Abstract
Free full text

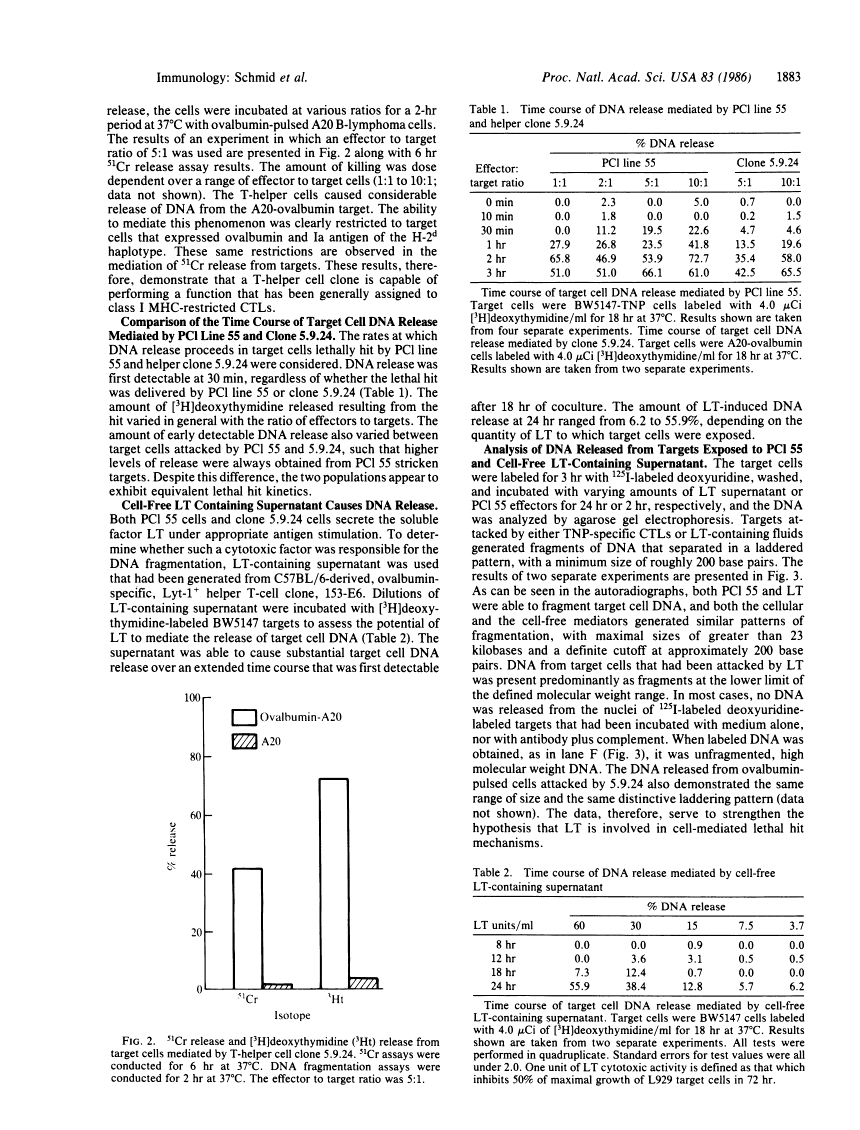
DNA fragmentation: manifestation of target cell destruction mediated by cytotoxic T-cell lines, lymphotoxin-secreting helper T-cell clones, and cell-free lymphotoxin-containing supernatant.
Abstract
A Lyt-2+, trinitrophenyl-specific, lymphotoxin-secreting, cytotoxic T-cell line, PCl 55, mediates the digestion of target cell DNA into discretely sized fragments. This phenomenon manifests itself within 30 min after effector cell encounter as measured by the release of 3H counts from target cells prelabeled with [3H]deoxythymidine and occurs even at very low effector to target cell ratios (0.25:1). A Lyt-1+, ovalbumin-specific, lymphotoxin-secreting T-helper cell clone, 5.9.24, is also able to mediate fragmentation of target cell DNA over a time course essentially indistinguishable from the cytotoxic T lymphocyte-mediated hit. Cell-free lymphotoxin-containing supernatants also cause release of DNA from targets, although they require a longer time course, on the order of 24 hr. In contrast, lysis of cells by antibody plus complement or Triton X-100 does not result in DNA release even after extended periods of incubation (24 hr). All three treatments that result in the release of DNA from cells cause fragmentation of that DNA into discretely sized pieces that are multiples of 200 base pairs. The results thus suggest that cytotoxic T cells, lymphotoxin-secreting helper clones with cytolytic activity, and lymphotoxin all effect target cell destruction by means of a similar mechanism and that observed differences in time course and the absence of target cell specificity in killing mediated by lymphotoxin may simply reflect differences in the mode of toxin delivery.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Schmid DS, Powell MB, Mahoney KA, Ruddle NH. A comparison of lysis mediated by Lyt 2+ TNP-specific cytotoxic-T-lymphocyte (CTL) lines with that mediated by rapidly internalized lymphotoxin-containing supernatant fluids: evidence for a role of soluble mediators in CTL-mediated killing. Cell Immunol. 1985 Jun;93(1):68–82. [Abstract] [Google Scholar]
- Russell JH, Masakowski VR, Dobos CB. Mechanisms of immune lysis. I. Physiological distinction between target cell death mediated by cytotoxic T lymphocytes and antibody plus complement. J Immunol. 1980 Mar;124(3):1100–1105. [Abstract] [Google Scholar]
- Russell JH, Dobos CB. Mechanisms of immune lysis. II. CTL-induced nuclear disintegration of the target begins within minutes of cell contact. J Immunol. 1980 Sep;125(3):1256–1261. [Abstract] [Google Scholar]
- Russell JH, Masakowski V, Rucinsky T, Phillips G. Mechanisms of immune lysis. III. Characterization of the nature and kinetics of the cytotoxic T lymphocyte-induced nuclear lesion in the target. J Immunol. 1982 May;128(5):2087–2094. [Abstract] [Google Scholar]
- Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [Abstract] [Google Scholar]
- Duke RC, Chervenak R, Cohen JJ. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. [Europe PMC free article] [Abstract] [Google Scholar]
- Dennert G, Podack ER. Cytolysis by H-2-specific T killer cells. Assembly of tubular complexes on target membranes. J Exp Med. 1983 May 1;157(5):1483–1495. [Europe PMC free article] [Abstract] [Google Scholar]
- Henkart PA, Millard PJ, Reynolds CW, Henkart MP. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984 Jul 1;160(1):75–93. [Europe PMC free article] [Abstract] [Google Scholar]
- Blumenthal R, Millard PJ, Henkart MP, Reynolds CW, Henkart PA. Liposomes as targets for granule cytolysin from cytotoxic large granular lymphocyte tumors. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5551–5555. [Europe PMC free article] [Abstract] [Google Scholar]
- Ruddle NH, Waksman BH. Cytotoxicity mediated by soluble antigen and lymphocytes in delayed hypersensitivity. 3. Analysis of mechanism. J Exp Med. 1968 Dec 1;128(6):1267–1279. [Europe PMC free article] [Abstract] [Google Scholar]
- Granger GA, Kolb WP. Lymphocyte in vitro cytotoxicity: mechanisms of immune and non-immune small lymphocyte mediated target L cell destruction. J Immunol. 1968 Jul;101(1):111–120. [Abstract] [Google Scholar]
- Russell SW, Rosenau W, Lee JC. Cytolysis induced by human lymphotoxin. Am J Pathol. 1972 Oct;69(1):103–118. [Europe PMC free article] [Abstract] [Google Scholar]
- Kramer SL, Granger GA. The role of lymphotoxin in target cell destruction induced by mitogen-activated human lymphocytes in vitro. II. The correlation of temperature and trypsin-sensitive phases of lymphotoxin-induced and lymphocyte-mediated cytotoxicity. J Immunol. 1976 Feb;116(2):562–567. [Abstract] [Google Scholar]
- Hessinger DA, Daynes RA, Granger GA. Binding of human lymphotoxin to target-cell membranes and its relation to cell-mediated cytodestruction. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3082–3086. [Europe PMC free article] [Abstract] [Google Scholar]
- Walker SM, Lucas ZJ. Role of soluble cytotoxins in cell-mediated immunity. Transplant Proc. 1973 Mar;5(1):137–140. [Abstract] [Google Scholar]
- Plaut M, Bubbers JE, Henney CS. Studies of the mechanism of lymphocyte-mediated cytolysis. VII. Two stages in the T cell-mediated lytic cycle with distinct cation requirements. J Immunol. 1976 Jan;116(1):150–155. [Abstract] [Google Scholar]
- Okamoto M, Mayer MM. Studies on the mechanism of action of guinea pig lymphotoxin. II. Increase of calcium uptake rate in LT-damaged target cells. J Immunol. 1978 Jan;120(1):279–285. [Abstract] [Google Scholar]
- Cerottini JC, Brunner KT. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. [Abstract] [Google Scholar]
- Zinkernagel RM, Doherty PC. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. [Abstract] [Google Scholar]
- Powell MB, Conta BS, Horowitz M, Ruddle NH. The differential inhibitory effect of lymphotoxin and immune interferon on normal and malignant lymphoid cells. Lymphokine Res. 1985 Winter;4(1):13–26. [Abstract] [Google Scholar]
- Conta BS, Powell MB, Ruddle NH. Activation of Lyt-1+ and Lyt-2+ T cell cloned lines: stimulation of proliferation, lymphokine production, and self-destruction. J Immunol. 1985 Apr;134(4):2185–2190. [Abstract] [Google Scholar]
- Okada CY, Rechsteiner M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell. 1982 May;29(1):33–41. [Abstract] [Google Scholar]
- Tite JP, Janeway CA., Jr Cloned helper T cells can kill B lymphoma cells in the presence of specific antigen: Ia restriction and cognate vs. noncognate interactions in cytolysis. Eur J Immunol. 1984 Oct;14(10):878–886. [Abstract] [Google Scholar]
- Tite JP, Powell MB, Ruddle NH. Protein-antigen specific Ia-restricted cytolytic T cells: analysis of frequency, target cell susceptibility, and mechanism of cytolysis. J Immunol. 1985 Jul;135(1):25–33. [Abstract] [Google Scholar]
- Männel DN, Moore RN, Mergenhagen SE. Macrophages as a source of tumoricidal activity (tumor-necrotizing factor). Infect Immun. 1980 Nov;30(2):523–530. [Europe PMC free article] [Abstract] [Google Scholar]
- Swain SL. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. [Abstract] [Google Scholar]
- Eardley DD, Shen FW, Gershon RK, Ruddle NH. Lymphotoxin production by subsets of T cells. J Immunol. 1980 Mar;124(3):1199–1202. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.83.6.1881
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc323188?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.83.6.1881
Article citations
Bispecific soluble cytokine receptor-nanobody fusions inhibit Interleukin (IL-)6 trans-signaling and IL-12/23 or tumor necrosis factor (TNF) signaling.
J Biol Chem, 299(11):105343, 13 Oct 2023
Cited by: 1 article | PMID: 37838173 | PMCID: PMC10652096
Lymphotoxin and TNF: how it all began-a tribute to the travelers.
Cytokine Growth Factor Rev, 25(2):83-89, 12 Feb 2014
Cited by: 49 articles | PMID: 24636534 | PMCID: PMC4027955
Review Free full text in Europe PMC
ELPylated anti-human TNF therapeutic single-domain antibodies for prevention of lethal septic shock.
Plant Biotechnol J, 9(1):22-31, 01 Jan 2011
Cited by: 49 articles | PMID: 20444206
Covalent dimerization of camelidae anti-human TNF-alpha single domain antibodies by the constant kappa light chain domain improves neutralizing activity.
Biotechnol Bioeng, 106(1):161-166, 01 May 2010
Cited by: 8 articles | PMID: 20047190
Transglutaminase-catalyzed covalent multimerization of Camelidae anti-human TNF single domain antibodies improves neutralizing activity.
J Biotechnol, 142(2):170-178, 14 Apr 2009
Cited by: 20 articles | PMID: 19439388
Go to all (75) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cytotoxic lymphokines produced by cloned human cytotoxic T lymphocytes. I. Cytotoxins produced by antigen-specific and natural killer-like CTL are dissimilar to classical lymphotoxins.
J Immunol, 135(6):4034-4043, 01 Dec 1985
Cited by: 15 articles | PMID: 2415596
A comparison of lysis mediated by Lyt 2+ TNP-specific cytotoxic-T-lymphocyte (CTL) lines with that mediated by rapidly internalized lymphotoxin-containing supernatant fluids: evidence for a role of soluble mediators in CTL-mediated killing.
Cell Immunol, 93(1):68-82, 01 Jun 1985
Cited by: 12 articles | PMID: 3873290
Immunoregulation by mouse T cell clones. III. Cloned H-Y-specific cytotoxic T cells secrete a soluble mediator(s) that inhibits cytotoxic responses by acting on both Lyt-2- and L3T4- lymphocytes.
Eur J Immunol, 15(8):773-783, 01 Aug 1985
Cited by: 3 articles | PMID: 2411568
Cellular mechanisms of lymphocyte-mediated lysis of tumor cells.
Ann Ist Super Sanita, 26(3-4):369-384, 01 Jan 1990
Cited by: 6 articles | PMID: 2151107
Review
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: CA 32447
Grant ID: CA 16885
Grant ID: P01 CA 29606