Abstract
Free full text

Remodeling T cell compartments during anti-CD3 immunotherapy of type 1 diabetes
Associated Data
Abstract
The immunological mechanism(s) of action whereby teplizumab preserves C-peptide levels in the progression of patients with recent onset type 1 diabetes (T1D) is still not well understood. In the present study, we evaluated the kinetics of T cell modulation in peripheral blood following two 14-day courses of teplizumab therapy one year apart in recent onset T1D participants in the AbATE clinical trial. Transient rises in PD-1+Foxp3+ Treg and potentially anergic (CD57−KLRG1−PD-1+) cells in the circulating CD4 T cell compartment were paralleled by more profound increases in circulating CD8 T cells with traits of exhaustion (CD57−KLRG1+PD-1+, TIGIT+KLRG1+, and persistent down-modulation of CD127). The observed phenotypic changes across cell types were associated with favorable response to treatment in the subgroup of study participants that did not develop anti-drug antibodies after the first course of therapy. These findings provide new insights on the duration and complexity of T cell modulation with teplizumab therapy in recent onset T1D, and in addition, suggest that coordinated immune mechanisms of tolerance that favor CD4 Treg function and restrain CD4 non-Treg and CD8 T cell activation may contribute to treatment success.
1. Introduction
Teplizumab is an FcR-nonbinding anti-CD3 monoclonal antibody that achieved partial and transient preservation of beta cell function in clinical trials in recent onset type 1 diabetes (T1D) [1–5]. In the AbATE (Autoimmunity-Blocking Antibody for Tolerance) trial, conducted by the Immune Tolerance Network, subjects with recent-onset T1D received two 14-day courses of therapy with teplizumab, and were evaluated after two years for preservation of plasma C-peptide, a surrogate for residual insulin-producing cells. The mechanism of action of teplizumab was initially thought to deplete T cells similar to other immunotherapies that target T cells, such as OKT3, thymoglobulin, and Campath (anti-CD52) [6]. More recently, we reported an increase in the frequency of circulating central memory CD8 T cells in clinical responders [7], and further investigation revealed that accumulation of partially exhausted CD8 T cells, characterized by co-expression of TIGIT and KLRG1 together with high levels of EOMES-associated gene networks, was associated with successful response to teplizumab therapy [8]. These studies suggested that teplizumab therapy, rather than acting as a depleting or blocking antibody, may have agonist properties that lead to changes in the composition of differentiation and activation status within the circulating pool of CD3-expressing T cells. Indeed, preclinical studies in murine models of T1D suggest that teplizumab may function as an agonist to induce expansion and/or regulatory function in T cell subsets [9–11], with margination to draining pancreatic lymph nodes, islets or the gastrointestinal tract [12].
In this follow-up study from the AbATE trial, we analyzed the effect of two 14-day courses of treatment with teplizumab one year apart on the circulating pool of T cell subsets. Specifically, we examined whether potential signatures of T cell tolerance, defined by CD4 regulatory T cells (Treg) and/or deletion, anergy, exhaustion, or senescence of non-Treg populations, were present over the 24-month trial period. Here, we report an initial overall reduction in frequencies of multiple lineages of T cells within the circulating pool of CD3-expressing lymphocytes, with a profound induction, expansion, or sparing of cells that express phenotypic markers associated with T cell inactivation or hyporesponsiveness. Some phenotypic changes were short-lived, returning to pre-treatment levels within ~2 months of each treatment course, while others persisted after the second course through 24 months, with the exception of subjects that developed anti-drug antibodies during therapy. These findings provide new insights on the duration and complexity of T cell modulation with teplizumab therapy in recent onset T1D, and in addition, suggest that coordinated immune mechanisms of tolerance that both promote CD4 Treg function and restrain CD4 non-Treg and CD8 T cell activation may contribute to treatment success.
2. Materials and Methods
2.1 Human subjects
Individuals between the ages of 8 and 30 were enrolled in the AbATE clinical trial within 8 weeks of diagnosis of T1D. Subjects were randomized to control or active therapy with teplizumab, which was administered via infusion over 14 days at study initiation and one year later. Details of the clinical trial design and schedule of drug administration have been previously reported [2]. The primary clinical outcome was the preservation of plasma C-peptide two years after initiation of the study measured as a 4-h MMTT-stimulated C-peptide AUC; the change in this value at 2 years compared to baseline was calculated, and responders were defined as individuals in the teplizumab arm of the study who maintained C-peptide above the level of all of the control subjects. This cut-off value assigned a Responder designation to 40% of the teplizumab-treated subjects in the trial [2]. Blood samples for flow cytometry analysis were collected periodically over these two years; all subjects gave informed consent for the immunological assays described herein. Immunological assays were performed on samples from subjects who completed the 2-year trial and—if in the treatment arm—had received both courses of teplizumab therapy per protocol.
2.2 Flow cytometry
Peripheral blood mononuclear cells were isolated and stored frozen until use. Vials of cryopreserved cells were thawed in a water bath before immediately diluting drop-wise into 1mL media with 10% serum and DNase. This 1mL was diluted more quickly with an additional 9mL of media prior to centrifugation, aspiration of media with DMSO, and resuspension in 2mL fresh media. After resting for 5min, cells were strained to remove clumps, counted, and distributed for staining. Viability (81% ± 18%) ranged from 28–100% and recovery (33% ± 19%) ranged from 2–137%. Surface markers were stained using cocktails prior to eBio FOXP3 fix/perm for intracellular staining. 16-parameter cytometry was performed on an LSR-Fortessa (BD Biosciences) with FACS Diva software and analyzed with FlowJo software version 9.5 (Tree Star, Ashland, OR). A panel defining Treg, anergic, senescent, and exhausted cells included monoclonal antibodies directed against the following markers: CD3, CD4, CD8, CD45RA, CD45RO, CCR7, FOXP3, CD127, PD-1, KLRG1, CD57, CD56, eomes, TIGIT, and Granzyme B (Supplemental Table 1). To permit direct comparisons between samples acquired across days, instrument standardization was performed using 8 peak rainbow calibration beads (Spherotech, Lake Forest, IL), adjusting PMT voltages so that 7th peak mean fluorescent intensities for each parameter were consistent. All samples from the same subject were run on the same day, and an internal control from the same subject was run each week to identify any machine or staining issues. An average of 580,000 live lymphocyte events were collected per sample and gated populations with <100 events were excluded from analysis.
2.3 Anti-drug antibodies (ADA)
Serum samples obtained from teplizumab-treated subjects before and 12 months after the first treatment course (one month prior to the second course of therapy), were analyzed for anti-teplizumab antibodies using an indirect ELISA by Esoterix Inc. (San Leandro, CA).
2.4 Statistical Considerations
For longitudinal assessment of lymphocyte subpopulations monitored by flow cytometry, values were log transformed and analyzed using mixed model for repeated measures. Pairwise comparisons were made between time points or groups and p-values were adjusted using Tukey’s multiple testing corrections. Paired t-test were used to compare fold changes of pre- and 2 weeks post-treatment between 1st and 2nd courses of therapy. R 3.3.2 was used for all analyses. Statistical significance was set at 5% (p < 0.05).
3. Results
3.1 Each course of teplizumab reduces the circulating pool of CD3-expressing lymphocytes
Teplizumab was administered to subjects in the treatment arm of the AbATE trial as a 14-day infusion on two occasions, one year apart. Transient reduction from peripheral blood was noted for multiple CD3-expressing lymphocyte populations, as shown in Figure 1. Overall, the first course of teplizumab reduced CD4 non-Treg and Treg, CD8, and NKT cell populations by approximately 35–60% immediately after infusion, and all returned to pre-treatment levels by month 2 (Fig. 1A). Initial reductions with the second course given one year later were similar in magnitude to that observed in the first course (Fig. 1B). Although frequencies of CD4 T cells remained significantly reduced at all follow-up visits assessed through month 24, percentages of CD8 and NK T cells recovered by month 15. Within the pool of circulating CD4 T cells, teplizumab reduced Treg more than non-Treg (~58% v ~35% change from baseline, respectively), which resulted in skewing of Treg/non-Treg (CD4 Tcm and Tem) balance in favor of non-Treg from 2 weeks through 24 months post-treatment. The degree of lymphocyte reduction from peripheral blood 2 weeks after each course did not correlate with CD3 expression levels (Supplemental Table 2), frequencies, or estimated absolute numbers of these four populations prior to treatment (data not shown). This contrasts with the T cell depleting mechanism of anti-CD3 antibodies observed in vivo in NOD mice [13], suggesting that teplizumab likely promotes T cell migration from circulation and modulation of T cell populations.
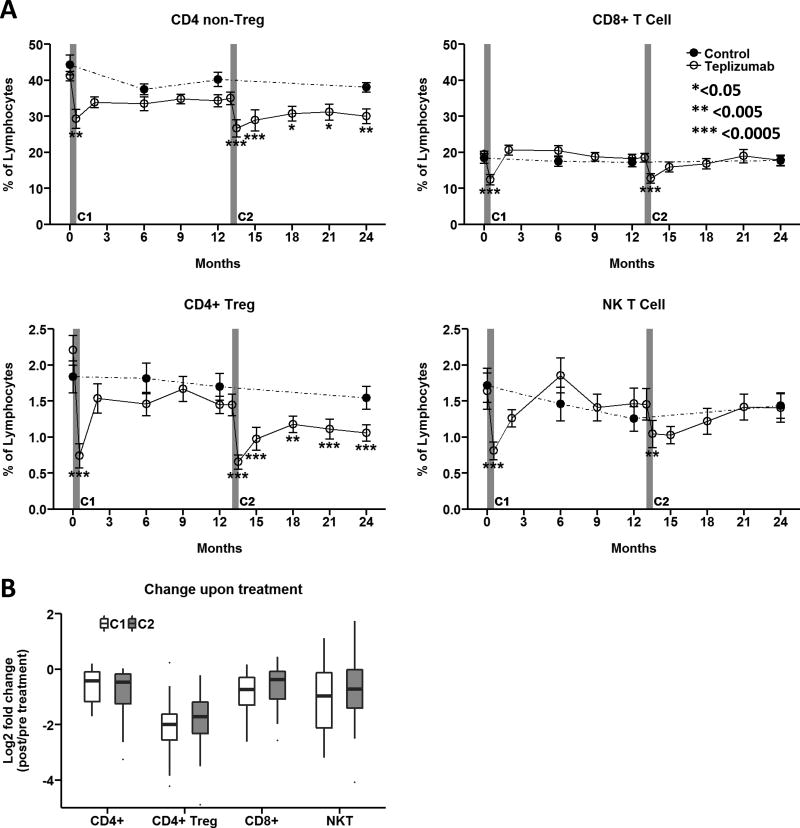
(A) CD4 non-Treg, CD4 Treg, CD8 T cells, and NKT frequencies as a % of live lymphocytes were determined as shown in Supplemental Figure 1. Data reported as mean ± SEM for Per Protocol participants in the teplizumab (n=24, solid lines with open circles) and control (n=17, dashed lines with closed circles) treatment arms. *<0.05, ** <0.005, ***<0.0005 adjusted p value. (B) Fold change of specific CD3-expressing lymphocyte populations upon treatment was determined by comparing month 0.5/pre and month 13.5/month 13. Box and whisker plots note 25–75th quartiles and range. C1, C2: Course 1 and 2. https://www.itntrialshare.org/AbATE_fig1.url
3.2 Teplizumab increases proportions of central memory cells and cells displaying an exhausted phenotype in circulating CD8 T cells
Significant shifts in circulating T cell subsets within the CD8 T cell compartment were observed following therapy. Immediately following teplizumab infusions, short-lived increases in CD8 central memory, and corresponding decreases in effector memory T cells occurred; the former persisted at a modestly elevated level, while the effector memory subsets rebounded within two months to levels higher than pre-treatment (Fig. 2A). Interestingly, during the second treatment course, the early teplizumab-induced changes in central memory and effector memory CD8 T cells were significantly diminished compared to the first course (Fig. 2B). No significant changes in percentages of naïve CD8 T cells were observed following either treatment course (Fig 2B).
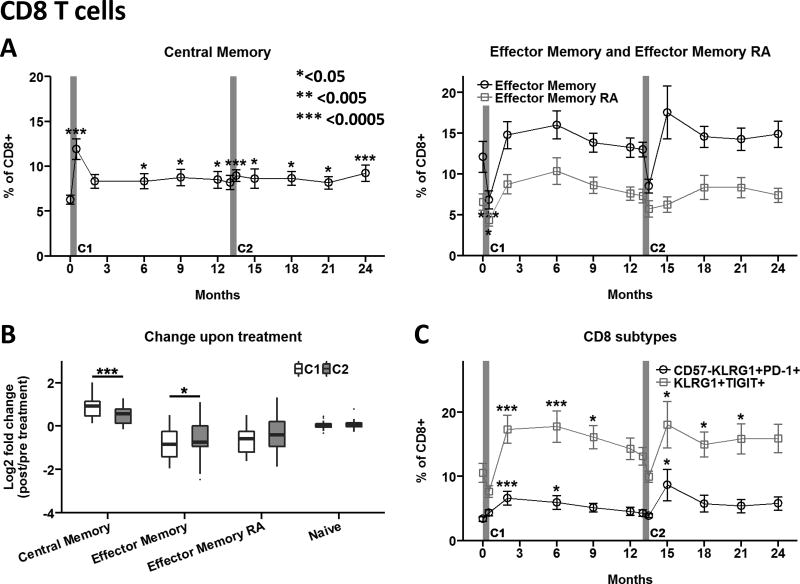
(A) CD8 T cell differentiation subsets were determined as shown in Supplemental Figure 2. (B) Fold-change upon treatment was determined by comparing month 0.5/pre and month 13.5/month 13. Box and whisker plots note 25–75th quartiles and range. (C) Phenotypes associated with CD8 T cell exhaustion or inactivation including CD57−KLRG1+PD-1+ and KLRG1+TIGIT+ were determined as shown in Supplemental Figure 2. Data in panels A and C are mean values ± SEM for Per Protocol participants in the teplizumab treatment arm. *<0.05, ** <0.005, ***<0.0005 adjusted p value. C1, C2: Course 1 and 2. https://www.itntrialshare.org/AbATE_fig2.url
We recently reported that partial exhaustion of CD8 T cells is associated with clinical response to teplizumab in the AbATE trial [8]. This cell population is characterized by co-expression of inhibitory receptors KLRG1 and TIGIT on the cell surface, together with high expression of the transcription factor EOMES, and variable surface expression of the co-inhibitory receptor PD-1. Percentages of partially exhausted KLRG1+TIGIT+ CD8 T cells increased within 2 months of teplizumab treatment and persisted for ~9 months after each course (Fig 2C). Similarly, CD8 T cells displaying the KLRG1+CD57−PD1+ phenotype, previously associated with CD8 T cell exhaustion in chronic viral infections and cancer [14–16], were also elevated.
3.3 Teplizumab modulates CD127 expression in circulating CD8 T cell subsets
The interleukin-7Rα, CD127, is an important mediator of T cell survival, and persistent down-modulation of this receptor is a key feature of exhausted antigen-specific CD8 T cells in human subjects with chronic viral infections [17–19]. Given that teplizumab increased the relative proportion of CD8 T cells expressing phenotypes associated with exhaustion, we examined the effect of teplizumab on CD127 surface expression on CD8 T cell differentiation subsets. Notably, percentages of CD127+ naive and central memory CD8 T cells were reduced 30–45% 2 weeks after the first treatment course, and returned to pre-treatment levels by 2 or 6 months, respectively (Fig. 3A). The kinetics of this response in early differentiation subsets are consistent with transient CD127 receptor shedding upon TCR engagement. A transient, but delayed, reduction in the percentage of CD127+ effector memory RA (Temra) CD8 T cells was observed ~6 months after the first course, consistent with persistent reduction in CD127 expression in more highly differentiated subsets through both receptor shedding and reduced transcription. The second course caused a temporary decline in frequencies of CD127+ naïve, central memory, and effector memory CD8 T cell subsets that rebound within ~5 months’ time (Fig. 3A). These CD127+ CD8 T cell subsets returned to pre-treatment levels by month 18, although densities of CD127 on the surface of all four CD8 T cell subsets remained significantly diminished following the second treatment course through 24 months (Fig. 3B).
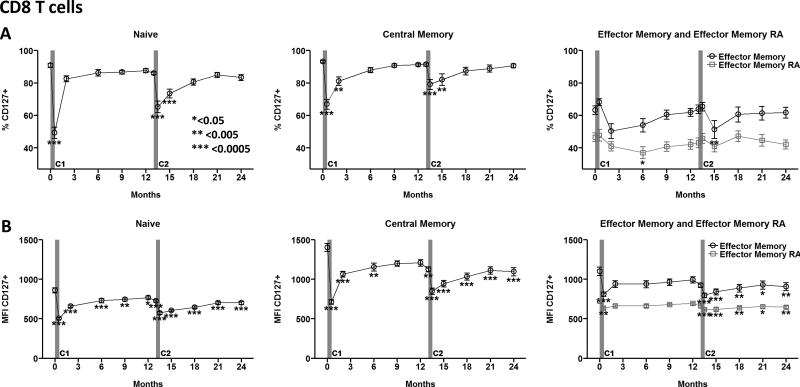
Representative gating for CD127 expression on CD8 T cell subsets is shown in Supplemental Figure 2. (A) Frequency and (B) level of expression (MFI) of CD127 on CD8 T cell subsets over time. Data reported as mean ± SEM for Per Protocol participants in the teplizumab treatment arm. *<0.05, **<0.005, ***<0.0005 adjusted p value. C1, C2: Course 1 and 2. https://www.itntrialshare.org/AbATE_fig3.url
3.4 Teplizumab increases proportions of effector memory and PD-1+ cells in circulating CD4 T cells
Effects on the CD4 T cell compartment following teplizumab therapy were less marked compared to those on the CD8 T cells. A small (2–5%) decline in central memory CD4 T cells and a comparable increase in effector memory CD4 T cells immediately after treatment were greatest with the second course as compared to the first course (Fig. 4A); this latter effect persisted through 24 months (Fig. 4B).
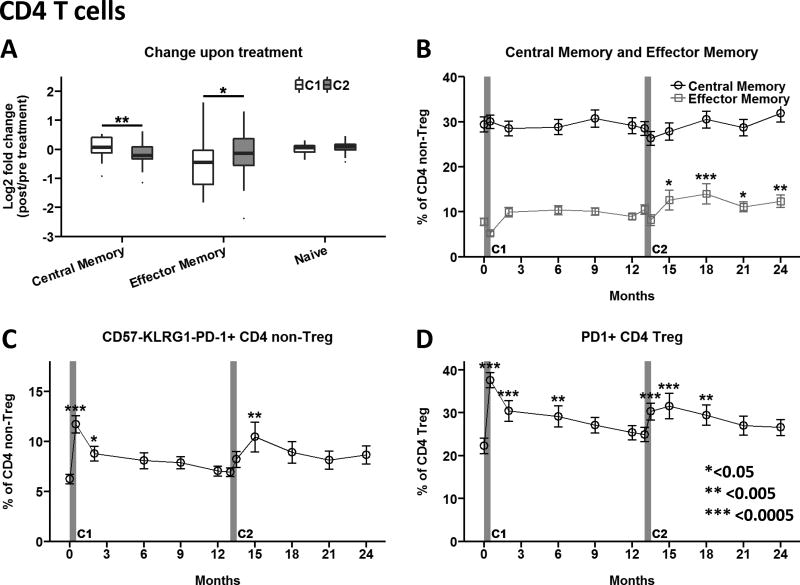
(A) Fold-change of CD4 differentiation subsets upon treatment was determined by comparing month 0.5/pre and month 13.5/month 13. Box and whisker plots note 25–75th quartiles and range. (B) Impact of teplizumab treatment on central memory (open circles) and effector memory (open squares) CD4 T cell subsets. (C) CD4 non-Treg cells displaying a potentially anergic phenotype CD57−KLRG1−PD-1+ and (D) PD-1 expression on CD4 Treg was determined as described in Supplemental Figure 3. Data in panels B–D are mean values ± SEM for Per Protocol participants in the teplizumab treatment arm. *<0.05, ** <0.005, ***<0.0005 adjusted p value. C1, C2: Course 1 and 2. https://www.itntrialshare.org/AbATE_fig4.url
To further explore the potential agonist effects of teplizumab, we assessed expression of surface molecules associated with down-modulation of CD4 T cell activation. We found that each course of teplizumab significantly increased percentages of CD4 T cells expressing the co-inhibitory receptor PD-1 in the absence of KLRG1 and CD57 (Fig. 4C), a phenotype that could indicate T cell anergy [15]. This transient increase in peripheral blood was observed for effector memory and central memory CD4 T cell subsets (data available at https://www.itntrialshare.org/AbATE.url). The percentage of Treg expressing PD-1 was also markedly increased following each treatment course, and returned to the pre-treatment profile within ~9 months (Fig. 4D).
3.5 Anti-teplizumab antibodies and T cell biomarkers of 24 month clinical outcome
In the Protégé clinical trial, anti-teplizumab (anti-drug) antibodies (ADA) were detected in ~50% of new-onset type 1 diabetic patients treated with two 14-day courses of teplizumab 6 months apart [20]. The biologic activity of ADA was shown by diminished bioavailability and increased teplizumab clearance, which was associated with reduced drug-induced transient depletion of circulating CD4 T cells [20]. In the AbATE trial, 7/34 (~20%) of the Per Protocol study population tested positive for ADA one month before starting the 2nd course at month 13. When we analyzed the 24-month clinical outcome data for these seven ADA-positive subjects, we found that six were clinical non-responders, compared to only one responder subject, suggesting that ADA may inhibit clinical efficacy of the teplizumab treatment (Suppl. Fig. 4). Since subjects in the AbATE trial received a second course of teplizumab a year following the first, we evaluated the impact of ADA-positivity resulting from the first course of this drug on the immunological effects of the second course, illustrated in Figure 5. There was an apparent impact from the presence of ADA, with ADA-positive subjects failing to show modulation of CD3 expression on both CD4 and CD8 T cells, respectively, at the time of the second treatment course (Fig. 5A, B).
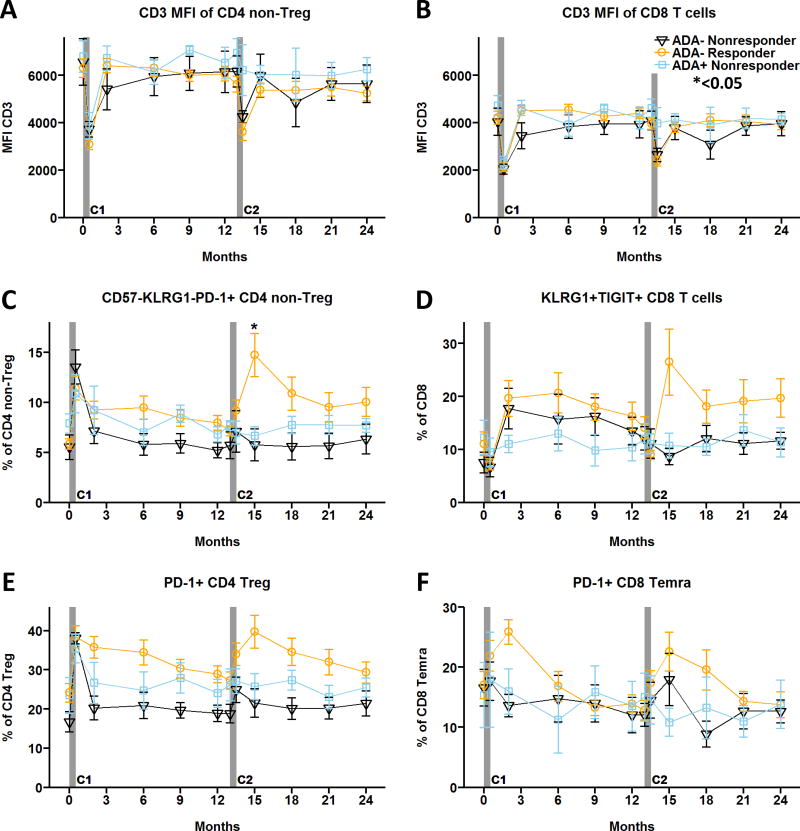
Of the thirty-four drug-treated subjects in the Per Protocol population tested for ADA at month 12, nineteen ADA-negative plus five ADA-positive subjects (~70%) had cryopreserved PBMC available for flow cytometric analysis. CD3 MFI of (A) CD4 non-Treg and (B) CD8 T cells. (C) CD4 non-Treg cells displaying a potentially anergic phenotype CD57−KLRG1−PD-1+. (D) CD8 T cells with KLRG1+TIGIT+ partial exhaustion signature. (E) PD-1+ CD4 Treg. (F) PD-1+CD8 Temra. Data reported are mean values ± SEM for Per Protocol participants in the teplizumab treatment arm. Responders were defined as participants with </= 40% decline from baseline C-peptide at 24 months [2]. ADA− Nonresponders (n=6, solid black line with open triangles); ADA− Responders (n=13, solid orange line with open circles); ADA+ Nonresponders (n=5, solid blue line with open squares). *<0.05 adjusted p value. C1, C2: Course 1 and 2. https://www.itntrialshare.org/AbATE_fig5.url
Notably, the magnitude of CD3 modulation by the second course of teplizumab in ADA-negative subjects was similar between drug-treated responders and non-responders, indicating that CD3 modulation impacted by ADA status was insufficient to explain clinical outcomes. Therefore, we also assessed the CD4 and CD8 anergy and exhaustion profiles relative to ADA status shown in Figure 5C–F. A clear distinction was identified in ADA-negative subjects, with a significant increase in CD57−KLRG1−PD-1+ CD4 T cells (Fig. 5C) and a parallel rise in KLRG1+TIGIT+ partially exhausted CD8 T cells (Fig. 5D) in drug-treated responders after the second treatment course. Interestingly, percentages of PD-1+ CD4 Treg (Fig. 5E) and PD-1+ CD8 Temra cells (Fig. 5F) tended to be higher in responders compared to non-responders among ADA-negative subjects after the first and second course of therapy. Since clinical response in the AbATE trial was based on plasma C-peptide levels at 24 months after initiation of therapy, this indicates that the presence of ADA is likely interfering with efficacy and induction of immunologic responses to teplizumab. Taken together, these data also suggest that increased expression of selected anergy or exhaustion T cell profiles may serve as early composite biomarkers of response to teplizumab therapy in ADA-negative subjects.
4. Discussion
There have been numerous clinical trials of anti-CD3 antibodies [4, 21], showing efficacy without a clear understanding of mechanism of action. Past studies [7–12] support the hypothesis that teplizumab does not function through deletion, but instead may promote migration and modulation of T cell subsets. In the present study, we evaluated the kinetics of T cell modulation in peripheral blood following two 14-day courses of teplizumab therapy, one year apart, in recent onset T1D participants in the AbATE clinical trial. Transient rises in PD-1+Foxp3+ Treg and potentially anergic (CD57−KLRG1−PD-1+) cells in the CD4 T cell compartment were paralleled by more profound increased percentages of cells with traits of exhaustion (CD57−KLRG1+PD-1+, TIGIT+KLRG1+, eomes expression, and persistent down-modulation of CD127) in the CD8 T cell compartment. These intriguing phenotypic changes across cell types were associated with favorable clinical response to teplizumab in the subgroup of study participants that did not develop anti-drug antibodies.
Complete participant-level raw data, analysis templates and analyzed datasets using 16-parameter flow cytometric analysis of viably frozen PBMC from the subjects in the AbATE trial were analyzed for these studies, and are available through open access on the Immune Tolerance Network data sharing website, https://www.itntrialshare.org/AbATE.url. These data provide a comprehensive survey of the kinetic changes in the composition and activation status of discrete CD4 and CD8 T cell subsets in patients receiving teplizumab, and in control participants. This high-dimensional flow analysis, and the results presented herein, build upon accumulating data for complex effects of anti-CD3, and support the notion that, in the absence of anti-drug antibodies, teplizumab modulation of T cells elicits mechanisms of immune response associated with coordinated induction of anergic and exhaustion characteristics in both CD4 and CD8 compartments that together may contribute to treatment success.
The appearance of CD8 T cells displaying phenotypes associated with exhaustion (KLRG1+TIGIT+ and CD57−KLRG1+PD-1+) was preceded by an early rise in central memory CD8 T cells with significantly reduced expression levels of CD127 that remained throughout 24 months. Our data, however, do not distinguish between a direct effect of anti-CD3 inducing these CD8 profiles and an indirect effect potentially involving impaired CD4 T cell help that contributes to CD8 T cell exhaustion [14, 18]; notably, potentially anergic PD-1+ CD4 T cells [22–24] also increased prior to expansion of KLRG1+TIGIT+ CD8 T cells. Although functional studies are warranted, this observation is reminiscent of the combined transcriptional signature of increased CD8 T cell exhaustion following decreased CD4 T cell costimulation associated with improved prognosis for autoimmunity [18], and is consistent with the EOMES+TIGIT+KLRG1+ signature demonstrated in peripheral blood transcriptional analysis in the AbATE subjects [8]. Therefore, one potential mechanism induced by anti-CD3 may include teplizumab acting as an agonist, and invoking a loss of T cell help through inactivation of CD4 T cells that promotes a rise in partially exhausted CD8 T cells, which associated with treatment success.
A unique feature of the current study was the ability to analyze cellular phenotypes after a second therapeutic course of teplizumab infusions. The T cell profiles elicited by the second course, one year after the first, clearly revealed that anti-drug antibodies present in treated subjects were associated with lack of immunological response. Notably, however, while not all ADA-negative subjects had favorable ‘responder’ clinical responses to therapy, within this group there was a prominent positive association between the immunological response during the second treatment course and the final clinical outcome measured a year later. This finding has implications both for improving therapeutic benefit by preventing anti-drug antibodies and for ‘personalizing’ prognosis by monitoring the induction of specific T cell biomarkers after treatment.
The characteristic flow cytometry profiles induced by teplizumab on both the CD4 and the CD8 T cell subsets in the AbATE subjects share PD-1 expression and some similarities with exhausted and anergic T cell phenotypes that have been extensively characterized in chronic viral infections and in cancer [14–16], both situations of profound immunomodulation. PD-1 is an inhibitory receptor that is rapidly upregulated on T cells following activation that serves as a negative regulator of T cell responses, proliferation, and survival [25, 26]. PD-1-mediated signaling promotes quiescence and immune suppression in activated effector T cells, and can also synergize with TGF-b-mediated signals for generation of induced CD4 Treg [27]. PD-1 signaling is essential for maintaining lymphocyte homeostasis by preventing immune-mediated damage and inducing T cell exhaustion to persistent antigens, as demonstrated in chronic viral infection and tumor models [14, 28]. Interestingly, our data reveal an early association between increases in PD-1+ Treg after the first treatment that persists through 24 months in drug-treated responders. While the underlying mechanisms for the relative rise in PD-1-expressing CD4 T cells are not clear, they may involve direct (CD3 agonist) and/or indirect upregulation of PD-1 on PD-1 negative cells [29–31], altered trafficking (if margination) [2, 12, 32, 33], survival (if depletion) [29], or expansion of PD-1-expressing CD4 T cells [23, 34]. As the immunobiology of PD-1 becomes better understood, we may be able to decipher between these potential underlying mechanisms creating additional opportunities for therapeutic approaches that improve the generation or persistence of CD4 T cells expressing PD-1 with potential for treating autoimmunity.
The induction of TIGIT-positive CD8 cells with profiles reminiscent of exhaustion is similarly intriguing. TIGIT is another ‘negative regulator’ ITIM-containing surface receptor well recognized for association with cancer-associated immunosuppression [35]. Thus, analogous to PD-1, the induction of TIGIT-positive CD8 T cells may mechanistically be a direct link to favorable therapeutic outcomes in autoimmunity. While CD8 T cell exhaustion has been most thoroughly described in cancer and chronic viral infections [14–16], the combination of markers induced by teplizumab on CD8 T cells in this study and in the previous work by Long, et al [8] strongly suggests that this pathway may be an important indicator of clinical outcome in T1D.
5. Conclusions
Although treatment with teplizumab leads to a transient reduction of CD3-positive cells from peripheral blood, its more durable effects correspond to an agonist activity that induces markers reminiscent of hyporesponsive cells. The impact on particular subsets of both CD4 and CD8 T cells following teplizumab treatment is clearly illustrated in this longitudinal cohort of well-characterized subjects followed over two years, through two 14-day courses of therapy. These data support an emerging concept that induction of cellular anergy and exhaustion may be a viable therapeutic strategy in autoimmunity, and that the partial efficacy of anti-CD3 drugs could be related to the expansion of multiple tolerogenic T cell populations in the subgroup of patients treated successfully. By identifying cells responsible for tolerance, more specific or combination treatment strategies in the future may be both safer and more efficacious. Use of comparable analyses in other trials will facilitate comparison of mechanisms of tolerance in different treatments and diseases.
Data and materials availability
Datasets and Figures from this study, along with clinical and laboratory data from the AbATE trial, are available on TrialShare, the Immune Tolerance Network data visualization portal, at: https://www.itntrialshare.org/AbATE.url.
Acknowledgments
We thank the members of the Human Immunophenotyping Core at Benaroya Research Institute for generating the longitudinal flow cytometry data.
Funding
Research reported in this publication was sponsored by the Immune Tolerance Network and supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1AI109565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
S.A.L, K.C.H, M.E., P.S.L., G.T.N., and K.M.H. contributed to concept development and experimental design. J.T. and N.L. performed experiments and collected data. N.L. helped with data analysis and visualization. S.A.L, J.T., K.C.H, P.S.L., S.S., G.T.N., and K.M.H. aided in interpretation of the data. K.M.H, G.T.N and S.A.L. wrote the manuscript. All authors made contributions to the final manuscript prior to submission.References
Citations & impact
Impact metrics
Article citations
Teplizumab's immunomodulatory effects on pancreatic β-cell function in type 1 diabetes mellitus.
Clin Diabetes Endocrinol, 10(1):23, 10 Aug 2024
Cited by: 0 articles | PMID: 39123252 | PMCID: PMC11316332
Review Free full text in Europe PMC
Inflammation in diabetes complications: molecular mechanisms and therapeutic interventions.
MedComm (2020), 5(4):e516, 12 Apr 2024
Cited by: 4 articles | PMID: 38617433 | PMCID: PMC11014467
Review Free full text in Europe PMC
CD8 T cells induce the peritubular capillary rarefaction during AKI to CKD transition.
Int J Biol Sci, 20(8):2980-2993, 19 May 2024
Cited by: 0 articles | PMID: 38904017 | PMCID: PMC11186369
Disease-modifying therapies and features linked to treatment response in type 1 diabetes prevention: a systematic review.
Commun Med (Lond), 3(1):130, 05 Oct 2023
Cited by: 4 articles | PMID: 37794169 | PMCID: PMC10550983
Combination treatment of a novel CXCR3 antagonist ACT-777991 with an anti-CD3 antibody synergistically increases persistent remission in experimental models of type 1 diabetes.
Clin Exp Immunol, 214(2):131-143, 01 Dec 2023
Cited by: 0 articles | PMID: 37458220 | PMCID: PMC10714188
Go to all (44) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Heterogeneous CD3 expression levels in differing T cell subsets correlate with the in vivo anti-CD3-mediated T cell modulation.
J Immunol, 194(5):2117-2127, 02 Feb 2015
Cited by: 18 articles | PMID: 25646305
Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis.
Diabetologia, 62(4):655-664, 19 Dec 2018
Cited by: 45 articles | PMID: 30569273 | PMCID: PMC6402971
KLRG1 expression identifies short-lived Foxp3+ Treg effector cells with functional plasticity in islets of NOD mice.
Autoimmunity, 50(6):354-362, 29 Aug 2017
Cited by: 18 articles | PMID: 28850267
Anti-CD3 clinical trials in type 1 diabetes mellitus.
Clin Immunol, 149(3):268-278, 11 May 2013
Cited by: 57 articles | PMID: 23726024
Review
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR001863
NIAID NIH HHS (1)
Grant ID: UM1 AI109565
NIDDK NIH HHS (1)
Grant ID: P30 DK063720




