Abstract
Introduction
Multiple studies now confirm that ∼40% of patients with fibromyalgia syndrome meet diagnostic criteria for small-fiber polyneuropathy (SFPN) and have objective pathologic or physiologic evidence of SFPN, whereas 60% do not. Given possibilities that tens or hundreds of millions globally could have SFPN, developing screening tools becomes important.Objectives
This analysis explored whether specific symptoms might help distinguish these fibromyalgia endophenotypes.Methods
With institutional review board approval, all adults tested for SFPN by distal-leg skin biopsy or autonomic function testing at Massachusetts General Hospital in 2014 to 2015 were queried about symptoms. Inclusion required a physician's fibromyalgia syndrome diagnosis plus meeting the American College of Rheumatology 2010 Fibromyalgia Criteria. The primary outcome was the validated Small-fiber Symptom Survey, which captures severity of all known SFPN-associated symptoms. The Composite Autonomic Symptom Score-31, Short-Form Health Survey-36, and Short-Form McGill Pain Questionnaires provided secondary outcomes.Results
Among the 39 participants, 14 had test-confirmed SFPN (SFPN+) and 25 did not (SFPN-). Their pain severity did not differ. Paresthesias ("tingling") were different (worse) in the SFPN+ group (3.14 ± 0.9 vs 2.28 ± 1.1; P = 0.16). Their component subscore for dysautonomia symptoms was also worse (10.42 ± 4.0 vs 7.16 ± 4.0; P = 0.019). Receiver operating characteristic analyses revealed that each item had fair diagnostic utility in predicting SFPN, with areas under the curve of 0.729. No secondary questionnaires discriminated significantly.Conclusion
Among patients with fibromyalgia, most symptoms overlap between those with or without confirmed SFPN. Symptoms of dysautonomia and paresthesias may help predict underlying SFPN. The reason to screen for SFPN is because-unlike fibromyalgia-its medical causes can sometimes be identified and definitively treated or cured.Free full text

Specific symptoms may discriminate between fibromyalgia patients with vs without objective test evidence of small-fiber polyneuropathy
Abstract
Introduction:
Multiple studies now confirm that ~40% of patients with fibromyalgia syndrome meet diagnostic criteria for small-fiber polyneuropathy (SFPN) and have objective pathologic or physiologic evidence of SFPN, whereas 60% do not. Given possibilities that tens or hundreds of millions globally could have SFPN, developing screening tools becomes important.
Objectives:
This analysis explored whether specific symptoms might help distinguish these fibromyalgia endophenotypes.
Methods:
With institutional review board approval, all adults tested for SFPN by distal-leg skin biopsy or autonomic function testing at Massachusetts General Hospital in 2014 to 2015 were queried about symptoms. Inclusion required a physician's fibromyalgia syndrome diagnosis plus meeting the American College of Rheumatology 2010 Fibromyalgia Criteria. The primary outcome was the validated Small-fiber Symptom Survey, which captures severity of all known SFPN-associated symptoms. The Composite Autonomic Symptom Score-31, Short-Form Health Survey-36, and Short-Form McGill Pain Questionnaires provided secondary outcomes.
Results:
Among the 39 participants, 14 had test-confirmed SFPN (SFPN+) and 25 did not (SFPN−). Their pain severity did not differ. Paresthesias (“tingling”) were different (worse) in the SFPN+ group (3.14 ± 0.9 vs 2.28 ± 1.1; P = 0.16). Their component subscore for dysautonomia symptoms was also worse (10.42 ± 4.0 vs 7.16 ± 4.0; P = 0.019). Receiver operating characteristic analyses revealed that each item had fair diagnostic utility in predicting SFPN, with areas under the curve of 0.729. No secondary questionnaires discriminated significantly.
Conclusion:
Among patients with fibromyalgia, most symptoms overlap between those with or without confirmed SFPN. Symptoms of dysautonomia and paresthesias may help predict underlying SFPN. The reason to screen for SFPN is because—unlike fibromyalgia—its medical causes can sometimes be identified and definitively treated or cured.
1. Introduction
Fibromyalgia syndrome (FMS) refers to a constellation of unexplained body-wide and multi-organ symptoms. The most common are widespread chronic pain, fatigue, exercise intolerance, gastrointestinal symptoms, and cognitive concerns. It is surprisingly common, with 1% to 5% prevalence in western countries.11,26 Fibromyalgia had no known biomedical causes until multiple laboratory results recently reported evidence consistent with small-fiber polyneuropathy (SFPN) in almost half of patients.1,12,19,24
Small-fiber polyneuropathy is a biologically plausible explanation for the FMS symptom complex. The small unmyelinated autonomic and C-fibers and thinly myelinated A-delta neurons mediate the nocifensive sensations, regulate many organs and tissues, and participate in inflammation and injury responses to help protect the body from external and internal dangers.13 The multifunctionality of small fibers explains why SFPN often causes multiple symptoms. The spontaneously firing nociceptive C-fibers identified in both fibromyalgia and SFPN19 appear to cause the widespread chronic pain characteristic of both illnesses. Other symptoms come from impaired small-fiber control of the circulation. Dysregulated blood flow within skeletal muscles (neurogenic myovasculopathy), also identified in both conditions,1,4 is the best current explanation for the chronic fatigue and exercise intolerance characteristic of them both. Small-fiber polyneuropathy is also increasingly appreciated to affect the central nervous system, a phenomenon often termed “brain fog.” This happens directly because the central axons of peripheral nervous system sensory fibers penetrate into the spinal cord and some ascend to the brain3,17 and through postsynaptic and network effects that can be radiologically imaged.8 Neurogenic vasculopathy can further impair cognitive function, as can tertiary effects including depression, reduced exercise, and poor sleep.15,18
Patients with fibromyalgia can be helped by detecting any underlying SFPN because it is a neurological disorder with identified pathobiology for which there are generally accepted, consensus-endorsed objective pathological and physiological tests.6,10 When these confirm the presence of SFPN, it can reduce concerns about psychosomatic causality, guide medical care, and improve insurer reimbursement. Treatments that raise blood pressure and improve organ perfusion can improve previously intractable symptoms including exercise intolerance and chronic fatigue.16 Most importantly, some of the medical causes of SFPN, eg, diabetes, can be tested for and sometimes definitively treated.9 Since small-fiber axons grow continuously throughout life, they can reinnervate their targets to restore function when neurotoxic conditions improve. However, with FMS so prevalent, it is impossible to test all patients with diagnostic tests for SFPN. More practical screening tools could find wide application.
The symptoms of fibromyalgia and SFPN overlap more than appreciated. Survey of 85 patients with objectively confirmed SFPN identified their most severe symptoms as “Tiredness (fatigue),” “Reduced endurance or strength for activities,” “Deep pains or aches,” “Tingling or Pins and needles,” and “Difficulty thinking, concentrating, or remembering.”23 Our group at the Massachusetts General Hospital (MGH) has been developing the patient-reported MGH Small-fiber Symptom Survey (MGH-SSS) to capture all common symptoms. It performed satisfactorily in initial validation, with good internal consistency, excellent test–retest reliability, and good-to-fair convergent validity.23 Here, we explored ability of the MGH-SSS to differentiate between patients with FMS with vs without objective evidence of SFPN. To the best of our knowledge, this is the first such attempt.
2. Methods
2.1. Source of subjects and allocation into study groups
In this retrospective project, data collected from participants in the recent validation study of the MGH-SSS were reanalyzed.23 These were patients who had been referred for multisymptom illnesses at the MGH, a tertiary-care university hospital that serves primarily the north-eastern United States. Eligibility required age 18 years and older, having fibromyalgia (defined below), English fluency, and having undergone at least one of the consensus-recommended objective diagnostic tests for SFPN: (1) a distal-leg, PGP9.5-immunolabeled skin biopsy, which requires density of epidermal nerve fibers ≤fifth centile of predicted and (2) diagnostic composite Autonomic Function Testing (AFT). This includes 4 domains: heart rate variability during deep breathing, heart and blood-pressure responses to Valsalva maneuver and tilt, and quantitative sudomotor axon reflex testing. The standard for AFT confirmation of the SFPN diagnosis is ≥2/4 domains with results outside the recommended reference range.12 These tests were conducted in MGH's accredited clinical diagnostic laboratories in 2014 to 2015. Allocation as SFPN+ required having at least one of the clinical test reports interpreted as diagnostic for SFPN. Participants were recruited using an invitation letter followed by a phone call, and all provided informed consent to a protocol approved by the hospital's institutional review board. Patients who completed the study received $15 compensation.
2.2. Fibromyalgia status
Inclusion into this study required having both a clinical diagnosis of FMS plus meeting the modified American College of Rheumatology (ACR) 2010 Fibromyalgia diagnostic criteria.27 The presence of a medical diagnosis of FMS was established by screening patients' paper and electronic medical records for this diagnosis. The ACR criteria are based on responses to the patient-reported fibromyalgia symptom severity (FSS) questionnaire and quantified with the Widespread Pain Index (WPI) and Symptom Severity (SS) scores (range 0–12).27 For the WPI, patients report areas that were painful during the last week. There are 19 areas (eg, left upper arm, right hip (buttock and trochanter), and left lower leg), thus WPI scores range between 0 and 19. For the SS, subjects rate the severity of 3 symptoms during the past week: fatigue, waking unrefreshed, and cognitive symptoms. All are rated from 0 (no problem) to 3 (severe: pervasive, continuous, and life-disturbing problems). The severity of somatic symptom is rated on a scale from 0 (no symptoms) to 3 (a great deal of symptoms). The FSS final score, which sums of the severity of the 3 symptoms plus the general severity of somatic symptoms, ranges between 0 and 12. To meet the diagnostic criteria for FMS, respondents must meet these 3 criteria: (1) WPI ≥7 and SS ≥5 or WPI 3-6 and SS ≥9, (2) symptoms present at a similar level for at least 3 months, and (3) no other disorder that would otherwise explain the pain.
2.3. Outcome measures
All qualifying participants completed the MGH-SSS, a 33-item survey designed to capture symptoms of SFPN of any or unknown cause.23 They reported the presence and severity of 32 potential symptoms using a 0 to 4 scale ranging between “0” = not at all to “4” = very much (0 not at all, 1 a little bit, 2 somewhat, 3 quite a bit, and 4 very much). The instructions were “Rate how much you have been affected by each symptom below in the last week.” The 33rd item is the 0 to 10 numerical pain rating scale asking respondents to rate “Intensity of your chronic widespread pain (on both sides of your body) at its worst during the last week.” The MGH-SSS generates a total score, and subscores for the 5 statistically driven distinct components derived from exploratory factor analysis.23 Component 1 was mainly gastrointestinal and component 2 mainly somatosensory symptoms. Component 3 contained miscellaneous symptoms without evident link. Component 4 comprised largely vascular symptoms, and component 5 contained all the urological symptoms.
To provide secondary outcomes, all participants also completed the Composite Autonomic Symptom Score-31, a validated survey of autonomic symptoms of neuropathy,20,22 the well-established Short-Form McGill Pain Questionnaire-2,5 and the Medical Outcomes Study Short-Form Health Survey (SF-36), a broadly used screen of overall patient-reported health.25
2.4. Data capture and analysis
All data were captured using the Research Electronic Data Capture System (REDCap), a secure web-based application for capturing medical data.7 Analyses were conducted with SPSS for Windows version 23 (Chicago, IL). Independent t tests were used to assess differences in measurements between the 2 study groups, and the chi-square statistic was used to assess differences in proportions. Receiver operating characteristic analysis was performed to assess the predictive value of symptoms in prediction of SFPN status. P values ≥0.05 were considered significant. Data were presented as mean ± SD. No corrections for multiple comparisons were applied.
3. Results
Among the original cohort of 159 patients,23 52 (33%) met the 2010 ACR FSS-based research criteria for FMS, and the medical records of 42 corroborated the diagnosis. Among them, 3 had medical diagnosis of FMS but did not meet the modified ACR 2010 Fibromyalgia diagnostic criteria. Thus, 39 patients with FMS meet inclusion criteria for this study. The results of skin biopsy and/or AFT confirmed the presence of SFPN in 14 patients with FMS (SFPN+), whereas in 25, they did not (SFPN−). There were no demographic differences (age, sex, and race) between the 2 groups.
Table Table11 reports the severity scores for each MGH-SSS–captured symptom in the entire cohort and stratified by study group. Among all participants, as well as in each of the study groups, the most severe symptoms were deep pains or aches, tiredness (fatigue), and reduced endurance or strength for activities. Among the individual MGH-SSS items, the presence of paresthesias (ie, tingling or “pins and needles”) was the only 1 with significant differential representation. It was more severe in the SFPN+ group (3.14 ± 0.9) than in the SFPN− group (2.28 ± 1.1; P = 0.16).
Table 1
Symptom severity scores from the MGH Small-Fiber Symptom Survey (MGH-SSS).
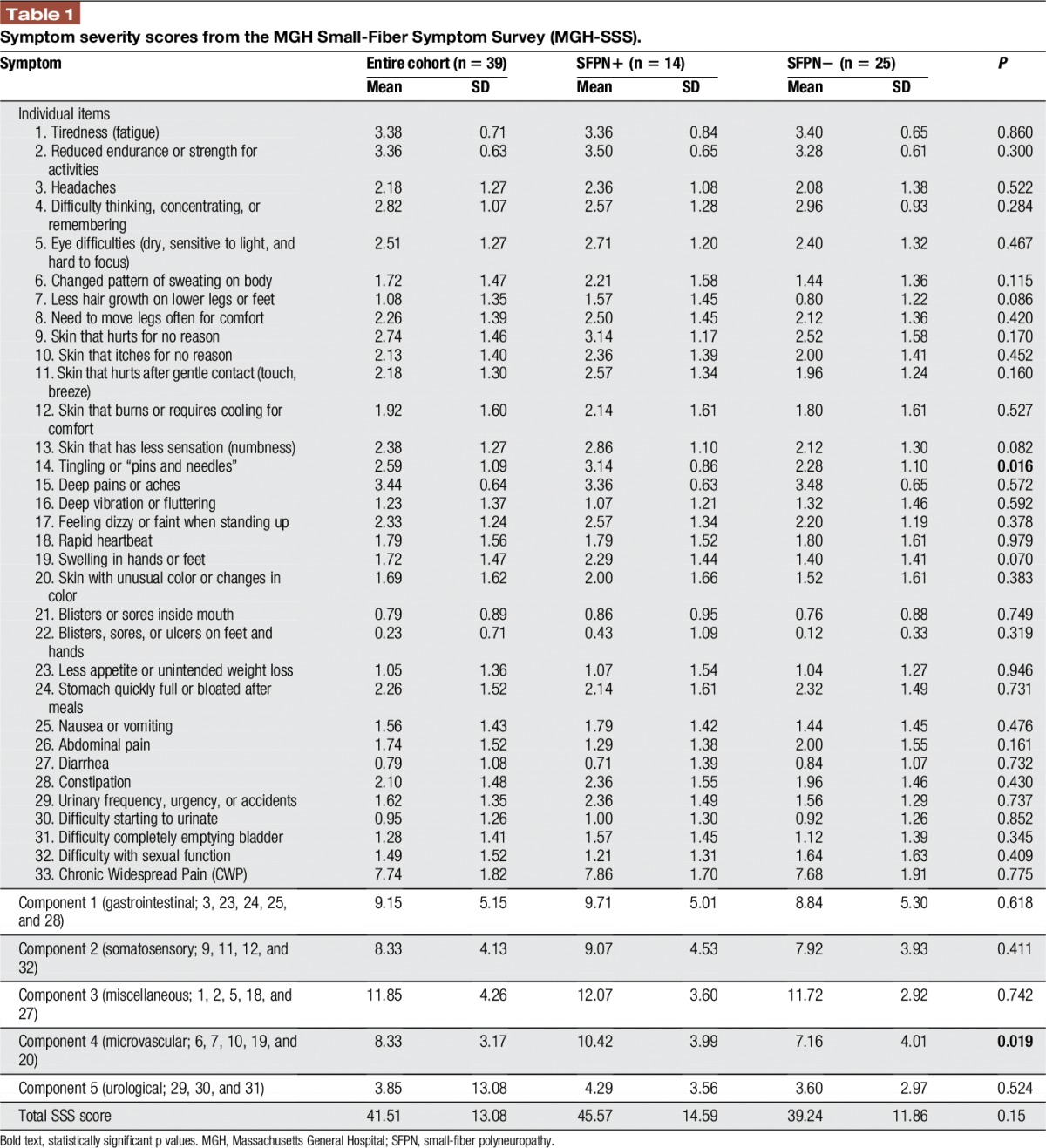
We then analyzed SSS symptoms grouped into the subscores generated by principal component analysis.23 The subscore of component number 4 was significantly higher in the SFPN+ (10.4 ± 4.0) than in the SFPN− groups (7.2 ± 4.0; P = 0.019). Component 4 clusters the symptoms: “Skin with unusual color or changes in color,” ”Less hair growth on lower legs or feet,” “Changed pattern of sweating on body,” “Swelling in hands or feet,” and “Skin that itches for no reason.” Diagnostic potential of the item “Tingling” and of component 4 subscore in predicting SFPN status was then evaluated by receiver operating characteristic analysis. As shown in Figure Figure1,1, each of these had an area under the curve of 0.729 (Fig. (Fig.1).1). Regarding the numeric pain rating score captured by the MGA-SSS, the entire cohort reported mean pain severity 7.74 ± 1.8, and there were no significant differences between the groups (P = 0.775, mean pain severity of 7.86 ± 1.7 in SFPN+ and 7.68 ± 1.9 in SFPN−).
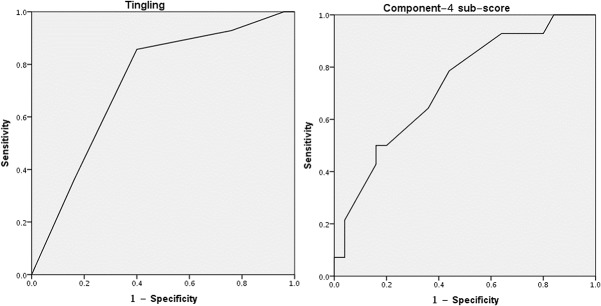
Receiver operating characteristic (ROC) plot for detecting SFPN status by component 4 subscore and by the item “Tingling.” SFPN, small-fiber polyneuropathy.
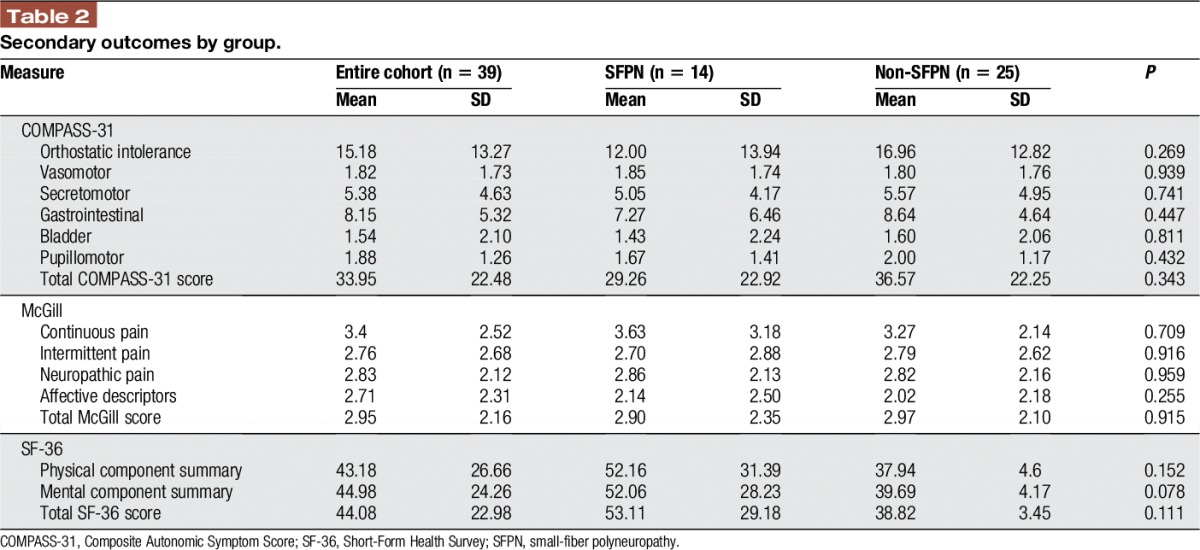
The total and subscores of secondary outcome measures are summarized in Table Table2.2. There were no differences in scores on the Composite Autonomic Symptom Score-31, McGill pain questionnaire, and the SF-36 between the 2 groups. The only trend (P = 0.078) was in the SF-36 mental component summary, where the SFPN+ group (52.06 ± 28.2) had higher scores than the SFPN− group (39.69 ± 4.2).
Table 2
Secondary outcomes by group.
4. Discussion
This is the first exploration of whether patient-reported symptoms might help screen patients with fibromyalgia for the presence or absence of SFPN, as defined by standard clinical interpretations of the consensus-recommended objective clinical diagnostic tests. This investigation used the MGH-SSS questionnaire, which quantitates the presence and severity of the most prevalent patient-reported symptoms of SFPN, regardless of their cause. The major findings are that 1 symptom (tingling) and 1 subscore (component 4, which sum the scores of vascular symptoms) demonstrated fair predictive value in predicting SFPN status in patients with fibromyalgia. Thus, screening for these particular symptoms might help identify those patients with fibromyalgia most likely to have biomarker evidence of SFPN.
Among individual symptoms, “Tingling or pins and needles” was more severe in the SFPN+ group. This is biologically plausible because paresthesias present in half of patients with SFPN2 are caused by spontaneous activity in myelinated cutaneous afferents.14,21 They are fairly specific for nerve involvement. Among the 5 MGH-SSS symptom clusters, component 4, which mostly reflects dysautonomic symptoms, was also more severe in the SFPN+ group.
The study's major limitation is recruitment bias. This was a single-hospital study, plus the comparator population was patients in whom their physicians had enough concern for the possibility of SFPN that they referred their patients for testing. The fact that these were a subset of patients originally recruited to validate the MGH-SSS adds additional likelihood of studying patients with fibromyalgia particularly interested in SFPN. Thus some “control” patients may also have SFPN. Although these biases reduce the chance of detecting real differences, the major initial group to target for screening is symptomatic patients, not asymptomatic controls in the community. Subsequent studies should include patients with fibromyalgia in whom neither physicians nor patients nor have concerns about possible SFPN.
Another limitation is that the study's small size only provided sufficient power to detect very large effects. For instance, it could not assess whether component 4 scores correlated with abnormal AFT results, which should be further explored. Although larger studies might have identified smaller significant differences, only large effects can be developed into screening tools, so they should remain the major focus. Furthermore, the fact that participants' diagnostic testing was nondiagnostic for SFPN does not entirely preclude this diagnosis but rather makes it far less likely. Because there is no consensus case definition of SFPN yet, the negative predictive value of these tests can not be measured. For instance, patients using high salt and hydration to treat orthostatic hypotension can test false normal on the tilt table. Regarding skin biopsies, patients with nonlength dependent neuropathies or those with patchy damage can have normal neurite densities in 1 small biopsy from a single location. Last, given that no correction for multiple comparisons was performed, our findings should be regarded as hypothesis that requires further testing.
However, the need for a screening questionnaire is greatest in patients in the population studied here, patients with fibromyalgia in whom there is clinical concern about the possibility of SFPN, and 1 goal of screening would be to predict who might benefit most from undertaking the actual skin biopsy and/or AFT. A semantic limitation also deserves mention. The current ACR diagnostic criteria for FMS state that the diagnosis of FMS can only be made if the subject does not have a disorder that would otherwise explain the symptoms. Based on this definition, patients with FMS with subsequent diagnoses of SFPN (the SFPN+ group in our study) are instantly no longer considered to have FMS, per definition. This does not correspond to real-world medical practice.
To conclude, among patients with diagnoses of fibromyalgia, most symptoms are present in similar severity between patients who do or do not have confirmed SFPN further evidence that these are overlapping populations. A few specific symptoms, namely the presence and severity of paresthesias and aggregate symptoms of peripheral autonomic dysfunction, might have predictive utility for screening among symptomatic patients. Further efforts are indicated.
Disclosures
The authors have no conflicts of interest to declare.
This work was supported in part by research grants from the National Institutes of Health (R01-NS093653 and UL1 TR001102), the U.S. Department of Defense (GW140169), and Lundbeckfonden Pre-Graduate Scholarship in Neurology.
Acknowledgments
The authors thank Heather M. Downs, BS and Kate O'Neill, BA for clinical research coordination.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
Articles from Pain Reports are provided here courtesy of Wolters Kluwer Health
Citations & impact
Impact metrics
Article citations
Small Fiber Neuropathy in Veterans With Gulf War Illness.
Fed Pract, 41(5):136-141, 15 May 2024
Cited by: 0 articles | PMID: 39398967 | PMCID: PMC11468626
Small fibre pathology, small fibre symptoms and pain in fibromyalgia syndrome.
Sci Rep, 14(1):3947, 16 Feb 2024
Cited by: 3 articles | PMID: 38365860 | PMCID: PMC10873371
Distinguishing fibromyalgia syndrome from small fiber neuropathy: a clinical guide.
Pain Rep, 9(1):e1136, 24 Jan 2024
Cited by: 1 article | PMID: 38283649 | PMCID: PMC10811691
The diagnostic assessment of fibromyalgia and the role of small fiber pathology.
Neurol Sci, 45(4):1789-1790, 22 Nov 2023
Cited by: 1 article | PMID: 37991641
Clinical criteria and diagnostic assessment of fibromyalgia: position statement of the Italian Society of Neurology-Neuropathic Pain Study Group.
Neurol Sci, 44(7):2561-2574, 24 May 2023
Cited by: 4 articles | PMID: 37222872 | PMCID: PMC10257633
Go to all (15) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia.
Pain, 154(11):2310-2316, 05 Jun 2013
Cited by: 160 articles | PMID: 23748113 | PMCID: PMC3845002
Initial Development and Validation of a Patient-Reported Symptom Survey for Small-Fiber Polyneuropathy.
J Pain, 18(5):556-563, 04 Jan 2017
Cited by: 15 articles | PMID: 28063957 | PMCID: PMC5947871
Validation of the composite autonomic symptom scale 31 (COMPASS-31) in patients with and without small fiber polyneuropathy.
Eur J Neurol, 22(7):1124-1130, 23 Apr 2015
Cited by: 53 articles | PMID: 25907824 | PMCID: PMC4464987
Small fiber polyneuropathy as a potential therapeutic target in interstitial cystitis/bladder pain syndrome.
Int Urogynecol J, 30(11):1817-1820, 25 Jun 2019
Cited by: 4 articles | PMID: 31240362 | PMCID: PMC7821096
Review Free full text in Europe PMC




