Abstract
Free full text

Tumor Mutational Burden and Response Rate to PD-1 Inhibition
Associated Data
TO THE EDITOR:
Inhibitors of programmed death 1 (PD-1) protein or its ligand (PD-L1) have shown remarkable clinical benefit in many cancers.1 One emerging biomarker of response to anti–PD-1 therapy is the tumor mutational burden (i.e., the total number of mutations per coding area of a tumor genome). This finding is supported by the clinical activity of anti–PD-1 therapy in colorectal cancer with mismatch repair deficiency, a tumor subtype with a high tumor mutational burden, as compared with the colorectal cancer subtype with mismatch repair proficiency, which has a significantly lower tumor mutational burden and a poor response to these agents.2,3
To evaluate the relationship between the tumor mutational burden and the objective response rate, we plotted the objective response rate for anti–PD-1 or anti–PD-L1 therapy against the corresponding median tumor mutational burden across multiple cancer types (Fig. 1). Through an extensive literature search, we identified 27 tumor types or subtypes for which data regarding the objective response rate are available. For each tumor type, we pooled the response data from the largest published studies that evaluated the objective response rate. We included only studies of anti–PD-1 or anti–PD-L1 monotherapy that enrolled at least 10 patients who were not selected for PD-L1 tumor expression. (Details about the methods are provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.) The median tumor mutational burden for each tumor type was obtained from a validated comprehensive genomic profiling assay performed and provided by Foundation Medicine.4
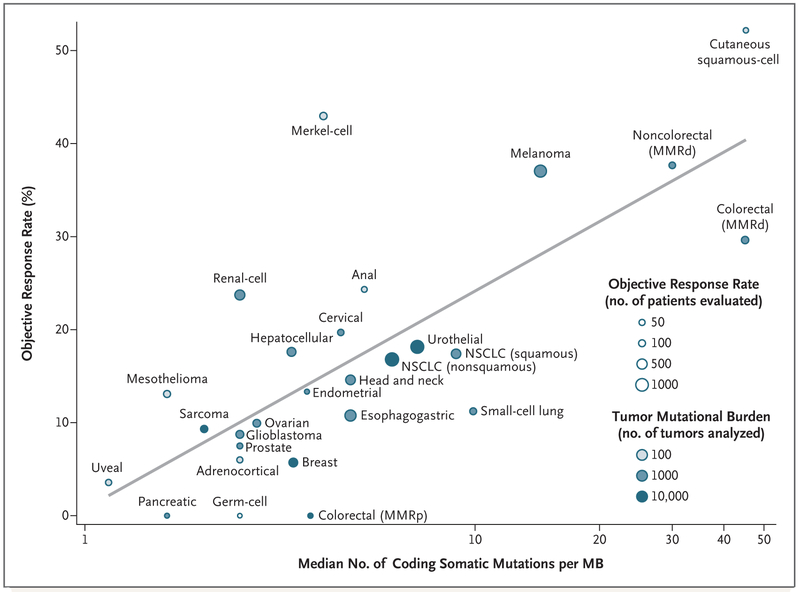
Shown is the median number of coding somatic mutations per megabase (MB) of DNA in 27 tumor types or subtypes among patients who received inhibitors of programmed death 1 (PD-1) protein or its ligand (PD-L1), as described in published studies for which data regarding the objective response rate are available. The number of patients who were evaluated for the objective response rate is shown for each tumor type (size of the circle), along with the number of tumor samples that were analyzed to calculate the tumor mutational burden (degree of shading of the circle). Data on the x axis are shown on a logarithmic scale. MMRd denotes mismatch repair-deficient, MMRp mismatch repair-proficient, and NSCLC non–small-cell lung cancer.
We observed a significant correlation between the tumor mutational burden and the objective response rate (P<0.001). The correlation coefficient of 0.74 suggests that 55% of the differences in the objective response rate across cancer types may be explained by the tumor mutational burden. Some cancer subtypes have a response to therapy that is better than would be predicted by the tumor mutational burden (e.g., Merkel-cell carcinoma), and some have a response that is worse than would be predicted (e.g., colorectal cancer with mismatch repair proficiency). The higher-than-anticipated objective response rates for Merkel-cell carcinoma and some other cancers that have been associated with viruses suggest that the presentation of viral antigens on certain tumor types may confer an increased response rate to anti–PD-1 therapy.5
Our linear correlation formula — objective response rate = 10.8 × loge(X) – 0.7, where “X” is the number of coding somatic mutations per megabase of DNA — can be used to make hypotheses with respect to the objective response rate in tumor types for which anti–PD-1 therapy has not been explored. For example, we anticipate a clinically meaningful objective response rate of 40.1% (95% confidence interval [CI], 31.2 to 50.6) for basal-cell carcinoma of the skin and of 20.6% (95% CI, 16.7 to 24.5) for sarcomatoid carcinoma of the lung on the basis of a median tumor mutational burden of 47.3 and 7.2, respectively.4 We anticipate a low objective response rate (<5%) for several other cancers (e.g., pilocytic astrocytoma and small-intestine carcinoid).4 A limitation of our analysis is that the sequenced tumor specimens were probably not the same ones for which clinical responses were assessed. Many different factors modulate the clinical response to an immune checkpoint inhibitor, but our findings highlight the strong relationship between the tumor mutational burden and the activity of anti–PD-1 therapies across multiple cancers.
Supplementary Material
Appendix
Disclosures
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
Full text links
Read article at publisher's site: https://doi.org/10.1056/nejmc1713444
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6549688?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1056/nejmc1713444
Article citations
TMED inhibition suppresses cell surface PD-1 expression and overcomes T cell dysfunction.
J Immunother Cancer, 12(11):e010145, 07 Nov 2024
Cited by: 0 articles | PMID: 39510795 | PMCID: PMC11552591
The T cell receptor β chain repertoire of tumor infiltrating lymphocytes improves neoantigen prediction and prioritization.
Elife, 13:RP94658, 28 Oct 2024
Cited by: 1 article | PMID: 39466298 | PMCID: PMC11517254
The Molecular Classification of Pheochromocytomas and Paragangliomas: Discovering the Genomic and Immune Landscape of Metastatic Disease.
Endocr Pathol, 28 Oct 2024
Cited by: 0 articles | PMID: 39466488
Review
Cancer Vaccines: Recent Insights and Future Directions.
Int J Mol Sci, 25(20):11256, 19 Oct 2024
Cited by: 0 articles | PMID: 39457036 | PMCID: PMC11508577
Review Free full text in Europe PMC
Combining ERAP1 silencing and entinostat therapy to overcome resistance to cancer immunotherapy in neuroblastoma.
J Exp Clin Cancer Res, 43(1):292, 22 Oct 2024
Cited by: 0 articles | PMID: 39438988 | PMCID: PMC11494811
Go to all (1,712) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Tumor mutation burden to tumor burden ratio and prediction of clinical benefit of anti-PD-1/PD-L1 immunotherapy.
Med Hypotheses, 116:111-113, 16 May 2018
Cited by: 15 articles | PMID: 29857892
Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade.
Cancer Immunol Res, 4(11):959-967, 26 Sep 2016
Cited by: 285 articles | PMID: 27671167 | PMCID: PMC5134329
Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors.
Mol Cancer, 17(1):129, 23 Aug 2018
Cited by: 413 articles | PMID: 30139382 | PMCID: PMC6107958
Review Free full text in Europe PMC
PDL-1/PD1 inhibitors: antibody or antinobody?
Future Oncol, 13(19):1669-1671, 23 Aug 2017
Cited by: 5 articles | PMID: 28831825





