcription precedes loss of Xist coating and depletion of H3K27me3 during X‐chromosome reprogramming in the mouse inner cell mass. Development 138, 2049–2057. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu, H. , Luo, J. , Yu, H. , Rattner, A. , Mo, A. , Wang, Y. , Smallwood, P. M. , Erlanger, B. , Wheelan, S. J. & Nathans, J. 2014. Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease. Neuron 81, 103–119. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1111/dgd.12365
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/dgd.12365
Citations & impact
Impact metrics
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/dgd.12365
Article citations
Visualization of X chromosome reactivation in mouse primordial germ cells in vivo.
Biol Open, 10(4):bio058602, 29 Apr 2021
Cited by: 4 articles | PMID: 33913476 | PMCID: PMC8096617
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Live imaging of X chromosome reactivation dynamics in early mouse development can discriminate naïve from primed pluripotent stem cells.
Development, 143(16):2958-2964, 28 Jul 2016
Cited by: 16 articles | PMID: 27471261
X-inactivation and X-reactivation: epigenetic hallmarks of mammalian reproduction and pluripotent stem cells.
Hum Genet, 130(2):265-280, 12 Jun 2011
Cited by: 44 articles | PMID: 21667284 | PMCID: PMC3744832
X-chromosome epigenetic reprogramming in pluripotent stem cells via noncoding genes.
Semin Cell Dev Biol, 22(4):336-342, 03 Mar 2011
Cited by: 23 articles | PMID: 21376830 | PMCID: PMC3175323
Review Free full text in Europe PMC
Live Imaging of X-Chromosome Inactivation and Reactivation Kinetics.
Methods Mol Biol, 1861:73-89, 01 Jan 2018
Cited by: 4 articles | PMID: 30218361
Funding
Funders who supported this work.
Japan Science and Technology Agency
Ministry of Education, Culture, Sports, Science and Technology (5)
Grant ID: 23500492
Grant ID: 15H01468
Grant ID: 17H05597
Grant ID: 17K07498
Grant ID: 26430087

 1
,
2
1
,
2