Abstract
Background
Abnormal amino acid metabolism is associated with vascular disease. However, the causative link between dysregulated tryptophan metabolism and abdominal aortic aneurysm (AAA) is unknown.Methods
Indoleamine 2,3-dioxygenase (IDO) is the first and rate-limiting enzyme in the kynurenine pathway of tryptophan metabolism. Mice with deficiencies in both apolipoprotein e (Apoe) and IDO (Apoe-/-/IDO-/-) were generated by cross-breeding IDO-/- mice with Apoe-/- mice.Results
The acute infusion of angiotensin II markedly increased the incidence of AAA in Apoe-/- mice, but not in Apoe-/-/IDO-/- mice, which presented decreased elastic lamina degradation and aortic expansion. These features were not altered by the reconstitution of bone marrow cells from IDO+/+ mice. Moreover, angiotensin II infusion instigated interferon-γ, which induced the expression of IDO and kynureninase and increased 3-hydroxyanthranilic acid (3-HAA) levels in the plasma and aortas of Apoe-/- mice, but not in IDO-/- mice. Both IDO and kynureninase controlled the production of 3-HAA in vascular smooth muscle cells. 3-HAA upregulated matrix metallopeptidase 2 via transcription factor nuclear factor-κB. Furthermore, kynureninase knockdown in mice restrained 3-HAA, matrix metallopeptidase 2, and resultant AAA formation by angiotensin II infusion. Intraperitoneal injections of 3-HAA into Apoe-/- and Apoe-/-/IDO-/- mice for 6 weeks increased the expression and activity of matrix metallopeptidase 2 in aortas without affecting metabolic parameters. Finally, human AAA samples had stronger staining with the antibodies against 3-HAA, IDO, and kynureninase than those in adjacent nonaneurysmal aortic sections of human AAA samples.Conclusions
These data define a previously undescribed causative role for 3-HAA, which is a product of tryptophan metabolism, in AAA formation. Furthermore, these findings suggest that 3-HAA reduction may be a new target for treating cardiovascular diseases.Free full text

Tryptophan-derived 3-Hydroxyanthranilic Acid Contributes to Angiotensin II-induced Abdominal Aortic Aneurysm Formation in Mice in vivo
Abstract
Background
Abnormal amino acid metabolism is associated with vascular disease. However, the causative link between dysregulated tryptophan metabolism and abdominal aortic aneurysm (AAA) is unknown.
Methods
Indoleamine 2,3-dioxygenase (IDO) is the first and rate-limiting enzyme in the kynurenine pathway of tryptophan metabolism. Mice with deficiencies in both apolipoprotein e (Apoe) and IDO (Apoe−/−/IDO−/−) were generated by cross-breeding IDO−/− mice with Apoe−/− mice.
Results
The acute infusion of angiotensin II (AngII) markedly increased the incidence of AAA in Apoe−/− mice, but not in Apoe−/−/IDO−/− mice, which presented decreased elastic lamina degradation and aortic expansion. These features were not altered by the reconstitution of bone marrow cells from IDO+/+ mice. Moreover, AngII infusion instigated interferon (IFN)-γ, which induced the expression of IDO and kynureninase (KNU) and increased 3-hydroxyanthranilic acid (3-HAA) levels in the plasma and aortas of Apoe−/− mice, but not in IDO−/− mice. Both IDO and KNU controlled the production of 3-HAA in vascular smooth muscle cells. 3-HAA upregulated MMP2 via transcription factor nuclear factor-kappa B (NF-κB). Furthermore, KNU knockdown in mice restrained 3-HAA, matrix metallopeptidase (MMP)2, and resultant AAA formation by AngII infusion. Intra-peritoneal injections of 3-HAA into Apoe−/− and Apoe−/−/IDO−/− mice for 6 weeks increased the expression and activity of MMP2 in aortas without affecting metabolic parameters. Finally, human AAA samples had stronger staining with the antibodies against 3-HAA, IDO, and KNU than those in adjacent nonaneurysmal aortic sections of human AAA samples.
Conclusions
These data define a previously undescribed causative role for 3-HAA, which is a product of tryptophan metabolism, in AAA formation. Furthermore, these findings suggest that 3-HAA reduction may be a new target for treating cardiovascular diseases.
Introduction
Abdominal aortic aneurysm (AAA) is a permanent, localized dilation of the abdominal aorta. It occurs in up to 9% of adults older than 65 years of age, with about 15,000 annual deaths after rupture in the United States.1, 2 Pathologically, AAA is characterized by a dilatation of all layers of the arterial wall due to elastin loss, smooth muscle cell apoptosis, and compensatory collagen deposition.3–5 Currently, no therapeutic strategies are proven to block AAA progression and rupture, and endovascular or open surgical repair appears to be the only available approach.2 Despite decades of research on AAA, there is a paucity of knowledge on the mechanisms and factors controlling AAA growth. Therefore, elucidating the molecular basis for this disease is imperative for the development of novel pharmacologic therapies.
The kynurenine (Kyn) pathway contributes to several fundamental biological processes and is the major route for the metabolism of essential amino acid tryptophan (Trp). Trp is constitutively oxidized by tryptophan 2,3-dioxygenase in liver cells. In other cell types, Trp is catalyzed by an alternative inducible indoleamine-pyrrole 2,3-dioxygenase (IDO) under certain pathophysiological conditions.6 The first stable intermediate from the Kyn pathway is Kyn. Subsequently, in eukaryotes, kynureninase (KNU) directly catalyzes the hydrolysis of Kyn or 3-hydroxykynurenine (3-HK) to form anthranilic acid (AA) or 3-hydroxyanthranilic acid (3-HAA), respectively.7, 8 Catabolites in the Kyn pathway of Trp metabolism play critical roles in vascular physiology and pathology9, 10 in addition to regulating the immune system11, 12 and inflammation.6, 13 Moreover, IDO is a potential novel contributor to vessel relaxation in systemic infections,14,15 which are also activated in acute severe heart attacks.16 Recently, IDO was reported to play a critical role in atherogenesis in mice.17, 18 All these findings have illustrated the key role of the Kyn pathway in the increased prevalence of cardiovascular disease (CVD).19 However, whether catabolites from the Kyn pathway contribute to AAA is unknown.
Angiotensin II (AngII) is a principal mediator for the development and progression of AAA.20 Many elements of human AAA are recapitulated in AngII-infused mice.21 Our previous study demonstrated that AngII infusion powerfully induces IDO expression and increases Kyn metabolites of Trp in the aortas of mice.10 More importantly, IDO deletion significantly inhibits AngII-triggered nicotinamide adenine dinucleotide phosphate-derived oxidative stress in vessels.10 Therefore, we sought to identify the roles of the Kyn pathway and its catabolites in AngII-induced AAA. AAA formation in AngII-treated apolipoprotein e-deficient (Apoe−/−) mice was significantly prevented in the IDO-deficient (IDO−/−) background and under the condition of in vivo KNU short-interfering RNA (siRNA) transfection. Mechanistically, the genetic inhibition of IDO and KNU substantially eliminated 3-HAA, which was the key Trp catabolite of the AngII-induced Kyn pathway, and intensely promoted matrix metallopeptidase 2 (MMP2) expression controlled by nuclear factor-kappa B (NF-κB) transcription factor in vascular smooth muscle cells (VSMCs).
Materials and Methods
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Oklahoma Health Sciences Center and Georgia State University. Human aortic samples used in this study were procured at Peking Union Medical College following patient consent according to Institutional Review Board-approved protocol.
IDO−/− mice were crossed with Aope−/− mice to generate Apoe−/−/IDO−/− mice. Apoe−/− and Apoe−/−/IDO−/− mice at age 8 weeks or 4 weeks after bone marrow transplantation or with KUN siRNA transfection on a chow diet were infused with AngII (1,000 ng/kg/min) or physiological saline (0.9% sodium chloride) infusion for 4 weeks or intraperitoneally injected with 3-HAA (200 mg/kg. d) or vehicle for 6 weeks.
Detailed descriptions of other unmentioned materials, methods, and experimental procedures are available in the online version of the paper.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism 6. Quantitative results are reported as means ± standard errors of the means. The comparisons of AAA incidence were made by Fisher’s Exact test. An unpaired or paired Student’s t-test was applied to detect significant differences between two groups. A two or three-way analysis of variance followed by Bonferroni’s multiple comparison tests was used to compare differences among more than two groups. A value of P< 0.05 was considered statistically significant.
Results
IDO Deletion Abrogates AngII-Induced AAA Formation
Recently, AngII-induced mouse AAA formation in the hypercholesterolemic mouse strain (Apoe−/−) has become the most widely used model.22–25 To unravel the role of the Trp-Kyn pathway in AAA formation, we generatedApoe−/−/IDO−/−(double-knockout) mice and detected the outcome of a 4-week AngII infusion (1000 ng/min/kg) in Apoe−/− and Apoe−/−/IDO−/−mice. As depicted in Supplementary Table 1 and 2, AngII infusion increased blood pressure without affecting the heart rate or any metabolic parameters. Morphologically, the aortas of saline-infused Apoe−/−/IDO−/−mice did not differ from those of saline-infused control Apoe−/− mice (Fig. 1a). In line with previous reports,3, 4, 26 the incidence of AngII-induced AAA in Apoe−/− mice was 73% (Fig. 1a, b). Both the maximal abdominal aortic diameter (Fig. 1c) and total aortic weight (Fig. 1d) were significantly higher in AngII-infused Apoe−/− mice than in saline-infused mice. Furthermore, the frequent disruption and increased degradation of elasticlaminas were observed in AngII-infused Apoe−/− mice but not in saline-infused mice (Fig. 1e, f). Markedly increased collagen deposition was also observed in AngII-infused Apoe−/− mice (Fig. 1e, g).
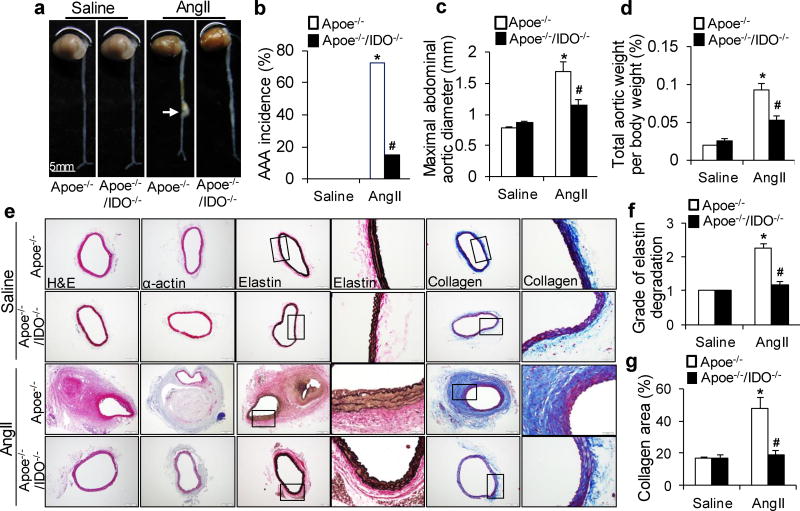
Saline or AngII (1000 ng/min per kg) was administered to Apoe−/− and Apoe−/−/IDO−/− mice for 4 weeks. (a) Representative photographs showing the macroscopic features of AngII-induced aneurysms. The arrow indicates typical AAA. (b–d) The incidence of AngII-induced AAA (b), maximal abdominal aortic diameter (c), and total aortic weight (d) in mice of the indicated genotypes after saline or AngII infusion. (e) Representative staining with hematoxylin and eosin (H&E), α-actin, Van Gieson’s (elastin), and Masson’s Trichrome (collagen) in the suprarenal aortas of mice after saline or AngII infusion. (f, g) Grade of elastin degradation (f) and collagen deposition (g) in the aortic wall of mice after saline or AngII infusion. *P<0.01 vs. saline-infused Apoe−/− mice, #P<0.01 vs. AngII-infused Apoe−/− mice. n=8 in each group of Apoe−/− and Apoe−/−/IDO−/− mice infused with saline. n=15 for AngII-infused Apoe−/− mice. n=12 for AngII-infused Apoe−/−/IDO−/− mice. P values were obtained by a Fisher’s Exact test in b and by a two-way analysis of variance (ANOVA) with following Bonferroni’s multiple comparisons in c, d, f, and g. The error bars in c, d, f, and g represent the standard error of the mean (s.e.m.).
In contrast, only 15% of AngII-infused Apoe−/−/IDO−/−mice developed AAA (Fig. 1a, b). The maximal abdominal aortic diameter (Fig. 1c) and total aortic weight (Fig. 1d) were remarkably reduced in AngII-treated Apoe−/−/IDO−/−mice compared with AngII-treated Apoe−/− mice. Neither the aortic expansion nor the increased aortic thickness was observed in AngII-infused Apoe−/−/IDO−/−mice (Fig. 1e). Moreover, AngII infusion inApoe−/−/IDO−/−mice did not cause aortic elastic lamina degradation or collagen deposition (Fig. 1f, g). These results suggest that IDO deletion confers protection from AngII-induced AAA formation in Apoe−/− mice in vivo.
IDO Deficiency Mitigates MMP2 Upregulation in AAA Mice
AngII infusion is intensely associated with vascular inflammation, which is considered a key mediator of AngII-induced AAA formation.25, 26 As shown in Supplementary Table 3, serum concentrations of inflammatory cytokines, including interferon (IFN)-γ, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and cyclophilin A (CyPA), were elevated in both AngII-infused Apoe−/− and Apoe−/−/IDO−/−mice. These data indicate that IDO deletion does not alter AngII-induced inflammation. Our previous study demonstrated that IFN-γ mediated AngII-induced Kyn pathway activation in vivo.10 Similarly, an increase in IFN-γ expression was evident in the aortas of AngII-treated Apoe−/− and Apoe−/−/IDO−/−mice. The consequent induction of IDO expression in the aortas of AngII-infused Apoe−/− mice was also observed (Fig. 2a, b, e). Accordingly, plasma levels of Kyn were significantly raised in AngII-infused Apoe−/− mice but not in IDO−/− mice (Fig. 2c), whereas AngII infusion did not alter plasma Trp levels in any mouse genotype (Fig. 2d). This may be due to the high content of Trp in mouse diets.
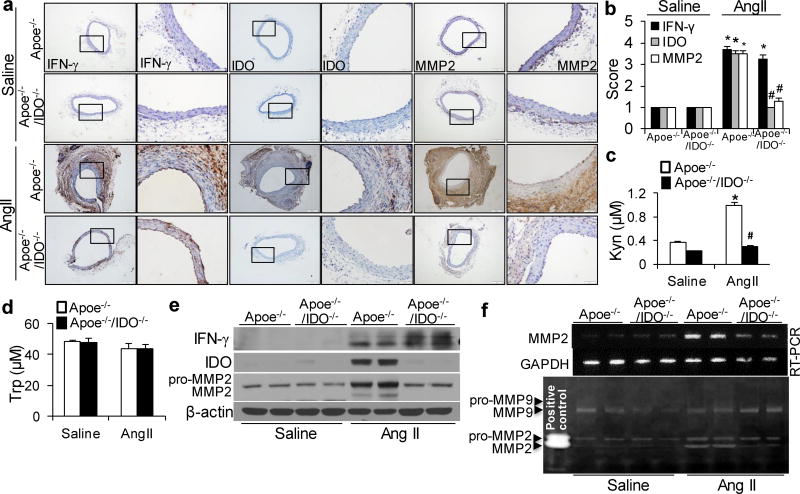
(a, b) Representative immunohistochemical staining (a) and quantification (b) for interferon (IFN)-γ, IDO, and MMP2 in the suprarenal aortas of saline- or AngII-infused mice. (c, d) Plasma concentrations of Kyn (c) and tryptophan (Trp) (d) detected in mice after saline or AngII infusion.(e, f) Protein expressions of IFN-γ, IDO, and MMP2 (e), as well as MMP2 mRNA (f) and activity (by zymography, f), in the suprarenal aortas of saline- or AngII-infused mice; β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as the loading control. *P<0.01 vs. saline-infused Apoe−/− mice, #P<0.01 vs. AngII-infused Apoe−/− mice. All results were obtained from 6–10 mice in each group. P values in b–d were obtained by a two-way ANOVA with following Bonferroni’s multiple comparisons. The error bars in b–d are s.e.m.
MMPs play a key role in the initiation and progression of AAA.27 In particular, VSMC-derived MMP228, 29 and macrophage-derived MMP930 are critical for AAA development. Thus, we next determined if IDO deletion affects the levels of MMP2 and MMP9 in AngII-induced AAA formation. MMP2 mRNA levels (Fig. 2f and Supplementary Fig. 1a), protein expressions (Fig. 2a, b, e) and activity (Fig. 2f) were substantially increased in AngII-infused Apoe−/− mice. In contrast, the increases in MMP2 expression and activity were dramatically abrogated in the aortas of AngII-infused IDO−/− mice (Fig. 2a, b, e, f). However, IDO deletion did not alter the AngII-induced increase in MMP9 activity (Fig. 2f). Therefore, MMP2 seems to be the most predominant MMP inhibited by IDO deletion in AngII-induced AAA formation.
Vascular IDO Deletion Inhibits AAA Formation
Based on the above-presented data, we postulated that the activation of the Kyn pathway in VSMCs promotes AAA formation. To test this hypothesis, we performed reciprocal bone marrow transplants between Apoe−/− and Apoe−/−/IDO−/−mice, in which bone marrow cells were transplanted into irradiated mice. After 6 weeks of engraftment, transplanted mice were treated with AngII (1000 ng/min per kg) for 4 weeks. This led to the formation of AAAs in Apoe−/− mice transplanted with either IDO−/− or IDO+/+ bone marrow cells, with a similar incidence of approximately 70% (Fig. 3a, b). In contrast, an inhibition of AAA formation (20% incidence) was observed in Apoe−/−/IDO−/− mice transplanted with either IDO−/− or IDO+/+ bone marrow cells (Fig. 3a, b).
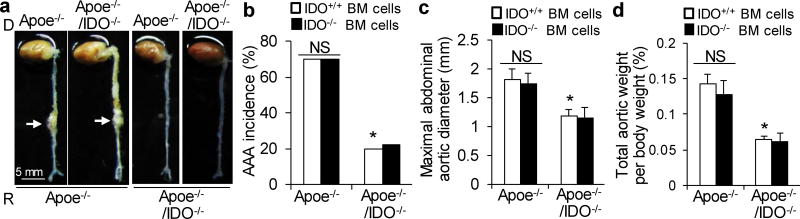
Apoe−/− and Apoe−/−/IDO−/− mice were reconstituted with Apoe−/− or Apoe−/−/IDO+/+ bone marrow cells, followed by 4 weeks of saline or AngII infusion (1000 ng/min per kg). (a) Representative photographs showing the macroscopic features of AngII-induced aneurysms. Arrows indicate typical AAA. (b–d) The incidence of AngII-induced AAA (b), maximal abdominal aortic diameter (c), and total aortic weight (d) in AngII-infused mice reconstituted with IDO+/+ or IDO−/− bone marrow cells. n=10–15 per group. BM, bone marrow; NS, not significant. *P<0.01 vs. AngII-infused Apoe−/− mice reconstituted with Apoe−/−/IDO+/+ bone marrow cells. P values were obtained by a Fisher exact test in b and by a two-way ANOVA with following Bonferroni’s multiple comparisons in c and d. The error bars in c and d are s.e.m.
No differences in the maximal abdominal aortic diameter (Fig. 3c) and total aortic weight (Fig. 3d) were observed between mice transplanted with IDO−/− bone marrow cells and mice transplanted with IDO+/+ bone marrow cells. These data suggest that IDO deficiency in vascular cells, rather than bone marrow-derived cells, is crucial for the development of AAA.
IFN-γ-induced MMP2 Expression with Additional Trp in Human Aortic Smooth Muscle Cells (HASMCs) is IDO Dependent
Next, we determined if Kyn pathway activation regulates MMP2 expression in HASMCs. As depicted in Supplementary Fig. 2a, IFN-γ (100 nM) powerfully induced IDO expression in HASMCs in a time-dependent manner. IFN-γ also induced the formation of Kyn and the consumption of Trp in the supernatant as a time dependent manner (Supplementary Fig. 2b). Interestingly, Trp was almost used up by the cells after 48 hours of treatment. HASMCs were then transfected with or without IDO siRNA for 48 hours and treated with IFN-γ (100 nM). Unexpectedly, MMP2 protein expression was decreased, along with IDO induction (Supplementary Fig. 2c), whereas the increase in Kynin the culture medium was accompanied by Trp depletion (Supplementary Fig. 2d). Trp depletion has been shown to inhibit MMP expression in human fibroblasts31 and to halt cell cycle progression in T cells.32 However, no reductions in Trp were observed in AngII-infused Apoe−/− mice (Fig. 2d). Therefore, different concentrations of exogenous Trp were supplemented in the culture medium of HASMCs with IFN-γ treatment for 48 hours to mimic thein vivo environment. MMP2 expression was markedly upregulated in HASMCs exposed to IFN-γ and >40 µMTrp (Supplementary Fig. 3b), whereas Trp addition did not alter MMP2 expression in HASMCs without IFN-γ (Supplementary Fig. 3a). Meanwhile, Kyn formation was intensely amplified in the presence of excessive Trp in the culture medium of IFN-γ-treated HASMCs (Supplementary Fig. 3b). Three-fold increases in Kyn were detected in the supernatant of IFN-γ-treated, Trp-supplemented (100 µM) HASMCs, compared with that in IFN-γ-treated HASMCs. When 50 µM Trp was added to the culture medium of IFN-γ-treated HASMCs, Trp levels were restored to control levels, and MMP2 expression was increased along with IDO induction (Supplementary Fig. 3c). These results suggest that IFN-γ induces MMP2 expression in vitro when sufficient amounts of Trp are present. As expected, IDO knockdown significantly inhibited IFN-γ-induced MMP2 expression, even in the presence of additional Trp (Supplementary Fig. 3c). This was further confirmed in IFN-γ-treated HASMCs with the addition of 100 µM Trp (Supplementary Fig. 3d).
Hypertryptophanemia33–35 is a rare autosomal recessive metabolic disorder that results in a massive buildup of Trp in the blood.36, 37 The addition of 500 µMTrp to HASMCs did not affect MMP2 expression (Supplementary Fig. 3e). However, MMP2 expression, MMP2 activity, and Kyn formation in the supernatant were increased in IFN-γ-treated HASMCs supplemented with Trp in a concentration- and time-dependent manner (Fig. 4a, b). In addition, no further increases in Kyn were detected in the supernatant of IFN-γ-treated HASMCs supplemented with >500 µM Trp (data not shown). Concomitantly, the upregulation of MMP2 by IFN-γ with 500 µM exogenous Trp was blocked by IDO knockdown (Fig. 4c). These results suggest that Kyn pathway activation potently promotes MMP2 expression and activity in HASMCs in vitro, thus indicating that Trp-derived Kyns may directly induce a concentration- and time-dependent induction of MMP2.
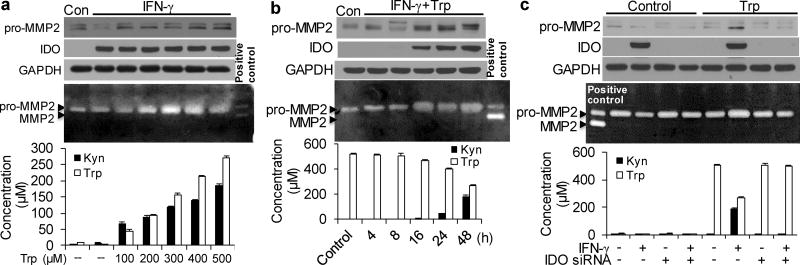
(a) Cultured HASMCs were either untreated (control; Con) or treated with IFN-γ combined with the indicated concentrations of exogenous Trp for 48 hours. (b) Cultured HASMCs were either untreated (control; Con) or treated with IFN-γ and 500µM Trp for the indicated time points. (c) HASMCs were transfected with scrambled siRNA or IDO siRNA and treated with DMSO (vehicle) or IFN-γ in the presence and absence of 500µM Trp for 48 hours. (a–c) Pro-MMP2, IDO, and GAPDH proteins were detected by immunoblotting. MMP2 activities in the culture medium were detected by zymography. Trp and Kyn levels in the culture medium were detected by high-performance liquid chromatography (HPLC). Three independent experiments for all quantitative data. The error bars in a–c are s.e.m.
3-HAA Promotes NF-κB-mediated MMP2 Expression in HASMCs
To identify Kyn pathway catabolites that upregulate MMP2, the expression and activity of MMP2 were detected in HASMCs incubated with major exogenous metabolites6, 37 of Trp degradation. A dramatic increase in MMP2 expression and activity was observed with 3-HAA,38–41 but not with Kyn,10 3-HK,10 kynurenic acid (KA),42 AA,43 xanthurenic acid (XA),44 or quinolinic acid (QA)45 (Fig. 5a). Furthermore, the expression and activity of MMP2 induced by 3-HAA appeared to be concentration- and time-dependent manner (Supplementary Fig.4a, b).
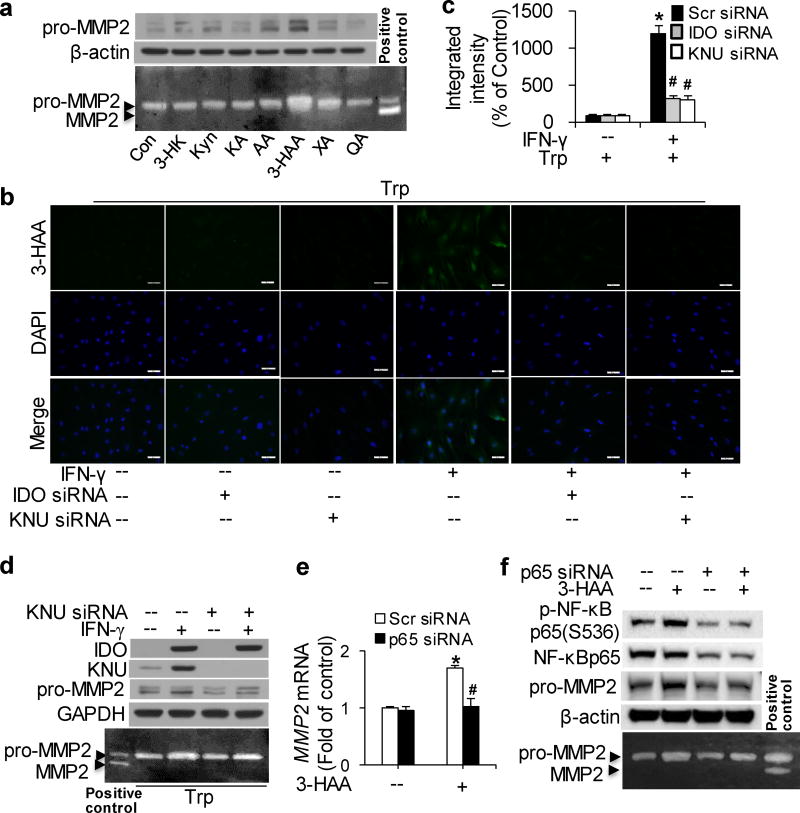
(a) Cultured HASMCs were either untreated (control; Con) or treated with the indicated metabolites of Trp degradation (Kyn: 100µM; 3-hydroxykynurenine [3-HK]: 100 µM; kynurenic acid [KA]: 75µM; anthranilic acid [AA]: 100µM; 3-HAA: 200µM; xanthurenic acid [XA]: 200µM; quinolinic acid [QA]: 1mM) for 48 hours. (b–d) Cultured HASMCs were transfected with scrambled siRNA, IDO siRNA, or kynureninase (KNU) siRNA and treated with vehicle or IFN-γ with the addition of 500 µM Trp for 48 hours. (e, f) Cultured HASMCs were transfected with scrambled siRNA or p65 siRNA and treated with 100 µM 3-HAA for 24 hours. pro-MMP2 (a, d, e), IDO (d), KNU (d), p-NF-κB p65(Ser536) (f) and NF-κB p65 (f) proteins were detected by immunoblotting; β-actin (a, f) and GAPDH (d) were used as loading controls, and MMP2 activities in the culture medium were detected by zymography. (b) A conjugated 3-HAA antibody was used to detect endogenous 3-HAA (green) in HASMCs. Cells were counterstained with a nuclear stain (DAPI; blue). (c) Quantitative analysis of the fluorescence intensity of intracellular 3-HAA.*P<0.01 vs. scrambled siRNA without IFN-γ, #P<0.01 vs. scrambled siRNA with IFN-γ treatment. (e) MMP2 and GAPDH mRNA were detected by real time polymerase chain reaction (PCR). In all panels, representative data from three independent experiments are shown. *P<0.05 vs Scr siRNA without 3-HAA, #P<0.01 vs Scr siRNA with 3-HAA treatment. P values in c and e were obtained by a two-way ANOVA with following Bonferroni’smultiple comparisons. The error bars in c and e are s.e.m. Scr siRNA: scrambled siRNA.
Following its synthesis by IDO in certain cell types, Kyn can be further metabolized bykynurenine-3-monooxygenase (KMO) into 3-HK, which is catabolized by KNU to form 3-HAA.6 To further validate the effects of 3-HAA on MMP2 induction in VSMCs, we detected endogenous 3-HAA and the expression of KNU. Intracellular 3-HAA and KNU expression emerged in IFN-γ-treated, Trp-supplemented HASMCs, along with MMP2 elevation (Fig. 5b–d). Accordingly, IDO or KNU knockdown blocked endogenous 3-HAA formation (Fig. 5b, c) which resulted in the inhibition of MMP2 expression and activity induced by IFN-γ with additional Trp (Fig. 5d). These results suggest that Trp-derived 3-HAA upregulates MMP2 expression in HASMCs in vitro.
Gene enrichment and positive immunostaining of NF-κB p65 and p50 have been observed in AAA tissues,46 besides; the modulation of NF-κB on MMP2 gene expression in vascular endothelium has been studied.47 We also observed 3-HAA-induced NF-κB p65 phosphorylation in HASMCs (Fig. 5f). Furthermore, NF-κB p65 knockdown in HASMCs by specific siRNA followed by 3-HAA treatment for 24 hours clearly blocked the elevation of MMP2 mRNA (Fig. 5e) and protein trigged by 3-HAA, as well as 3-HAA-accelerated MMP2 activity (Fig. 5e, f). These results demonstrate that NF-κB is implicated in 3-HAA-induced MMP2 upregulation.
IDO Deletion Abolishes 3-HAA Generation in AAA Mice
Next, we detected endogenous 3-HAA in vivo. In line with our in vitro data, increased levels of 3-HAA were observed in the aortas of AngII-treated Apoe−/− mice, but not in those of Apoe−/−/IDO−/− mice (Fig. 6a, b). Plasma3-HAA levels were notably raised in AngII-treated Apoe−/−mice (Fig. 6c), suggesting that IDO deficiency suppresses MMP2 expression in aortas by inhibiting endogenous 3-HAA formation.
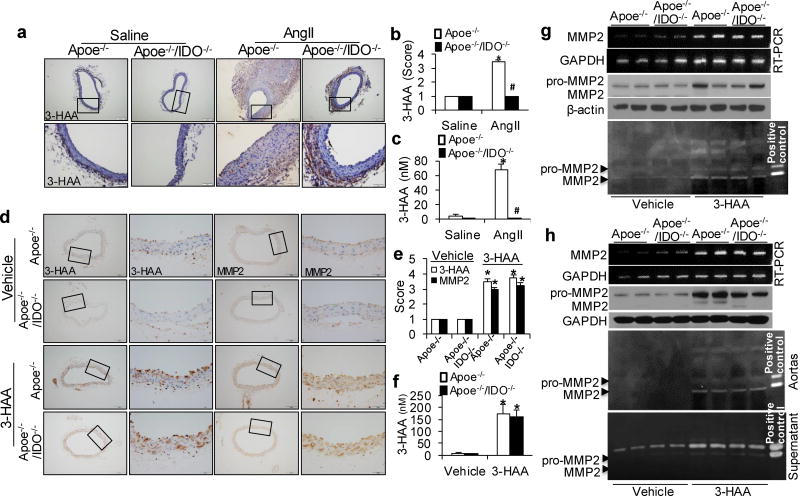
(a–c) Saline or AngII (1000 ng/min per kg) was administered to Apoe−/− and Apoe−/−/IDO−/− mice for 4 weeks. Representative immunohistochemical staining (a) and quantification (b) for 3-HAA in the suprarenal aortas of saline- or AngII-infused mice of the indicated genotypes. (c) Serum levels of 3-HAA were detected by HPLC in mice after saline or AngII infusion. *P<0.01 vs. saline-infused Apoe−/− mice, #P<0.01 vs. AngII-infused Apoe−/− mice. n=8–10 per group. (d–g) Apoe−/− and Apoe−/−/IDO−/− mice were intra-peritoneally injected with vehicle (100 µl of 20% DMSO in Captisol) or 3-HAA (200 mg/kg/d) for 6 weeks. Representative immunohistochemical staining (d) and quantification (e) for 3-HAA and MMP2 in the aortas of vehicle- or 3-HAA-injected mice of the indicated genotypes. (f) Plasma levels of 3-HAA were detected by HPLC in mice with or without 3-HAA injection. (g) The mRNA and protein expressions and activities (by zymography) of MMP2 in the aortas of vehicle- or 3-HAA-injected mice, β-actin and GAPDH were used as the loading control. *P<0.01 vs. vehicle-injected Apoe−/− mice. All results represent 8–12 mice per group.(h) The aortas isolated from Apoe−/− and Apoe−/−/IDO−/− mice were treated with vehicle or 400 µM 3-HAA for 48 hours ex vivo. MMP2 and GAPDH mRNA were detected by RT-PCR, and MMP2 and β-actin protein expressions in the aortas were detected by immunoblotting, and MMP2 activity in the aortas and supernatants was detected by zymography. All results were obtained from eight mice in each group. P values in b, c, e, and f were obtained by a two-way ANOVA with following Bonferroni’s multiple comparisons. The error bars in b, c, e, and f are s.e.m.
3-HAA Accelerates MMP2 Expression in Mouse Aortas
To ascertain the effect of 3-HAA in MMP2 expression in vivo, Apoe−/− and Apoe−/−/IDO−/− mice were intra-peritoneally treated with vehicle or 3-HAA (200mg/kg/d)38–41 for 6 weeks. Corresponding increases in 3-HAA were detected in the plasma (Fig. 6f) and aortas (Fig. 6d, e) of 3-HAA-injected Apoe−/− and Apoe−/−/IDO−/− mice. As depicted in Supplementary Table 4, 3-HAA injection did not affect any metabolic parameters in Apoe−/− or Apoe−/−/IDO−/− mice. Consequently, MMP2 mRNA levels (Fig. 6g and Supplementary Fig. 1b), protein expressions (Fig. 6d, e, g) and activity (Fig. 6g) were remarkably increased in both 3-HAA-treated Apoe−/− and Apoe−/−/IDO−/− mice, as compared with vehicle-treated mice. Aortas isolated from Apoe−/− and Apoe−/−/IDO−/− mice were incubated with or without 400 µM 3-HAA for 48 hours. Coincidently, 3-HAA treatment significantly upregulated MMP2 mRNA levels (Fig. 6h and Supplementary Fig. 1c), protein expressions and activity of the aortas from both Apoe−/− and Apoe−/−/IDO−/− mice (Fig. 6h). A similar increase in MMP2 activity was observed in the culture medium of 3-HAA-treated aortas (Fig. 6h). These results suggest that exogenous 3-HAA upregulates MMP2 expression in mouse aortas in vivo and ex vivo. Furthermore, 3-HAA-mediated MMP2 induction was independent on IDO.
KNU Deficiency Attenuates AngII-Induced AAA Formation
To further identify the effects of endogenous 3-HAA on AAA development, we treated AngII-infused Apoe−/− mice with KNU siRNA. There were no differences in blood pressure or heart rate between scrambled siRNA- and KNU siRNA-transfected, AngII-infused mice (Supplementary Table 5). Changes in metabolic parameters were also not observed (Supplementary Table 6). Only 22% of AngII-infused, KNU siRNA-transfected Apoe−/− mice developed AAA compared with a 75% incidence of AAA in AngII-infused, scrambled siRNA-transfected Apoe−/− mice (Fig. 7a, b). The maximal abdominal aortic diameter (Fig. 7c) and total aortic weight (Fig. 7d) were remarkably lower in mice with KNU knockdown. Moreover, AngII infusion did not cause significant elastic lamina degradation and aortic expansion in KNU siRNA-transfected Apoe−/− mice (Fig. 7e, f). Collagen deposition was dramatically reduced in KNU siRNA-transfected mice compared with control mice (Fig. 7e, g). These results suggest that KNU knockdown protects Apoe−/− mice from AngII-induced AAA formation in vivo.
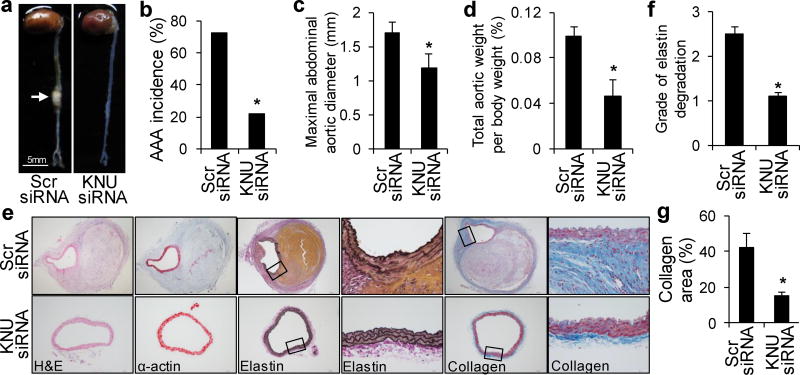
After transfection with scrambled (Scr) siRNA or KNU siRNA, Apoe−/− mice were infused with AngII (1000 ng/min per kg) for 4 weeks. (a) Representative photographs showing the macroscopic features of AngII-induced aneurysms. The arrow indicates typical AAA. (b–d) The incidence of AngII-induced AAA (b), maximal abdominal aortic diameter (c), and total aortic weight (d) in mice with the indicated siRNA transfections after AngII infusion. (e) Representative staining with H&E, α-actin, Van Gieson’s, and Masson’s Trichrome stain in the suprarenal aortas of mice with the indicated siRNA transfections after AngII infusion. (f, g) Grade of elastin degradation (f) and collagen deposition (g) in the aortic wall of mice with the indicated siRNA transfections after AngII infusion. *P<0.01 vs. scrambled siRNA-transfected Apoe−/− mice. n=10–12 in each group. P values were obtained by a Fisher’sExact test in b and by a ttest in c, d, f, and g. The error bars in c, d, f, and g are s.e.m.
Inhibition of Trp-Derived 3-HAA Abolishes MMP2 Upregulation in AAA Mice
As shown in Supplementary Table 7, KNU silencing did not alter serum concentrations of inflammatory cytokines in AngII-infused Apoe−/− mice. Accordingly, both IFN-γ and IDO expression levels were markedly enhanced in the aortas of AngII-treated Apoe−/− mice with or without KNU knockdown (Fig. 8d and Supplementary Fig. 5a, b). As expected, AngII only increased KNU expression in Apoe−/− mice transfected with scrambled siRNA (Fig. 8a, b, d). In line with this, high plasma levels of Kyn were detected in both groups of mice (Supplementary Fig. 5d), whereas high 3-HAA levels were only observed in the plasma (Fig. 8c) and aortas (Fig. 8a, b) of AngII-treated Apoe−/− mice with scrambled siRNA but not in KNU siRNA-transfected Apoe−/− mice. Because of compensatory Trp in the mouse diet, no differences in plasma Trp levels were found between scrambled siRNA- and KNU siRNA-transfected mice (Supplementary Fig. 5c).
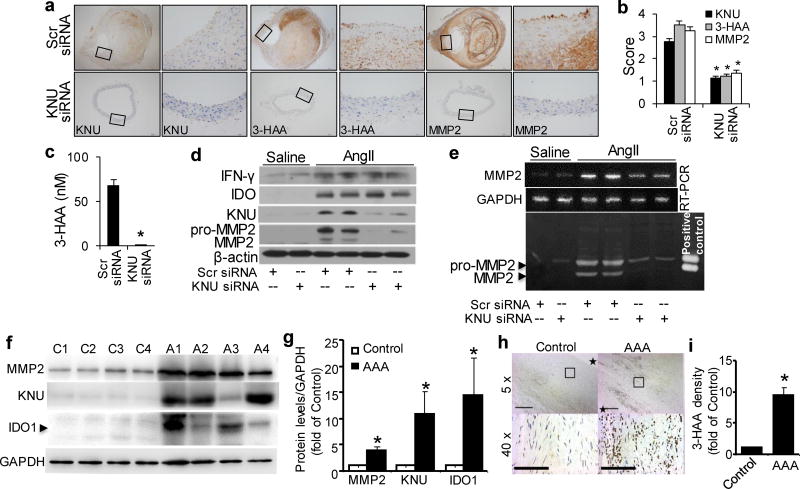
After transfections with scrambled (Scr) siRNA or kynureninase (KNU) siRNA, Apoe−/− mice were infused with saline or AngII (1000 ng/min per kg) for 4 weeks. (a–b) Representative immunohistochemical staining (a) and quantification for KNU, 3-HAA, and MMP2 (b) in the suprarenal aortas of AngII-infused mice with the indicated siRNA transfections. (c) Plasma concentrations of 3-HAA detected by HPLC in mice with the indicated siRNA transfections after AngII infusion.(d, e) The protein expression levels of IFN-γ, IDO, KNU, MMP2, and β-actin (d), as well as MMP2 mRNA (e) and activity (by zymography, e), in the suprarenal aortas of saline- or AngII-infused mice with the indicated siRNA transfections. *P<0.01 vs. scrambled siRNA-transfected Apoe−/− mice. All results were obtained from 6–10 mice in each group. (f) IDO1, KNU, and MMP2 increased in patient AAA samples. Representative Western blots were shown. C1~C4 indicates 4 control adjacent nonaneurysmal aortic sections; A1~A4 indicates 4 human AAA samples. (g) Quantification data for (f). n=4, *P<0.01 vs respective control group. (h) Anti-3-HAA staining in patient AAA area. *in control group indicates aortic lumen, * in AAA sample indicates aortic lumen side. (i) Quantification data for (h) (40X). *P<0.01 vs control group. P values in b and c, were obtained by a t test. P values in g and i were obtained by a paired t test. The error bars in b, c, g andi are s.e.m.
Next, we determined whether KNU silencing affects MMP2 levels in AngII-induced AAA formation. Compared with AngII-infused and scrambled siRNA-transfected Apoe−/− mice, MMP2 mRNA levels (Fig. 8e and Supplementary Fig.1d), protein expressions (Fig. 8a, b, d) and activity (Fig. 8e) were significantly reduced in AngII-infused, KNU siRNA-transfected Apoe−/− mice, indicating that endogenous 3-HAA reduction lowers MMP2 expression to inhibit AAA formation.
Kynurenine Pathway Activation in Human AAA Formation
To establish the clinical relevance of Kyn pathway activation and AAA formation, we further examined the expression of Kyn pathway key enzymes IDO and KNU in human AAA samples. Human AAA tissues and their control adjacent aortic sections without an aneurysm were obtained from patients undergoing open surgery. As expected, pro-aneurysmal molecules, such as MMP2 was dramatically elevated in human AAA sections compared with adjacent nonaneurysmal aortic sections (Fig. 8 f & g). Importantly, both IDO1 and KNU were significantly upregulated in human AAA samples (Fig. 8 f & g). Moreover, human AAA samples had stronger anti-3-HAA staining than adjacent nonaneurysmal aortic sections (Fig. 8h & i).
Discussion
This study is the first to show that IDO deletion or KNU knockdown in vivo restrained AngII-induced AAA in Apoe−/− mice. In VSMCs, AngII-mediated IFN-γ induced the expression of IDO and KNU, which are two key enzymes that regulate 3-HAA formation in the kynurenine pathway of Trp metabolism. Elevated 3-HAA upregulated NF-κB p65 phosphorylation at Ser536, resulting in the aberrant expression and activation of MMP2 and consequent extracellular matrix degradation (Supplementary Fig. 6). This mechanism not only provides an evident link between the Trp-Kyn pathway and AAA but also identifies a specific Trpmetabolite (3-HAA) that promotes AAA formation.
Activation of the Kyn pathway by AngII triggered AAA formation in Apoe−/− mice, and either IDO or KNU inhibition had a remarkably protective effect. These results might provide further insight into the emerging role of the Kyn pathway in the prevalence of CVD. Positive correlations between increased Kynurenines (Kyn, 3-HK, 3-HAA, KA, AA, or QA) and oxidative stress, immunoinflammatory responses, or endothelial dysfunction in CVD have been shown.48–53 In addition, Kyn, QA, and MMPs were significantly higher in continuous peritoneal dialysis patients with CVD than in patients without CVD and controls.52 QA and the QA/Kyn ratio have been identified to be factors that are independently associated with MMP2, and QA is positively correlated with MMP2.52 Furthermore, Kyn increases MMP1 and MMP3 in dermal fibroblasts.54 These observations support our findings for the direct connection between Kyn pathway activation and upregulated MMPs.
VSMCs are essential for AngII-induced AAA formation.3, 4 Regarding the dominant MMPs (MMP2 and MMP9) in vascular tissue,55 our results show that IDO deletion did not affect AngII-induced MMP9 but significantly reduced AngII-induced MMP2, which is exclusively secreted by VSMCs. The bone marrow transplantation experiments validated that vascular cells were the key targets of Kyns in the initiation and progression of AAA. Similar findings were observed in cultured HASMCs. Treatment with IFN-γ, along with the addition of exogenous Trp, induced MMP2 activation in vitro. The additional Trp not only rectified Trp depletion in the presence of IFN-γ but also dramatically augmented the cascade of Kyn generation in HASMCs.
Most importantly, we identified the specific product of Trp metabolism that is responsible for AngII-induced AAA. Exogenous 3-HAA treatment increased the mRNA and protein expressions of MMP2 in HASMCsin vitro and in mouse aortas in vivo, as well as MMP2activity. Subsequently, NF-κB mediated 3-HAA-instigated MMP2 induction in HASMCs in vitro. As a product of 3-HK, 3-HAA is prone to auto-oxidation in a process that favors the formation of superoxide anions.56 In experimental models, the pattern of 3-HAA in mitochondrial processes involves the inhibition of oxygen uptake by mitochondrial respiration with nicotinamide adenine dinucleotide-dependent substrates, uncoupling of the respiratory chain, and oxidative phosphorylation.57 In patients with chronic kidney disease, 3-HAA is independently associated with monocyte chemoattractant protein-1 and macrophage inflammatory protein-1β.58 Additionally, 3-HAA induces the depletion of intracellular glutathione in activated T cells without increasing formation of reactive oxygen species (ROS).59 The results from these studies provide us with the background needed to conduct further studies about 3-HAA-mediated VSMC activation and MMP2 secretion. 3-HAA might promote VSMC senescence with or without ROS generation. It has also been shown to inhibit atherosclerosis by regulating lipid metabolism and inflammation, and can significantly decrease plasma cholesterol and triglyceride levels in low-density lipoprotein receptor-deficient mice.38, 39 However, we did not observe any decreases in lipid levels in 3-HAA-treatedApoe−/− mice.
Results from the current study have shed light on the role of endogenous 3-HAA in AngII-induced AAA. Both in vitro and in vivoKNU silencing substantially lessened MMP2 activation in VSMCs. The in vivo silencing of KNU eliminated AngII-induced AAA development in Apoe−/− mice. Our investigation into the effects of exogenous and endogenous 3-HAA on MMP2 upregulation in VSMCs has clarified the role of 3-HAA in AngII-induced AAA.
Overall, we have established a causative correlation between Kyn pathway activation and AAA formation in vivo and identified the contribution of 3-HAA in AngII-triggered AAA. The identification of 3-HAA in AngII-triggered AAA and in human patients with AAA suggest that tryptophan-derived metabolites might be a biomarker for AAA diagnosis and the agents that prevent 3-HAA generation may have therapeutic potential for AAA and other CVD.
Acknowledgments
Sources of Funding
This study was supported by the National Institutes of Health grants (HL079584, HL080499, HL089920, HL110488, HL128014, HL132500, HL137371, HL140954, AG047776, and CA213022). This work was, in part, supported by the Georgia Research Alliance. Dr. Zou is a Georgia Research Alliance Eminent Scholar in Molecular Medicine. Dr. H.Z. Chen is supported by the Youth Top-notch Talent Support Program and the Youth Yangtze River Scholar Program in China.
Abbreviations used
| AAA | abdominal aortic aneurysm |
| AngII | angiotensin II |
| 3-HAA | 3-hydroxyanthranilic acid |
| 3-HK | 3-hydroxykynurenine |
| IDO1 | indoleamine-2, 3-dioxygenase 1 |
| IFN-γ | interferon-gamma |
| KMO | kynurenine-3-monooxygenase |
| KNU | kynureninase |
| NF-κB | factor nuclear factor-kappa B |
| MMP | matrix metalloproteinase |
| TIMP | tissue inhibitor of metalloproteinase |
| VSMC | vascular smooth muscle cell |
References
Full text links
Read article at publisher's site: https://doi.org/10.1161/circulationaha.117.030972
Read article for free, from open access legal sources, via Unpaywall:
https://www.ahajournals.org/doi/pdf/10.1161/CIRCULATIONAHA.117.030972
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1161/circulationaha.117.030972
Article citations
Harnessing intestinal tryptophan catabolism to relieve atherosclerosis in mice.
Nat Commun, 15(1):6390, 29 Jul 2024
Cited by: 0 articles | PMID: 39080345 | PMCID: PMC11289133
Progress and perspectives of metabolic biomarkers in human aortic dissection.
Metabolomics, 20(4):76, 13 Jul 2024
Cited by: 0 articles | PMID: 39002042
Review
Cellular metabolism changes in atherosclerosis and the impact of comorbidities.
Front Cell Dev Biol, 12:1446964, 12 Aug 2024
Cited by: 0 articles | PMID: 39188527 | PMCID: PMC11345199
Review Free full text in Europe PMC
Tryptophan and Its Derived Metabolites as Biomarkers for Tuberculosis Disease: A Systematic Review
Iran Biomed J, 28(4):140-147, 01 Jul 2024
Cited by: 0 articles | PMID: 39034495 | PMCID: PMC11444479
Review Free full text in Europe PMC
Dual treatment with kynurenine pathway inhibitors and NAD+ precursors synergistically extends life span in Drosophila.
Aging Cell, 23(4):e14102, 13 Mar 2024
Cited by: 2 articles | PMID: 38481042 | PMCID: PMC11019140
Go to all (41) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Tauroursodeoxycholic Acid Attenuates Angiotensin II Induced Abdominal Aortic Aneurysm Formation in Apolipoprotein E-deficient Mice by Inhibiting Endoplasmic Reticulum Stress.
Eur J Vasc Endovasc Surg, 53(3):337-345, 24 Nov 2016
Cited by: 30 articles | PMID: 27889204
Intermedin1-53 Attenuates Abdominal Aortic Aneurysm by Inhibiting Oxidative Stress.
Arterioscler Thromb Vasc Biol, 36(11):2176-2190, 15 Sep 2016
Cited by: 30 articles | PMID: 27634835
RANKL-mediated osteoclastogenic differentiation of macrophages in the abdominal aorta of angiotensin II-infused apolipoprotein E knockout mice.
J Vasc Surg, 68(6s):48S-59S.e1, 21 Apr 2018
Cited by: 12 articles | PMID: 29685509 | PMCID: PMC6558660
Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases.
Cell Mol Life Sci, 74(16):2899-2916, 17 Mar 2017
Cited by: 110 articles | PMID: 28314892 | PMCID: PMC5501999
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: R01 CA213022
NHLBI NIH HHS (9)
Grant ID: R01 HL074399
Grant ID: R01 HL128014
Grant ID: R01 HL140954
Grant ID: R01 HL079584
Grant ID: R01 HL089920
Grant ID: R01 HL110488
Grant ID: R01 HL137371
Grant ID: R01 HL080499
Grant ID: R01 HL132500
NIA NIH HHS (1)
Grant ID: R01 AG047776





