Abstract
Free full text
Regulation of neuronal development and function by ROS
Abstract
Reactive oxygen species (ROS) have long been studied as destructive agents in the context of nervous system ageing, disease and degeneration. Their roles as signalling molecules under normal physiological conditions is less well understood. Recent studies have provided ample evidence of ROS‐regulating neuronal development and function, from the establishment of neuronal polarity to growth cone pathfinding; from the regulation of connectivity and synaptic transmission to the tuning of neuronal networks. Appreciation of the varied processes that are subject to regulation by ROS might help us understand how changes in ROS metabolism and buffering could progressively impact on neuronal networks with age and disease.
Abbreviations
BH4, tetrahydrobiopterin
CamKII, calcium/calmodulin‐dependent kinase II
CRMP2, collapsin response mediator protein 2
ER, endoplasmic reticulum
ERK, extracellular signal–regulated kinase
Grx1, glutaredoxin 1
HFS, high‐frequency stimulation
IP3Rs, inositol‐3‐phosphate receptors
JNK, Jun‐N‐terminal Kinase
LTD, long‐term depression
LTP, long‐term potentiation
MICAL, molecule interacting with CasL
MsrB, methionine sulfoxide reductase
NGF, nerve growth factor
PKC, protein kinase C
PP, protein phosphatase
PTEN, phosphatase and tensin homolog
PVIs, parvalbumin‐expressing inhibitory interneurons
redox, reduction–oxidation
ROS, reactive oxygen species
RyRs, ryanodine receptors
The increase in atmospheric oxygenation is presumed to have set the pace of evolutionary change. The symbiotic acquisition of mitochondria 1.45 billion years ago generating the eukaryota further allowed diversification via the efficient metabolic use of diatomic oxygen. Reactive oxygen species (ROS), highly reactive molecules and free radicals derived from molecular oxygen are produced as natural by‐products of normal respiratory metabolism, with the major source being the mitochondria. Mitochondria leak bursts of ROS as a function of respiration 1 pointing to a link between metabolism and ageing‐related damage. Other subcellular locations of ROS production continue to be identified and include the endoplasmic reticulum (ER) 2, peroxisome 3, the cytosol 4, plasma membrane 5 and extracellular space 6. However, there is a growing opinion and body of evidence that ROS can act as physiological signalling molecules. In this review we will focus on the role of ROS as a physiological signal in the nervous system during development and as a regulator of neuronal function.
By their highly reactive nature, ROS are damaging to the cell, oxidising proteins, lipids and DNA and are normally regarded as detrimental to cell function. Indeed, an overwhelming of the defences against ROS is termed oxidative stress and is commonly associated with cellular damage seen in neurodegenerative disorders, including Parkinson's 7 and Alzheimer's disease 8. ROS and the accumulation of ROS‐related damage are also associated with ageing; oxidised lipids, DNA damage and the accumulation of lipofuscin (the ‘aging pigment’, autofluorescent material found in endosomes consisting of oxidised lipids, proteins, transition metals and senescent mitochondria) 9. Increasing evolutionary complexity and concomitant demand for oxygen‐dependent energy production via reduction–oxidation (redox) reactions also produced a diversification in defence mechanisms against ROS. This is particularly notable in energy demanding tissues such as heart and liver. The nervous system is anomalous in this framework as nerve cells are very energy demanding yet at the same time inadequately equipped with antioxidant defence 10, 11. Interestingly, much of the ROS defence within the nervous system occurs in glia 12. It is becoming increasingly apparent, however, that evolution may have made a virtue out of a necessity and co‐opted ROS for cellular signalling mechanisms. For such a framework lowered antioxidant defence or ROS buffering in neurons would be permissive and necessary.
Regulation of ROS in the nervous system, the case for the defence
Studies of ROS in the nervous system have primarily focused on ROS as damaging agents and the defence against ROS. Protection against ROS is mediated by multilayered constitutive and adaptive forms of defence. In the brain, static defences against ROS generally are seen to be (a) constitutive and enzymatic as seen in the standing high concentrations of enzymes such as superoxide dismutases, catalases, thioredoxin reductases and glutathione peroxidases or (b) constitutive and nonenzymatic as mediated by defence molecules (termed ROS scavengers) present in the cell, via synthesis or diet, such as alpha‐tocopherol (vitamin E), ascorbic acid, ß‐carotene and tetrahydrobiopterin (BH4). BH4 is a molecule of particular interest. For example, BH4 is a highly sensitive scavenger of H2O2 and Hydroxyl ions, while also important for the synthesis of the neurotransmitters dopamine, serotonin and noradrenaline. This suggests a functional link between ROS abundance, buffered by BH4 levels, and neurotransmitter function (for review see 13). A second set of defence mechanisms is adaptive and mediated by transcription factors 14. Two pathways are prominent: (a) the NRF2/Keap system, predominant in glial cells 12 and (b) the Jun‐N‐terminal Kinase (JNK)/AP‐1 system, seen to be a major protective mechanism in neurons. Both promote the transcription of genes encoding antioxidant response proteins. For example, NRF2 promotes the expression of glutathione‐S‐transferases in glia 15 while in neurons AP‐1 activation upregulates sulfiredoxin 16. NRF2 activity in glia mediates neuronal protection in a nonautonomous manner, partly through the ensheathing nature of symbiotic glial–neuron interactions. Indeed, keeping NRF2 function low in neurons allows dendritic and synaptic development and their regulation via redox‐sensitive signalling pathways, such as JNK/AP‐1 and WNT 17.
Neuronal polarity
Most neuronal cell types are explicitly polarised, endowed with a major axonal neurite that mediates long‐range connectivity and is primarily presynaptic, dedicated to passing on information, while the somato‐dendritic compartment of the cell is composed of branched smaller diameter neurites that are largely postsynaptic. The establishment of neuronal polarity has until recently mostly been studied in vitro, using low‐density cultures of cortical and hippocampal neurons. Under such conditions neuronal polarity first manifests with the emergence of a primary neurite, which extends more rapidly than others and develops into the axon, while the other minor neurites adopt postsynaptic dendritic characteristics. Several signalling pathways, including TGF‐ß, growth factors (e.g. BDNF), LKB and PI3 kinases, have been implicated in bringing about and maintaining asymmetries of the cytoskeleton. Characteristically, axons contain microtubules whose plus ends face away from the cell body, while dendrites have microtubules of mixed (mammals) or opposite polarity (e.g. Drosophila and Caenorhabditis elegans; for reviews see 18, 19). Perhaps inspired by work from other systems that have associated NADPH oxidase‐generated ROS with the regulation of cell polarisation and growth, for example, Arabidopsis hair cell outgrowth 20 and the enforcement of apical dominance in Aspergillus hyphae 21, ROS have been investigated as signals regulating the polarisation of neurons. Indeed, in vitro studies suggest that ROS produced by NADPH oxidases were required alongside growth factors for the differentiation of neuronal characteristics by PC12 and SH‐SY5Y cells, such as axonal outgrowth 22, 23, 24, 25. In vivo, gene expression profiles show that subunits of the NOX2 NADPH oxidase complex are present at the right place and time in mouse and rat embryonic hippocampal neurons 26, 27. In the context of neuronal differentiation mediated by nerve growth factor (NGF), Neuregulin or Retinoic Acid ROS appear to be permissive, acting in parallel to these growth factors. As documented for several growth factors, ROS can enhance downstream kinase signalling by inhibition of phosphatases, whose active sites contain cysteines that are susceptible to oxidation 28. The activity of protein kinases is critical during cellular polarisation, meditating positive feedback loops and signal amplification. Multiple pathways converge onto PI3 kinase, which is enriched at the tip of the future axon as neurons adopt a polarised morphology 29, 30 (reviewed in 18). Interestingly, PI3 kinase signalling can be regulated by ROS, by redox‐mediated inhibition of the phosphatase and tensin homolog (PTEN), which antagonises the PI3 kinase product phosphoinositide‐3‐phosphate 31, 32.
Studies on embryonic hippocampal neurons and cerebellar granule cells in culture have underpinned the idea that ROS contribute to the establishment of neuronal polarity. As cerebellar granule cells differentiate during the first 3 days in vitro overall ROS levels increase and become specifically enriched at growth cones and other cytoskeletally dynamic sites. This is paralleled by a ROS‐dependent rise in Tau and MAP2 expression levels, which are representative of axonal and dendritic cytoskeletal specialisation 33. Underlining a requirement for ROS in these processes, neurons derived from NOX2 knockout mice showed reduced neurite length compared to control cells 33. Similarly, in embryonic hippocampal neurons pharmacological or genetic reduction in NADPH oxidase activity, e.g. by expression of the dominant negative DNp22phox regulatory subunit, led to delayed and reduced axon outgrowth 26. Conversely, overactivation of NADPH oxidase by overexpression of the accessory protein p47phox promoted axonal growth 34. These studies suggest that NOX2 activity could indeed contribute to neuronal polarisation and promote neurite outgrowth.
The mechanisms by which physiological levels of ROS support neurite outgrowth and axon specification appear to involve release of calcium from intracellular stores, a potent second messenger regulating cytoskeletal organisation and dynamics (reviewed in 35, 36). Short respiratory bursts of NADPH oxidase‐generated ROS promote calcium release by redox modification of ryanodine (RyRs) and inositol‐3‐phosphate receptors (IP3Rs). These in turn lead to increased expression of Rac1, an activator of the NOX2 complex, thus generating a positive feedback loop that can convert initially transient ROS bursts into sustained ROS activation and high levels of intracellular calcium 34; (Fig. (Fig.1).1). However, the extent to which ROS signalling promotes the establishment of neuronal polarity in vivo remains to be determined. Phenotypes of NADPH oxidase knockout and RNAi knockdown experiments in several experimental animal models suggest that removal of any one NADPH oxidase still allows the nervous systems to form and function remarkably well, at least to a level sufficient for survival under laboratory conditions 37, 38. This could indicate a degree of functional redundancy. It also suggests that ROS might have a modulatory role rather than being critically required for the establishment of neuronal polarity. This would not be entirely surprising, as during nervous system development neural progenitor cells and their progeny are invariably located within asymmetric environments where local enrichment of numerous cues, including cell adhesion and extracellular matrix proteins, can contribute to cellular polarisation 39, 40, 41 (for review see 18).
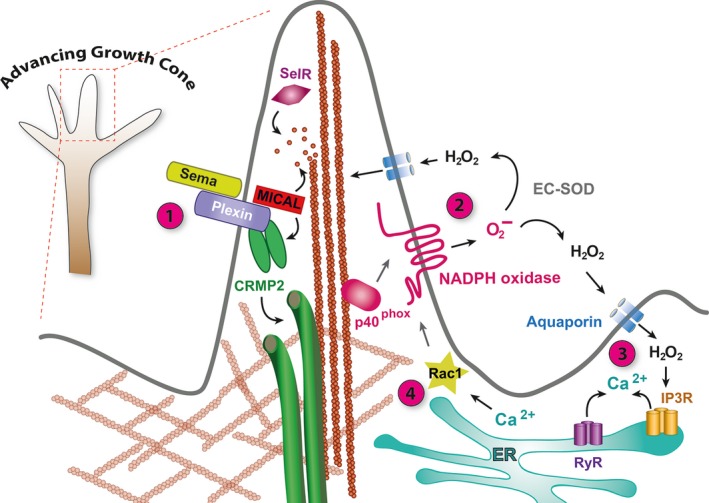
Regulation of the growth cone cytoskeleton by ROS. (1) Semaphorin binding to Plexin activates MICAL monooxygenase. MICAL interactions with F‐actin lead to oxidation of the conserved amino acid Met44 64. Oxidation of actin destabilises F‐actin filaments and promotes binding of the actin severing protein Profilin, thus promoting F‐actin disassembly 67. Oxidised actin monomers have a reduced propensity for polymerisation. MICAL redox activity is opposed by the MsrB enzyme SelR, which specifically reduces Met‐44‐R‐sulfoxide 66. MICAL activation via Semaphorin‐Plexin binding also generates H2O2 that can oxidise CRMP2, enabling it to form a disulfide‐linked homodimer and to transiently interact with Thioredoxin, which stimulates phosphorylation of CRMP2 by glycogen synthase kinase‐3, promoting CRMP2 modification of microtubules and growth cone collapse 71. (2) Superoxide produced by the NOX2 NADPH oxidase catalytic subunit (gp91phox) is regulated by translocation of the p40phox regulatory subunit from its association with F‐actin to the plasma membrane upon growth cone engagement with a substrate or guidance cue 58. Converted into hydrogen peroxide by extracellular superoxide dismutase (EC‐SOD), H2O2 enters the cytoplasm via aquaporins and can oxidise cytoskeletal proteins (e.g. F‐actin) 132, 133. (3) H2O2 also modifies RyRs and IP3Rs triggering release of calcium from internal stores in the ER. (4) Changes in intracellular calcium modify activities of cytoskeletal regulatory proteins, directly or indirectly, e.g. via the regulation of Calcium/calmodulin‐dependent kinase II (CamKII) or the phosphatase calcineurin or activation of the protease calpain. Elevated calcium levels also lead to expression of the cytoskeletal and NADPH oxidase regulator Rac1, thus generating a positive feedback loop that can amplify and sustain transient respiratory bursts 34.
Cytoskeletal modifications by ROS and growth cone pathfinding
Reactive oxygen species can regulate cytoskeletal change at multiple levels; directly via redox modification of structural cytoskeletal proteins and indirectly by modification of proteins or signalling pathways that regulate cytoskeletal dynamics. For example, actin and tubulin monomers contain multiple cysteine and methionine residues exposed to the cytoplasm that are subject to redox modifications, notably glutathionylation, nitrosylation and carbonylation 42, 43, 44, 45, 46, 47, 48, 49. Indeed, all major cytoskeletal elements and many cytoskeleton‐associated proteins are subject to direct redox modifications of some kind 50, 51, 52, with substantial fractions of actin, tubulin and neurofilaments found glutathionylated under normal physiological conditions 53. The question of which residues are modified, under what conditions and how this impacts on protein function and dynamics remains live. For alpha‐actin purified from rabbit muscles, in vitro and cell culture studies have shown Cys 374 sensitive to glutathionylation, leading to a reduced rate of actin polymerisation and altered actin dynamics 44, 54. Actin Cys 374 glutathionylation has been proposed to occur in response to growth factor and integrin‐stimulated signalling following interactions with the extracellular matrix 54. This actin modification is thought to regulate the disassembly of the actinomyosin complex during cell spreading 52. Along the same lines, disruption of protein deglutathionylation by mutation of glutaredoxin 1 (Grx1), the gene coding for an enzyme that catalyses actin deglutathionylation, led to reduced actin polymerisation and impaired polarisation, chemotaxis, adhesion, and phagocytosis by neutrophils. Conversely, blocking NOX activity led to increased formation of filamentous actin 55. Thus, ROS have increasingly been recognised as important regulators of actin dynamics.
One of the principal ROS sources in neurons is NAPDH oxidases. Their activity is highly regulated, making them prime candidates as regulators of growth cone cytoskeletal dynamics (for reviews see 56 and 57). How NADPH oxidase‐generated ROS regulate cytoskeletal dynamics in neuronal growth cones remains poorly understood. Pharmacological inhibition of NADPH oxidase activity or lowering cytosolic ROS levels led to reduced F‐actin content, retrograde flow and neurite outgrowth. Few studies have documented the localisation of NOX complex components in neuronal growth cones in vitro, none as yet in vivo 58, 59. In cultured Aplysia bag cell neurons the main enzymatic subunit of a NOX2‐type NADPH oxidase, NOX2/gp91phox, was seen localised to the plasma membrane, largely distinct from the regulatory subunit p40phox found associated with filopodial actin bundles (Fig. (Fig.1).1). Interestingly, a local stimulus of growth cone interaction with apCAM‐coated beads triggered colocalisation of both subunits to the site of growth cone–substrate interaction. Thus, in growth cones NADPH oxidase subunits are localised to the periphery at sites of actin assembly 58. Their activity is regulated by multiple pathways, including activation following translocation of regulatory subunits, such as p40phox or Rac1, but also by other signalling pathways, such as protein kinase C (PKC) 59. The extent to which local NAPDH oxidase activation regulates growth cone dynamics directly, via oxidation of actin and tubulin, or indirectly, through modulation of other signalling pathways, remains to be seen. Both are likely. A study using cultured marsupial kidney cells demonstrated that hydrogen peroxide‐induced chemotaxis and filopodial dynamics were in large part indirectly regulated by ROS via local extracellular signal–regulated kinase (ERK) pathway activation, which promoted actin retrograde flow by differential activation and recruitment of cofilin and the Arp2/3 nucleator at the leading edge 60. It is conceivable that ROS signalling in the nervous system might similarly be utilised for noncell autonomous communication, either during the development of synaptic connections or their subsequent adjustment. For example, in a mouse model for multiple sclerosis, persistent activation of NOX2/gp91phox in microglia leads to the impairment of synaptic plasticity in adjacent hippocampal neurons 61.
Thus far, the clearest evidence for post‐translational redox modification of cytoskeletal proteins directing growth cone pathfinding derives from studies of Semaphorin‐Plexin signalling. The cytoplasmic tail of Plexin interacts with the NADPH‐dependent monooxygenase, molecule interacting with CasL (MICAL), which is activated upon binding of Semaphorin guidance cues 62, 63, 64, 65, 66, 67 (for review see 68; Fig. 1). Elegant experiments by the Terman laboratory and collaborators demonstrated that the amino‐terminal NADPH‐dependent redox domain of MICAL‐1 binds F‐actin and directly oxidises the methionine residues Met44 and Met47 64, 65. These redox modifications are regulated and can be reversed by a methionine sulfoxide reductase, (MsrB/SelR), which catalyses the reduction of methionine sulfoxide to methionine 66. MICAL‐1‐mediated oxidation of actin weakens inter‐actin contacts while simultaneously increasing the binding affinity of the F‐actin severing protein cofilin by more than an order of magnitude. This synergistic effect of actin destabilisation by oxidation and concomitantly increased binding of cofilin promotes F‐actin disassembly. In addition, MICAL‐mediated oxidation decreases the capacity of actin for repolymerisation, thus further impacting on the dynamics of the actin cytoskeleton 67. Thus, Semaphorin‐Plexin guidance cue–receptor interactions are directly transduced to redox modification of the actin cytoskeleton, leading to F‐actin disassembly and altered reassembly dynamics.
In addition, MICAL activation also impacts on the microtubule cytoskeleton via another binding partner, collapsin response mediator protein 2 (CRMP2). CRMP2 is responsible for Semaphorin‐induced growth cone collapse 69 and interacts with tubulin heterodimers regulating microtubule dynamics 1 62, 70. Current data suggest that MICAL‐1 binds CRMP2, and that upon MICAL monooxygenase activation (e.g. by Semaphorin binding to Plexin), hydrogen peroxide is produced that oxidises CRMP2. Oxidation of CRMP2 promotes formation of disulfide‐linked CRMP2 homodimers, which interact with thioredoxin, which in turn promotes their phosphorylation by GSK‐3ß, microtubule disassembly and growth cone collapse 71; (Fig. (Fig.1).1). In summary, direct redox modifications of cytoskeletal elements, notably actin and tubulin, as well as of regulators of cytoskeletal dynamics, such as CRMP2, lie at the heart of Semaphorin‐Plexin growth cone guidance. It is conceivable that redox modification of cytoskeletal proteins extends to other guidance cue signalling pathways, either directly or in a modulatory capacity where the cellular redox state determines growth cone responses to extracellular cues.
Connectivity and structural plasticity
For decades ROS have been implicated in neurodegenerative conditions, largely thought of as destructive agents 72, 73. Increasingly ROS have also been viewed as regulators and modulators of signalling pathways and gene expression, of which many are known to regulate neuronal growth and plasticity (reviewed in 56, 57, 74). Several years ago we provided the first direct in vivo evidence of ROS as regulators of synaptic terminal growth, under pathological conditions in an experimental animal model (Drosophila) for lysosomal storage disease 75. The study demonstrated that oxidative stress resulting from lysosomal storage dysfunction led to activation of the JNK cascade and activation of the immediate early genes c‐Jun and c‐Fos (AP‐1) which in turn led to altered growth of neuromuscular junction terminals 75. Along with a prior study by Sanyal and colleagues 76, this work identified AP‐1 as the major adaptive response to ROS in neurons. The JNK/AP‐1 signalling pathway has long been known to be critical in many neuronal functions and is a well‐known mediator of synaptic and oxidative stress responses (reviewed in 74). AP‐1 is a heterodimer composed of the leucine‐zipper transcription factors Fos and Jun. Fos is one of the major immediate early transcription factors mediating long‐term synaptic changes during long‐term potentiation (LTP) 77, 78, although the actual mechanism inducing JNK phosphorylation and subsequent AP‐1 activation in this process remains obscure. Activation of JNK/AP‐1 by oxidative stress is thought to reinforce autophagy, and many genes encoding autophagy proteins are direct transcriptional targets of AP‐1 79. Some evidence suggests that activation of autophagy via the oxidative stress‐induced JNK/AP‐1 pathway can regulate synaptic terminal size and strength at the Drosophila larval neuromuscular synapse 75, 76, 80. The data point to the importance of the JNK/AP‐1 pathway as regulating synaptic function and to ROS as critical upstream signals during normal physiological conditions as well as under oxidative stress.
In a follow‐up study we since asked whether ROS also act as regulators of synaptic terminal growth and plasticity under normal physiological conditions, which until now has remained largely unexplored. Indeed, we found that ROS, in particular hydrogen peroxide, are necessary for activity‐induced synaptic terminal growth and are sufficient to drive synaptic terminal growth 81. Specifically, overactivation of motoneurons leads to increased mitochondrial ROS levels at the presynaptic neuromuscular junction of Drosophila larvae, previously also reported for cultured hippocampal neurons 82, 83. In Drosophila larvae, activity‐generated ROS promote altered synaptic terminal growth, generating more, albeit smaller synaptic varicosities (boutons) along with a reduction in the number of synaptic release sites. Postsynaptic dendrites similarly undergo homeostatic structural adjustments in response to activity‐generated ROS, leading to smaller dendritic arbours, which we previously showed equates to reduced synaptic input sites and reduced synaptic drive 84. The signalling and downstream effector pathways of neuronal activity‐generated ROS are only just being sketched out. We found that neuronal ROS signal via the conserved redox‐sensitive protein DJ‐1ß, a homologue of vertebrate DJ‐1 (PARK7) 85, 86, which appears to act as a neuronal redox sensor. Oxidation of DJ‐1ß increases inhibitory interactions with the phosphatase PTEN thus leading to disinhibition of PI3kinase signalling, a known regulator of synaptic terminal growth 87, 88, 89, 90. Whether changes in dendritic growth are also regulated by PTEN‐PI3kinase as a ROS effector pathway, and how synaptic terminal growth might be coupled to synaptic connectivity remain to be determined. Importantly, ROS are obligate signals for activity‐regulated structural plasticity of synaptic terminals, either instructive or permissive. Targeted abrogation of neuronal ROS signalling in Drosophila larval motoneurons, by expression of a modified form of the DJ‐1β redox sensor (the conserved Cysteine 106 mutated to a nonoxidisable Alanine 86, 91) prevented the locomotor network from adjusting homeostatically in response to increased levels of network activity, resulting in abnormal motor output 81.
Precisely how neuronal activation leads to the generation of ROS signals is not clear and is likely context specific. In general, ROS are formed as obligate by‐products of mitochondrial respiratory ATP synthesis, by ‘leakage’ of the electron transport chain 72. Thus, mitochondrial ROS could potentially provide neurons with a readout of their energetic demand. In addition, NMDA receptor stimulation has been shown to trigger ROS generation by either mitochondria 92, 93 or NADPH oxidases 94. NADPH oxidase activity is subject to complex regulatory pathways, of which many are associated with neuronal activation, such as elevated intracellular calcium levels, Protein kinases C and A, as well as calmodulin and calcium/calmodulin‐dependent kinase II (CamKII) 34, 56, 95, 96, 97, 98.
It has become increasingly evident that during normal nervous system development and function ROS act as second messengers that regulate multiple aspects, from neuronal polarity and axon growth cone behaviour to structural plasticity. As such ROS have more recently been investigated as potentially involved in the aetiology of psychiatric disorders with neurodevelopmental origins. For example, in mammalian brains cortical parvalbumin‐expressing inhibitory interneurons (PVIs) are critical to the excitation–inhibition balance and therefore function of many cortical networks. These fast‐spiking neurons are arguably highly susceptible to ROS generated by mitochondrial ATP metabolism and it is thought that their surrounding perineuronal nets confer protection of the PVIs to oxidative challenges 99. Imbalances in ROS metabolism and buffering are thought of as potentially impacting on the development of cortical networks during so‐called critical periods, leading to sub‐optimal network performance and neuropsychiatric disorders 100. Supporting this hypothesis are reports of post mortem brain tissue from patients with schizophrenia, bipolar or autism spectrum disorder exhibiting reductions in cortical PVIs 101, 102, 103. Moreover, a recent study did indeed find that conditions of elevated oxidative stress preceded and correlated with reduced integrity of PVIs in mouse models for these disorders 104.
Synaptic transmission and plasticity
Synaptic plasticity describes the ability of synapses to adjust their strength, connectivity and structure in response to previously experienced activity. The inherent plasticity of neurons is key to neuronal network development and in networks allows for adaptation, memory and learning. Synaptic strength may be enhanced or reduced depending upon the neuronal context and the nature of stimulation, the best‐studied examples being LTP and long‐term depression (LTD). LTP was originally described following repetitive stimulation of the perforant path fibres to the dentate area of the hippocampus 105. The high‐frequency stimulation (HFS) used for induction of LTP results in opening of NMDA receptors and thus elevated intracellular Ca2+. This leads to the adjustment of synaptic strength via direct and transcriptionally regulated modification of synaptic proteins, and changes in the composition of synaptic protein complexes (for review see 57, 106).
A role for ROS in synaptic plasticity has been demonstrated in various model systems and areas of the nervous system (Fig. 2). ROS production is elevated in hippocampal slice preparations following increased neuronal activity, NMDA receptor activation and subsequent LTP 92. In mouse hippocampus NMDA receptor activation triggers ROS generation through the NOX2 NADPH oxidase, regulated by PKC 94. Importantly, acute application of cell permeable superoxide scavengers can block HFS‐induced LTP in hippocampal slices. Dysregulation of ROS via transgenic mis‐expression of SOD1 or Catalase similarly blocked LTP, suggesting that LTP requires ROS and at the same time is sensitive to the cellular redox state 107, 108, 109, 110. Conversely, bath applied elevation of ROS in hippocampal slices can be sufficient to induce LTP in the CA1 region 111. ROS are also required and sufficient for the induction and maintenance of spinal cord LTP, contributing to central sensitisation and chronic neuropathic pain 112. Interestingly, in cerebellar Purkinje neurons superoxide is required for LTD, although in these cells synaptic depression (as opposed to potentiation) requires elevated intracellular calcium concentration 113.
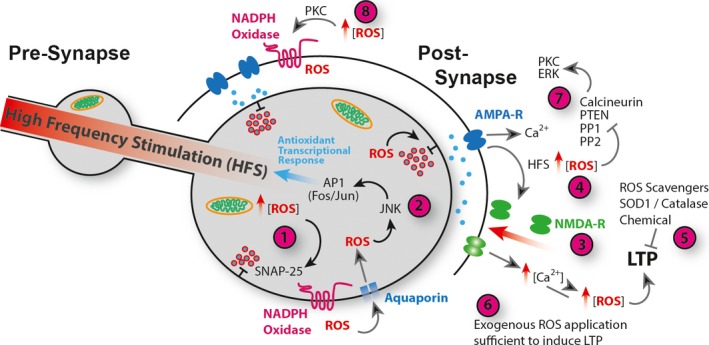
ROS regulation of Synaptic Plasticity. (1) Presynaptic ROS, derived from mitochondria or NADPH oxidase activity regulates vesicle release via oxidation of SNAP‐25 124. ROS regulate release probabilities with subsynaptic terminal resolution 122, 123. (2) Increases in ROS lead to activation of JNK and AP‐1, which promote expression of antioxidant encoding genes 14 and others required for autophagy 79. AP‐1 mediates neuronal adaptive responses to ROS 75, 76. (3) Postsynaptic LTP, in response to HFS, drives recruitment and opening of NMDA receptors and consequent elevation of intracellular Ca2+ concentration. HFS causes elevated ROS production and a shift towards an oxidative environment in the synaptic terminal (4) 83, 92. HFS‐induced LTP requires ROS (5) 108 and exogenous application of ROS (6) is sufficient to induce LTP in the absence of HFS 111. ROS regulate canonical synaptic plasticity pathways via direct oxidative modification, and inhibition of phosphatases PP1, PP2, PTEN and Calcineurin resulting in increased kinase signalling including ERK and PKC (7) 114, 115, 116, 117, 118, 119, 120, 121. Also, ROS‐activated PKC stimulates NADPH‐oxidase activation and exacerbated ROS production (8) 107, 111.
The specific protein targets of ROS for regulating synaptic plasticity and the interplay between Ca2+ and ROS‐regulated pathways are exciting and thriving research fields 57. In particular, the link between ROS and Ca2+‐regulated plasticity signalling pathways raises the possibility that synaptic redox state provides a permissive environment that, depending upon context, positively or negatively tunes neuronal plasticity in response to Ca2+ signalling. One of the recurrent challenges is to identify the mechanisms through which ROS act, whether these are direct redox modification of effector proteins, or whether ROS impact on cellular function indirectly by modulating other signalling pathways. For example, several protein phosphatases (PP), including PP1 and PP2, PTEN and calcineurin (PP 2B), are regulated by ROS, perhaps suggesting a general role for ROS as negative regulators of kinase cascade pathways 114, 115, 116, 117, 118, 119. In the capacity of phosphatase inhibitors ROS have modulatory access to pathways known to regulate synaptic plasticity. Indeed, in response to H2O2 application the phosphorylation state of ERK is upregulated in hippocampal slices, cortical neurons and PC12 cells 120, 121. Similarly, activation of PKC, which is required for hippocampal LTP, is triggered by ROS 111 (Fig. 2).
Direct regulation of synaptic function by redox modification of synaptic proteins also appears likely. For example, exposure of frog NMJs to H2O2 revealed their capability to directly regulate synaptic release probabilities. While synaptic strength remained unchanged, the authors observed altered quantal release synchronicity when comparing proximal vs. distal parts of the NMJ, suggesting a modulatory role for ROS or redox state with subsynaptic resolution 122, 123. In a follow‐up study the same group identified SNAP‐25, a component of the SNARE complex and regulator of synaptic vesicle fusion, as a direct ROS target, whose oxidation by H2O2 mediated downregulation of release synchronicity 124. Excitingly, a recent study demonstrated a role for targeted redox modifications in regulating presynaptic homeostatic adjustments of quantal content. Postsynaptic Semaphorin to presynaptic Plexin signalling activates the MICAL monooxygenase. This is known to specifically modify the actin cytoskeleton, which might impact on the presynaptic cytoskeleton in general and actin‐mediated vesicle tethering in particular 125 (Fig. 2).
In summary, current evidence suggests that synaptic plasticity is regulated by both direct and indirect modes of ROS action. It will almost certainly be determined by context, such as local redox state of pre‐ or postsynaptic sites, the nature (e.g. hydrogen peroxide vs. superoxide vs. nitric oxide), source and subcellular localisation of ROS (e.g. mitochondrial vs. plasma membrane localised NADPH oxidase vs. cytoskeleton associated monooygenase). In addition, different protein modifications might be subject to distinct ROS concentration thresholds, which could endow ROS signalling with a further degree of flexibility and complexity.
Conclusions
A large body of work has demonstrated the importance of ROS as signals that regulate numerous processes during neuronal development and nervous system function. With the majority of work to date having been carried out in cultured cells, it will be important to verify these by studying ROS signalling in vivo. While whole animal knockout mutants, for example, of NADPH oxidases, are informative, future work would ideally use cell‐specific mosaic manipulations with which to unambiguously determine cell type specificity of ROS requirements. One central aspect will be to determine how ROS generation is regulated, for example, the subcellular localisation of NADPH oxidases and other ROS generators. Previous studies have reported cue‐dependent changes in regulatory subunits as mechanisms for controlling ROS production 58, 126. Equally important for our understanding will be genetically encoded tools for visualising ROS and cellular redox potential, ideally with specificity for different ROS species 127, 128, 129, 130. Under normal physiological conditions ROS signals are expected to be confined in space and time. In the nervous system, in particular, there is a need for new reagents that will allow genetically targeted in vivo manipulation of ROS generators and scavengers with spatial and temporal control appropriate for studying neuronal cell behaviour and synaptic transmission. Following the success of optogenetic modulators of neuronal excitability, this might take the route for engineering optogenetic solutions for ROS generation and sequestration 131.
Acknowledgements
We apologise to all colleagues whose work we were unable to include due to space limitations. This work was supported by BBSRC studentship to NG and BBSRC research grants to ML (BB/IO1179X/1, BB/M002934/1) and to STS (BB/I012273/1, BB/M002322/1).
Contributor Information
Matthew C. W. Oswald, Email: ku.ca.mac@463om.
Sean T. Sweeney, Email: [email protected].
Matthias Landgraf, Email: ku.ca.mac@60001lm.
References
Full text links
Read article at publisher's site: https://doi.org/10.1002/1873-3468.12972
Read article for free, from open access legal sources, via Unpaywall:
https://febs.onlinelibrary.wiley.com/doi/pdfdirect/10.1002/1873-3468.12972
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/1873-3468.12972
Article citations
Photobiomodulation effects on neuronal transdifferentiation of immortalized adipose-derived mesenchymal stem cells.
Lasers Med Sci, 39(1):257, 11 Oct 2024
Cited by: 0 articles | PMID: 39390299 | PMCID: PMC11466999
Signaling Pathways Concerning Mitochondrial Dysfunction: Implications in Neurodegeneration and Possible Molecular Targets.
J Mol Neurosci, 74(4):101, 28 Oct 2024
Cited by: 0 articles | PMID: 39466510
Review
Ribosome heterogeneity in development and disease.
Front Cell Dev Biol, 12:1414269, 17 Jul 2024
Cited by: 0 articles | PMID: 39086661 | PMCID: PMC11288964
The relationship between hypoxia and Alzheimer's disease: an updated review.
Front Aging Neurosci, 16:1402774, 17 Jul 2024
Cited by: 0 articles | PMID: 39086755 | PMCID: PMC11288848
Review Free full text in Europe PMC
GPR50 regulates neuronal development as a mitophagy receptor.
Cell Death Dis, 15(8):591, 15 Aug 2024
Cited by: 0 articles | PMID: 39143050 | PMCID: PMC11324738
Go to all (92) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Bidirectional interactions between NOX2-type NADPH oxidase and the F-actin cytoskeleton in neuronal growth cones.
J Neurochem, 130(4):526-540, 25 Apr 2014
Cited by: 45 articles | PMID: 24702317 | PMCID: PMC4126878
The role of mitochondrial ROS in the aging brain.
FEBS Lett, 592(5):743-758, 15 Nov 2017
Cited by: 157 articles | PMID: 29106705
Review
Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders.
Neuromolecular Med, 2(2):215-231, 01 Jan 2002
Cited by: 123 articles | PMID: 12428812
Review
Oxygen radicals elicit paralysis and collapse of spinal cord neuron growth cones upon exposure to proinflammatory cytokines.
Biomed Res Int, 2014:191767, 23 Jun 2014
Cited by: 9 articles | PMID: 25050325 | PMCID: PMC4090484
Funding
Funders who supported this work.
Biotechnology and Biological Sciences Research Council (7)
Grant ID: 1312476
Grant ID: BB/M002322/1
Grant ID: BB/M002322/1
0 publications
Grant ID: BB/I012273/1
Grant ID: BB/IO1179X/1
Grant ID: BB/M002934/1
Grant ID: BB/I01179X/1

 1
1




