Abstract
Free full text

Ultrasound-mediated piezoelectric differentiation of neuron-like PC12 cells on PVDF membranes
Associated Data
Abstract
Electrical and/or electromechanical stimulation has been shown to play a significant role in regenerating various functionalities in soft tissues, such as tendons, muscles, and nerves. In this work, we investigate the piezoelectric polymer polyvinylidene fluoride (PVDF) as a potential substrate for wireless neuronal differentiation. Piezoelectric PVDF enables generation of electrical charges on its surface upon acoustic stimulation, inducing neuritogenesis of PC12 cells. We demonstrate that the effect of pure piezoelectric stimulation on neurite generation in PC12 cells is comparable to the ones induced by neuronal growth factor (NGF). In inhibitor experiments, our results indicate that dynamic stimulation of PVDF by ultrasonic (US) waves activates calcium channels, thus inducing the generation of neurites via a cyclic adenosine monophosphate (cAMP)-dependent pathway. This mechanism is independent from the well-studied NGF induced mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPK/ERK) pathway. The use of US, in combination with piezoelectric polymers, is advantageous since focused power transmission can occur deep into biological tissues, which holds great promise for the development of non-invasive neuroregenerative devices.
Introduction
Neurotrauma and neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, have devastating effects on the life of more than 30 million people worldwide1–3. In general, these diseases result in irreversible structural disruption of the neuronal network accompanied by cell death4, 5. Unfortunately, adults have limited capability to actively regenerate or replace neuronal tissue6. A landmark study in the field of neurobiology by Richardson et al. demonstrated that the deficiency of neuronal regeneration is not an intrinsic cell property, but is rather caused by an unfavourable growth environment within the damaged tissue7. As a consequence, if neurons are provided with the correct set of biological and physical stimuli, regeneration of the damaged neuronal tissue can occur8. The most prominent group of neuronal stimulation factors used today are neurotrophins, such as nerve growth factor (NGF), inducing intracellular pathways that stimulate cell proliferation, survival, and differentiation9. Incorporation of neurotrophins, e.g. NGF, into polymer scaffold has been examined for efficient nerve repair stimulation in vitro 10, 11. However, the short half-life time and fast diffusion of NGF, significantly limits their efficiency in vivo 12.
As an alternative to conventional NGF treatments, studies have demonstrated the potential of electrical charges for the differentiation of multiple cell types13 including neurons14, 15. Enhanced neuronal outgrowth has been shown on conductive materials, such as polypyrrole, carbon nanotubes and indium tin oxide under electrostimulating conditions16–19. However, applicability of these materials in clinical devices has been hampered by the necessity of using wired electrical stimulation with external power supplies, which makes these devices invasive, causing chronic wounds with increased risk of infection and inflamation in vivo. Therefore, identifying new electroactive scaffold materials for enhanced wireless neuroregeneration remains a major challenge for neuronal biomedicine.
Piezoelectric materials, which can generate transient charges on their surfaces upon mechanical stimulation, have attracted increasing attention because of their potential application for remote control, regenerative therapy20, 21. They have been used as piezoelectric nanoparticles, such as boron nitride and barium titanate, to demonstrate enhanced neurite outgrowth under ultrasound (US) stimulation in neuronal-like and neuroblastoma-derived cells22, 23. To make use of this unique feature, these nanoparticles were embedded into polymer nanocomposites through two photon lithography or into hydrogels to fabricate scaffolds24, 25. Piezoelectric polymers, e.g. PVDF and its copolymers, have also been successfully used in this context. Such polymeric materials, which possess a superior processability compatible with current scaffold shaping techniques, can easily be used to fabricate piezoelectric scaffolds. Arinzeh’s group demonstrated the feasibility of using electrospun piezoelectric P(VDF-TrFE) scaffolds for enhanced proliferation and stimulation of stem and neuron cells26–28. Nevertheless, those studies were conducted under static conditions where the influence of the piezoelectric P(VDF-TrFE) property on the cell fate under dynamic stimulation was not investigated. The suitability of polymer/ceramic piezoelectric composite films under US for neuronal stimulation through direct piezoelectric effect was demonstrated by Genchi et al. using P(VDF-TrFE) and BaTiO3 29. Besides, a recent work from Lanceros-Mendez group demonstrated wireless stimulation of cells using P(VDF-TrFE)/Terfenol-D composite film through magnetoelectric coupling effect30.
In this work, we investigate the role of the piezoelectric polymer β-polyvinylidenfluorid (β-PVDF) as a candidate to support neuronal differentiation. The piezoelectric properties of β-PVDF allow for the generation of electrical charges on its surface as a result of mechanical deformation31. Compared with P(VDF-TrFE), which is piezoelectric in any case, the non-piezoelectric α-PVDF provides a counterpart that allows for ruling out the influence of the surface chemistry and/or mechanical factors on the induction of PC12 differentiation using β-PVDF. Besides, PVDF is more accessible due to its low cost and high production volume. By using the PC12 model for neuronal differentiation of mammalian cells32, we examine the capability of the piezoelectric surface characteristic for the initiation and growth of cell neurites (Fig. 1a). Ultrasound (US) is utilized as a power transmission system for wireless dynamic stimulation of β-PVDF. US has been widely employed in biomedical devices due to its superior biocompatibility and deeply focused penetration in biological tissues33. The electrical charge generation by β-PVDF is focused solely around the polymer, allowing for enhanced spatial control of the neuronal stimulation area. In addition, the molecular differentiation pathway induced by the electrostimulation of PC12 cells is investigated through dedicated experiments with biochemical inhibitors specifically targeting alternative differentiation pathways. The capability of US stimulated β-PVDF membranes to induce neuronal differentiation is non-invasive and allows for spatially precise, long-term therapy, making it an ideal candidate for the development of novel neuroregenerative devices.
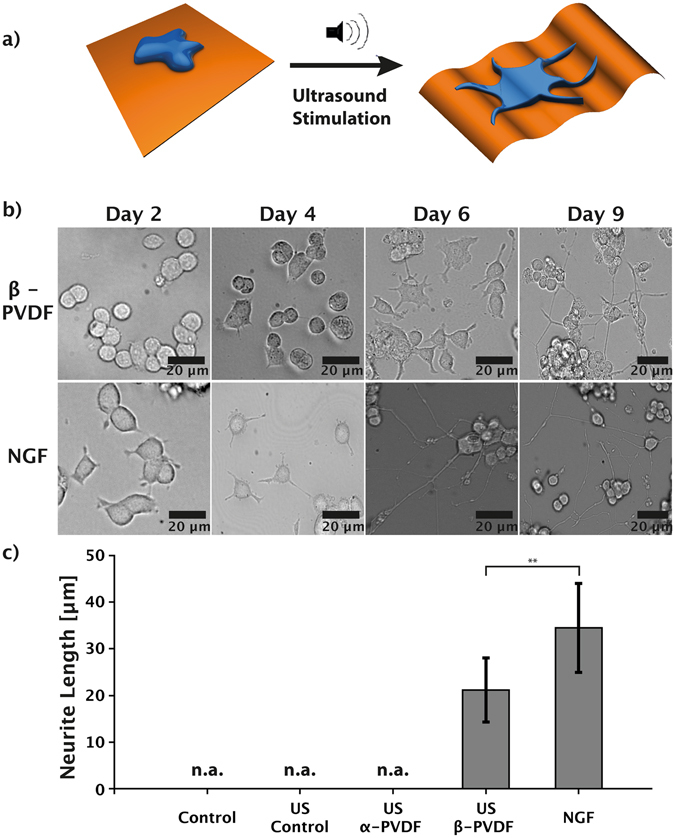
(a) Schematic illustration: Ultrasound (US) stimulation of the piezoelectric β-PVDF membrane induces neuronal differentiation of PC12 cells due to wireless, mechanical deformation of the β-PVDF membrane. (b) Time-lapse images of PC12 cells stimulated by NGF or piezoelectric β-PVDF upon US stimulation. (c) Comparison of average neurite length of PC12 cells cultured in media (control), media with US (US control), cultured on non-piezoelectric α-PVDF with US stimulation, cultured on piezoelectric β-PVDF with US stimulation, and cultured with NGF. **p <
< 0.01; n.a.: measured protrusions lower than 10
0.01; n.a.: measured protrusions lower than 10 µm – not considered as neurites.
µm – not considered as neurites.
Results and Discussion
In vitro neuronal-like cell stimulation by piezoelectric PVDF
To promote cell adhesion, β-PVDF membranes were pre-treated with poly-L-lysine. In the stimulation experiment, PC12 cells cultured on the piezoelectric β-PVDF membranes were exposed to US for 10 minutes, five times per day. Negative control experiments, where cells were cultured with or without US stimulation on β-PVDF, α-PVDF, or directly on well plates coated with PLL were carried out. The phase of β-PVDF and α-PVDF is almost pure according to the calculation using the method reported in literature34 (see Supplementary Fig. S1). Positive control experiments (NGF stimulation) were also performed for comparison. Further experimental details can be found in the experimental section.
minutes, five times per day. Negative control experiments, where cells were cultured with or without US stimulation on β-PVDF, α-PVDF, or directly on well plates coated with PLL were carried out. The phase of β-PVDF and α-PVDF is almost pure according to the calculation using the method reported in literature34 (see Supplementary Fig. S1). Positive control experiments (NGF stimulation) were also performed for comparison. Further experimental details can be found in the experimental section.
Initially, PC12 cells were seeded on pre-treated β-PVDF membranes or on normal tissue-culture plates with NGF. Phase-contrast optical microscope images (Fig. 1b) after two days of US stimulation showed no morphological change in PC12 cells on the piezoelectric β-PVDF membrane. First protrusion formation of piezoelectrically stimulated PC12 cells was observed at day 4, and continuous exposure to the US led to further growth of the neurite length. Maximum neurite outgrowth was reached at day 9 (see Supplementary Fig. S2). In comparison, PC12 cells stimulated with NGF rapidly showed small protrusion formation after day 2, and continued to increase in length until day 6. Continued stimulation until day 9 did not induce further neurite outgrowth.
Figure 1c shows the average neurite length measure in PC12 cells cultured under different conditions after nine days of stimulation. Here, only the cells with neurites longer than 10 μm are considered as differentiated35. US treatment of PC12 cells on a piezoelectric β-PVDF membrane induced differentiation with an average neurite outgrowth of 22.9
μm are considered as differentiated35. US treatment of PC12 cells on a piezoelectric β-PVDF membrane induced differentiation with an average neurite outgrowth of 22.9 μm
μm ±
± 6.8
6.8 μm. PC12 cells subjected to NGF stimulation showed neurite formation with an average length of 34.5
μm. PC12 cells subjected to NGF stimulation showed neurite formation with an average length of 34.5 μm
μm ±
± 9.5
9.5 μm. In contrast, unstimulated PC12 cells and cells stimulated only by US (without a PVDF substrate) showed only small protrusions (average length 2.3
μm. In contrast, unstimulated PC12 cells and cells stimulated only by US (without a PVDF substrate) showed only small protrusions (average length 2.3 μm
μm ±
± 1.8
1.8 μm and 3.1
μm and 3.1 μm
μm ±
± 1.5
1.5 μm, respectively) and no formation of neurites. Cells cultured on non-piezoelectric α-PVDF with US stimulation also showed no neurite formation (average protrusion length 4.6
μm, respectively) and no formation of neurites. Cells cultured on non-piezoelectric α-PVDF with US stimulation also showed no neurite formation (average protrusion length 4.6 μm
μm ±
± 3.1
3.1 μm). Typical cell morphology in these control experiments are shown in Supplementary Fig. S3.
μm). Typical cell morphology in these control experiments are shown in Supplementary Fig. S3.
These results indicate that β-PVDF membranes are able to induce neuronal differentiation with comparable efficiency to commonly used NGF protocols. Control samples (PC12 on tissue culture plates with and without US stimulation) showed short membrane protrusions but no genuine neurites36. From these results, we can further conclude that the stimulation offered by US alone has no significant influence on PC12 differentiation.
It has been reported that surface and mechanical properties of substrates can also affect cell dynamics37, 38. In order to exclude the influence of surface/mechanical factors on the induction of PC12 differentiation, non-piezoelectric α-PVDF sheets (same surface chemistry/similar mechanical properties as β-PVDF) were used for control experiments. Also the crystallinity of the α- and β-PVDF films used in this study are very similar, with measured values of 44.3% and 50.0%, respectively (Supplementary Fig. S4). The Young’s modulus of α-PVDF is slightly lower than that of β-PVDF but they are still of the same magnitude (~GPa, Table S1). In any case, the moduli of both films are much higher than the moduli range (10 Pa ~750
Pa ~750 kPa)39 in which neuronal cells are extensively studied regarding their mechanoconduction behavior. On substrates with Young’s modulus in the GPa range, the cell ability to deform the substrate and thus sense gradients of stiffness is well beyond saturation. To our knowledge, no previous work describes the mechanoconduction behavior of PC12 cells on such stiff substrates. However, it has been found that decreased substrate rigidity in the range of kPa~MPa leads to an increase in neurite branching and extension40, 41. In our case, α-PVDF has a lower modulus compared to β-PVDF. Therefore, differences in mechanical properties cannot be the main reason for promoting cell differentiation. We also investigated the surface morphology of both films (Supplementary Fig. S5). The root mean square surface roughness of the α-PVDF films (7.418
kPa)39 in which neuronal cells are extensively studied regarding their mechanoconduction behavior. On substrates with Young’s modulus in the GPa range, the cell ability to deform the substrate and thus sense gradients of stiffness is well beyond saturation. To our knowledge, no previous work describes the mechanoconduction behavior of PC12 cells on such stiff substrates. However, it has been found that decreased substrate rigidity in the range of kPa~MPa leads to an increase in neurite branching and extension40, 41. In our case, α-PVDF has a lower modulus compared to β-PVDF. Therefore, differences in mechanical properties cannot be the main reason for promoting cell differentiation. We also investigated the surface morphology of both films (Supplementary Fig. S5). The root mean square surface roughness of the α-PVDF films (7.418 nm) is somewhat smaller than that of the β-PVDF films (11.807
nm) is somewhat smaller than that of the β-PVDF films (11.807 nm), indicating a slightly smoother surface. However, according to previous studies42, neither neurite number per cell nor neurite length is affected by surface roughness when it varies from 3.5 to 16
nm), indicating a slightly smoother surface. However, according to previous studies42, neither neurite number per cell nor neurite length is affected by surface roughness when it varies from 3.5 to 16 nm. Therefore, significant differences in the induction of neurite formation between α- and β-PVDF under US stimulation clearly indicate that differentiation is caused by the piezoelectric properties of β-PVDF. This result is consistent with recent studies conducted by Lanceros-Mendez’s group, where the proliferation and differentiation of osteogenic cells were promoted by piezoelectric PVDF exposure to dynamic mechanical stimulation43, 44. The advantageous feature of wireless deep tissue penetration in the human body makes ultrasound an attractive choice over direct mechanical transduction for in vivo medical applications.
nm. Therefore, significant differences in the induction of neurite formation between α- and β-PVDF under US stimulation clearly indicate that differentiation is caused by the piezoelectric properties of β-PVDF. This result is consistent with recent studies conducted by Lanceros-Mendez’s group, where the proliferation and differentiation of osteogenic cells were promoted by piezoelectric PVDF exposure to dynamic mechanical stimulation43, 44. The advantageous feature of wireless deep tissue penetration in the human body makes ultrasound an attractive choice over direct mechanical transduction for in vivo medical applications.
Mechanism of neuronal differentiation induced by piezoelectric stimulation
Inhibitor experiments were conducted to investigate the underlying molecular mechanism of the PC12 differentiation by piezoelectric β-PVDF stimulation. Figure 2a illustrates the two different molecular signaling pathways of PC12 differentiation: the MAPK/ERK (mitogen-activated protein kinases/extracellular signal-regulated kinases) pathway and the cAMP (cyclic adenosine monophosphate) – dependent pathway45, 46. It is known that NGF induced differentiation relies on the MAPK/ERK pathway. NGF molecules bind to tyrosine kinase receptors (TrkA), causing intracellular phosphorylation. Consequently, the activation of Ras/Rap1 initiates the downstream signaling cascade MEK→ERK→Cdk5, promoting PC12 neuronal differentiation. Another pathway, i.e. cAMP-dependent pathway, also known as the adenylyl cyclase (AC) pathway, can be triggered by a variety of extracellular stimuli. For example, it has been demonstrated that an elevated intracellular concentration of calcium ions (Ca2+) leads to the activation of adenylyl cyclase (AC) either directly or indirectly via complex formation with calmodulin47. As a key regulatory enzyme, AC catalyzes the conversion of ATP to cyclic AMP (cAMP)48, a secondary messenger molecule that is responsible for multiple regulatory signaling pathways, such as the activation of the protein kinase A (PKA) dependent neuronal differentiation pathway46. Previous studies have shown that electrical pulses can activate calcium channels in cells, resulting in an increased cellular Ca2+ influx49, 50. Therefore, it is likely that the observed PC12 differentiation upon piezoelectric stimulation happens via the AC signalling cascade.
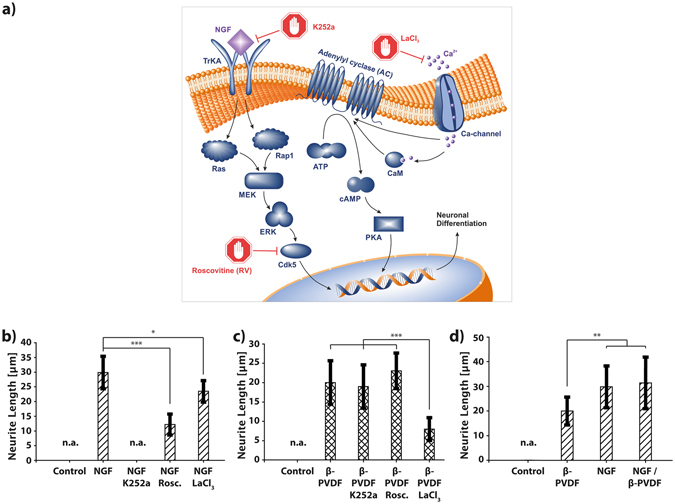
(a) Illustration of intracellular pathways affecting PC12 differentiation. On the left, the well-studied TrKA/Ras/MEK/ERK pathway. On the right, the proposed differentiation pathway induced by piezoelectric depolarization of the PC12 cell membrane. (b,c) Inhibitor experiments, showing average neurite length of PC12 cells stimulated by β-PVDF and US or NGF under the influence of the inhibitors K252a, LaCl3, and Roscovitine (RV). (d) Comparison of the stimulation effect of β-PVDF, NGF, and β-PVDF and NGF on the differentiation of PC12 cells. ***p <
< 0.001; n.a.: measured protrusions lower than 10
0.001; n.a.: measured protrusions lower than 10 µm – not considered as neurites.
µm – not considered as neurites.
To test our hypothesis, PC12 cells were treated with different inhibitors such as K252a, roscovitine (RV), and lanthanum chloride (LaCl3). K252a can block the TrkA receptor51, and RV can block p35/cyclin dependent kinase (Cdk5)52. They are both known inhibitors for neuronal differentiation via the NGF triggered differentiation pathways. LaCl3 was used as a Ca2+ channel blocker in order to investigate the possible effect of piezoelectric stimulation on the activation of Ca2+ channels53. Figure 2b, shows that the average neurite length of NGF stimulated PC12 cells was significantly inhibited in the case of RV and K252a due to the inhibition of Cdk5 or TrkA, respectively. K252a inhibition almost completely suppressed the PC12 differentiation, whereas RV primarily reduced the neurite growth. The use of LaCl3 showed only a minor influence on the NGF dependent differentiation effect. For piezoelectric-dependent PC12 differentiation, an opposite inhibition scheme was observed (Fig. 2c). Here, the TrkA and Cdk5 inhibitors of the NGF signaling pathway, i.e. K252a and RV did not show any influence on the development and growth of the neurites. However, using the Ca2+ channel blocker, LaCl3, caused a significant reduction of neurite outgrowth, comparable to the inhibition observed for K252a and RV for the NGF stimulation experiments. These results suggest that the piezoelectric stimulation causes the activation of Ca2+ channel, and the increased Ca2+ influx plays a substantial role in the differentiation of PC12 cells on piezoelectric β-PVDF. Furthermore, the inhibitor experiments suggest that both mechanisms of piezoelectric β-PVDF and NGF dependent differentiation are independent from each other, since NGF inhibitors did not show any influence on β-PVDF stimulation and vice versa.
To further elucidate the independent action of NGF and piezoelectric stimuli, we investigated simultaneous stimulation of PC12 cells by both NGF and piezoelectric β-PVDF. Figure 2d, shows the average neurite length for PC12 cells stimulated by β-PVDF, by NGF, and NGF/β-PVDF. The results show that simultaneous stimulation of PC12 by NGF and piezoelectric β-PVDF did not significantly increase neurite outgrowth. These results indicate that both stimulation mechanisms are independent and mutual activation does not affect differentiation efficiency.
Polarization
The polarization of neuron cells characterizes the formation of axons and dendrites in neuron cells and is crucial for neuronal functionality54, 55. Many extra – and intracellular processes are responsible for the establishment and maintenance of cell polarity, such as protein diffusion and scaffold formation, secretory pathways, and ion channel activity56. To assess the influence of piezoelectric stimulation on the polarity of PC12 cells, we investigated the polarity distribution of stimulated PC12 cells on β-PVDF and under NGF stimulation. Figure 3a shows that NGF induced differentiation leads to a higher formation of multipolar cells (69%) compared to β-PVDF (48%) stimulated cells. Formation of bipolar (27%) and monopolar (4%) cells was rarely observed for NGF stimulated cells, whereas piezoelectric stimulation showed an increase in the formation of bipolar cells (40%). Figure 3b provides an overview of the neurite length and orientation of all investigated PC 12 cells stimulated by NGF and PVDF. The results confirm that the stimulation with PVDF is isotropic and does not provide a preferential direction to neurite initiation or outgrowth, although the surface of the β-PVDF shows some aligned grooves (Supplementary Fig. S5). Several investigations have evaluated the influence of topographic cues on neurite growth. The majority of these studies used microgrooves with depths ranging from 200 nm to 69
nm to 69 μm57. While the grooves used in previous studies usually have well-defined shapes, i.e., square cross section with sharp edges, the aligned polymer structures on our β-PVDF films are much less ordered and exhibit small heights (<50
μm57. While the grooves used in previous studies usually have well-defined shapes, i.e., square cross section with sharp edges, the aligned polymer structures on our β-PVDF films are much less ordered and exhibit small heights (<50 nm, Supplementary Fig. S5). Previous reports demonstrated that adherent eukaryotic cells can sense and respond to gratings with vertical size down to 35
nm, Supplementary Fig. S5). Previous reports demonstrated that adherent eukaryotic cells can sense and respond to gratings with vertical size down to 35 nm. Shallower topographies are equivalent to flat surfaces in terms of contact guidance58. In addition, it has been proven that noisy nanotopographies with specific directionality are not able to induce a preferential neurite extension59. These reports may explain why neurite guided extension was not observed on our β-PVDF films. Such stimulation has the potential to be coupled with substrates inducing directionality in neurite growth by means of topography60 or gradients of rigidity or adhesion points61. Fluorescence images (Fig. 3c) further confirm this observation on cell polarity, where cells stimulated on PVDF showed a lower degree of neurite formation compared to NGF treated PC12 cells. Similar results can be seen in the SEM image (Fig. 3d) of two interconnected and differentiated PC12 cells stimulated on β-PVDF having a bipolar morphology.
nm. Shallower topographies are equivalent to flat surfaces in terms of contact guidance58. In addition, it has been proven that noisy nanotopographies with specific directionality are not able to induce a preferential neurite extension59. These reports may explain why neurite guided extension was not observed on our β-PVDF films. Such stimulation has the potential to be coupled with substrates inducing directionality in neurite growth by means of topography60 or gradients of rigidity or adhesion points61. Fluorescence images (Fig. 3c) further confirm this observation on cell polarity, where cells stimulated on PVDF showed a lower degree of neurite formation compared to NGF treated PC12 cells. Similar results can be seen in the SEM image (Fig. 3d) of two interconnected and differentiated PC12 cells stimulated on β-PVDF having a bipolar morphology.
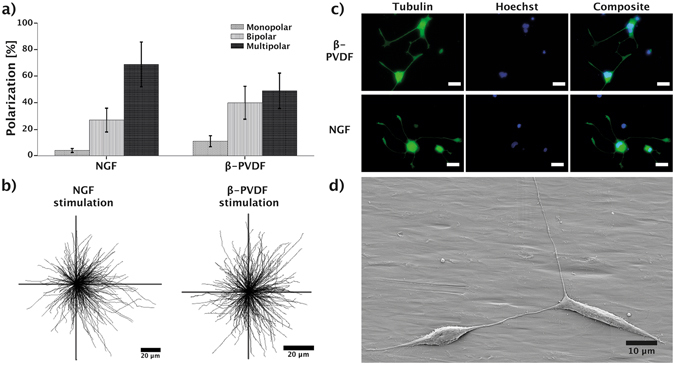
(a) Polarization of differentiated PC12 cells stimulated by NGF or β-PVDF/US. (b) Tracing of the measured neurites, showing their length and directionality. (c) Fluorescence images of stained tubulin (green) and nucleus (blue), differentiated PC12 cells stimulated by β-PVDF/US or NGF; scale bar represents 10 μm. (d) SEM image showing differentiated PC12 cells on the β-PVDF membrane after nine days of US stimulation.
μm. (d) SEM image showing differentiated PC12 cells on the β-PVDF membrane after nine days of US stimulation.
Conclusions
Inducing differentiation of neuronal cells by wirelessly stimulated piezoelectric polymers can create a new therapeutic avenue for contactless, controlled neuroregenerative therapies. As a proof of concept, we provide an in vitro analysis on the application of the piezoelectric polymer β-PVDF for the initiation of PC12 cell differentiation. The results show that externally applied US waves are sufficient to induce polarization in piezoelectric polymer sheets, hence, inducing differentiation of PC12 cells. The differentiation efficiency is comparable to conventional in vitro differentiation protocols using NGF. The neurite outgrowth was uniform in all directions, which suggests that the electrical stimulus by the β-PVDF membrane induces an overall differentiation pressure without directional neurite extension. Additionally, inhibitor experiments elucidate the underlying molecular pathway responsible for piezoelectric β-PVDF induced differentiation, which has been shown to be independent from the well-known NGF induction process. Our results indicate that the electrical polarization of the β-PVDF membrane induces a repolarization of the cell membrane, resulting in an enhanced influx of Ca2+. Subsequently, downstream mechanisms cause the activation of the cAMP/PKA pathway, which leads to the induced differentiation potential of PC12 cells. The results obtained in this study indicate that piezoelectric β-PVDF can be fabricated and integrated into cost-effective scaffolds and devices that can be stimulated by US for minimally invasive neuronal regeneration. In addition, the polymeric material used in this study allows for facile doping with drugs or neuronal growth factors for enhanced tissue regeneration by a multi stimulus system.
Materials and Methods
Materials and characterization
Poled β-PVDF membrane was purchased from Precision Acoustics Ltd, UK. The piezoelectric coefficient d33 is −30 ±
± 2 pC/N. α-PVDF membrane was purchased from CS Hyde. Both β-PVDF and α-PVDF membranes have a thickness of 25~30
2 pC/N. α-PVDF membrane was purchased from CS Hyde. Both β-PVDF and α-PVDF membranes have a thickness of 25~30 μm. The crystalline phase was confirmed by X-Ray diffraction pattern (Bruker D8 Advance). Infrared spectra were measured using a VERTEX 70 (Bruker, Germany) Fourier transformed infrared spectrometer equipped with a silicon ATR crystal. Spectra were recorded in the range of 4000 ~ 400
μm. The crystalline phase was confirmed by X-Ray diffraction pattern (Bruker D8 Advance). Infrared spectra were measured using a VERTEX 70 (Bruker, Germany) Fourier transformed infrared spectrometer equipped with a silicon ATR crystal. Spectra were recorded in the range of 4000 ~ 400 cm−1 at 4
cm−1 at 4 cm−1 resolution averaged from 64 scans. Thermal analysis was conducted using a differential scanning calorimeter (DSC 822e, Mettler Toledo, Switzerland) calibrated with Indium for temperature and enthalpy. DSC thermograms were recorded under N2 purge at standard heating and cooling rates of 10
cm−1 resolution averaged from 64 scans. Thermal analysis was conducted using a differential scanning calorimeter (DSC 822e, Mettler Toledo, Switzerland) calibrated with Indium for temperature and enthalpy. DSC thermograms were recorded under N2 purge at standard heating and cooling rates of 10 °C min−1, using a sample weight of ca. 5
°C min−1, using a sample weight of ca. 5 mg. Tensile test measurements of the PVDF films were performed on a static mechanical tester (Instron® 5864, USA) fitted with a 0.1
mg. Tensile test measurements of the PVDF films were performed on a static mechanical tester (Instron® 5864, USA) fitted with a 0.1 kN load cell. Dumbbell-shaped specimens with a gauge length and width of 12
kN load cell. Dumbbell-shaped specimens with a gauge length and width of 12 mm and 2
mm and 2 mm (ISO 527-2), respectively, were cut out and tested at 37
mm (ISO 527-2), respectively, were cut out and tested at 37 °C. The value of the Young’s modulus, refers to an average value of at least three measurements.
°C. The value of the Young’s modulus, refers to an average value of at least three measurements.
To measure the voltage generated upon ultrasonic stimulation, the membrane was coated with 100 nm gold on both sides using thermal evaporation. A copper wire was attached to the gold coating on each side to provide connection with an oscilloscope (LeCroy WJ324). The membrane was covered with a thin layer of polystyrene by dip-coating in polystyrene/toluene solution (2% w/v) and the copper wires are sealed with kapton tape in order to prevent short-circuit. A photograph of the device and a typical output voltage profile is shown in Supplementary Fig. S6.
nm gold on both sides using thermal evaporation. A copper wire was attached to the gold coating on each side to provide connection with an oscilloscope (LeCroy WJ324). The membrane was covered with a thin layer of polystyrene by dip-coating in polystyrene/toluene solution (2% w/v) and the copper wires are sealed with kapton tape in order to prevent short-circuit. A photograph of the device and a typical output voltage profile is shown in Supplementary Fig. S6.
Sample preparation
The PVDF membrane was cut into 1 cm2 sheets. The sheets were placed three times for 10
cm2 sheets. The sheets were placed three times for 10 min in 70% ethanol. Afterwards, films were air dried and exposed to ultraviolet light (UV) for 20
min in 70% ethanol. Afterwards, films were air dried and exposed to ultraviolet light (UV) for 20 min.
min.
The PVDF sheets were placed in a 24 well-plate and incubated with poly-l-lysine (PLL) solution overnight. Then, the PLL was removed and the sheets were dried for 1 h at room temperature. Finally, the sheets were washed with PBS.
h at room temperature. Finally, the sheets were washed with PBS.
Cell culture
PC12 cells were grown in RPMI medium supplemented with 10% horse serum (HS), 5% fetal bovine serum (FBS), 2 mM glutamine, and 1 x antibiotic antimycotic solution and were maintained at 37
mM glutamine, and 1 x antibiotic antimycotic solution and were maintained at 37 °C, and 5% CO2.
°C, and 5% CO2.
For piezoelectric stimulation experiments, PC12 cells were harvested and transferred to the differentiation media composed of RPMI with 5% HS, 1% FBS, 2 mM glutamine, and 1 x antibiotic antimycotic solution. Cells were then placed in 24 well-plates containing pretreated PVDF sheets and incubated overnight at 37
mM glutamine, and 1 x antibiotic antimycotic solution. Cells were then placed in 24 well-plates containing pretreated PVDF sheets and incubated overnight at 37 °C, 5% CO2 in order to allow cells to attach. For the following nine days, the cells were stimulated by ultrasound five times a day for 10
°C, 5% CO2 in order to allow cells to attach. For the following nine days, the cells were stimulated by ultrasound five times a day for 10 min within an ultrasonic bath (VWR USC300DF, nominal power and frequency: 80
min within an ultrasonic bath (VWR USC300DF, nominal power and frequency: 80 W, 132
W, 132 kHz) at 37
kHz) at 37 °C. The media was replaced daily.
°C. The media was replaced daily.
For chemical stimulation experiments, PC12 cells were harvested and transferred to a PLL coated 24 well plate in differentiation media supplemented with 50 ng/mL neuronal growth factors (NGF). PC12 cells were maintained at 37
ng/mL neuronal growth factors (NGF). PC12 cells were maintained at 37 °C, 5% CO2 for nine days and the media was replaced daily.
°C, 5% CO2 for nine days and the media was replaced daily.
Inhibitor experiments were conducted by supplementing differentiation media of piezoelectrically or chemically stimulated PC12 cells with 50 nM K252a, 50
nM K252a, 50 μM lanthanum (III) chloride (LaCl3), or 10
μM lanthanum (III) chloride (LaCl3), or 10 μM roscovitine. Media was exchanged daily.
μM roscovitine. Media was exchanged daily.
Imaging
In general, all bright field and fluorescence images were obtained using an inverted optical microscope (Olympus IX-81).
Prior to imaging, cell media was removed, the cells were washed three times with PBS, and fixed in 4% paraformaldehyde solution for 15 min. Then, the cells were permeabilized with 0.1% Triton – X for 10
min. Then, the cells were permeabilized with 0.1% Triton – X for 10 min. For tubulin staining, the cells were incubated with monoclonal antibody to β3 – tubulin for 3
min. For tubulin staining, the cells were incubated with monoclonal antibody to β3 – tubulin for 3 h at room temperature and, subsequently, labeled with goat-anti-mouse secondary antibody conjugated with Alexa Fluor® 568. Cells were washed three times before 2
h at room temperature and, subsequently, labeled with goat-anti-mouse secondary antibody conjugated with Alexa Fluor® 568. Cells were washed three times before 2 μg/mL Hoechst 333452 solution was added. Afterwards, cells were washed again with PBS three times and used for imaging.
μg/mL Hoechst 333452 solution was added. Afterwards, cells were washed again with PBS three times and used for imaging.
For SEM images, PC12 cells differentiated on PVDF membrane were fixed for 15 min in 4% paraformaldehyde. The fixed cells were washed three times in DI water and placed in 1-butyl-3-methylimidazolium tetrafluoroborate for 30
min in 4% paraformaldehyde. The fixed cells were washed three times in DI water and placed in 1-butyl-3-methylimidazolium tetrafluoroborate for 30 s. The cells were then washed in a container with DI water for 30
s. The cells were then washed in a container with DI water for 30 sec and air dried, before imaging with SEM (Zeiss Ultra) operating at 3
sec and air dried, before imaging with SEM (Zeiss Ultra) operating at 3 kV.
kV.
Data analysis
The quantitative results of neurite outgrowth were obtained by analyzing microscopy images in ImageJ (National Institute of Health, USA) using the NeuronJ plug-in software. All quantitative measurements are expressed as mean values, and error bars indicate the standard deviation of the mean. Statistical comparison of neurite length under different stimulation conditions was conducted by using the non-parametric Mann-Whitney U test.
Acknowledgements
This work has been financed by the European Research Council Starting Grant “Magnetoelectric Chemonanorobotics for Chemical and Biomedical Applications (ELECTROCHEMBOTS)”, by the ERC grant agreement no. 336456. X.Z. Chen would like to acknowledge the ETH Career Seed Grant (Grant No. SEED-14 16-1). The authors would like to acknowledge the Scientific Center for Optical and Electron Microscopy (ScopeM) and the FIRST laboratory, ETH Zurich for their technical support. X.Z. Chen would also like to thank Ms. Lu Jin for her support on XRD measurements. This work is part of the CINDY project, supported by the Swiss National Science Foundation (SNSF 2-77795-13). The authors are also grateful to Mr. Thomas Vasileiou for fruitful discussions and his advice on experimental procedures.
Author Contributions
M.H., X.C., F.M.T.T. and G.G. did the experiments. M.H., X.C., and A.F. drafted the manuscript. X.C. and S.P. conceived the idea. D.P., B.J., and S.P. supervised this work. All authors reviewed and revised the manuscript.
Notes
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-03992-3
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiang-Zhong Chen, Email: hc.zhte@naixnehc.
Aldo Ferrari, Email: hc.zhte@irarrefa.
Salvador Pané, Email: hc.zhte@pladiv.
References
Articles from Scientific Reports are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41598-017-03992-3
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/s41598-017-03992-3.pdf
Citations & impact
Impact metrics
Article citations
Engineering 3D Scaffold-Free Nanoparticle-Laden Stem Cell Constructs for Piezoelectric Enhancement of Human Neural Tissue Formation and Function.
Adv Sci (Weinh), 11(40):e2310010, 25 Jul 2024
Cited by: 1 article | PMID: 39049737 | PMCID: PMC11516115
Biomimetic electrospun PVDF/self-assembling peptide piezoelectric scaffolds for neural stem cell transplantation in neural tissue engineering.
RSC Adv, 14(30):21277-21291, 05 Jul 2024
Cited by: 0 articles | PMID: 38974226
From electricity to vitality: the emerging use of piezoelectric materials in tissue regeneration.
Burns Trauma, 12:tkae013, 02 Jul 2024
Cited by: 0 articles | PMID: 38957661 | PMCID: PMC11218788
Review Free full text in Europe PMC
Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration.
Int J Mol Sci, 25(11):6012, 30 May 2024
Cited by: 3 articles | PMID: 38892199 | PMCID: PMC11172494
Review Free full text in Europe PMC
An electromechanical stimulation regulating model with flexoelectric effect of piezoelectric laminated micro-beam for cell bionic culture.
Sci Rep, 14(1):6130, 13 Mar 2024
Cited by: 0 articles | PMID: 38480822
Go to all (48) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Sustained activation of M-Ras induced by nerve growth factor is essential for neuronal differentiation of PC12 cells.
Genes Cells, 11(9):1097-1113, 01 Sep 2006
Cited by: 37 articles | PMID: 16923128
Induction of neurite outgrowth in PC12 cells treated with temperature-controlled repeated thermal stimulation.
PLoS One, 10(4):e0124024, 16 Apr 2015
Cited by: 15 articles | PMID: 25879210 | PMCID: PMC4399938
Enhanced neuronal differentiation by dynamic piezoelectric stimulation.
J Biomed Mater Res A, 111(1):35-44, 07 Sep 2022
Cited by: 0 articles | PMID: 36069387
Physical Stimulation Methods Developed for In Vitro Neuronal Differentiation Studies of PC12 Cells: A Comprehensive Review.
Int J Mol Sci, 25(2):772, 07 Jan 2024
Cited by: 0 articles | PMID: 38255846 | PMCID: PMC10815383
Review Free full text in Europe PMC
Funding
Funders who supported this work.
European Research Council (1)
MAGNETOELECTRIC CHEMONANOROBOTICS FOR CHEMICAL AND BIOMEDICAL APPLICATIONS (ELECTROCHEMBOTS)
Dr Salvador Pané Vidal, Swiss Federal Institute of Technology Zurich (ETH Zurich)
Grant ID: 336456

 1
1 



