Abstract
Free full text

American Association of Physicists in Medicine Task Group 263: Standardizing Nomenclatures in Radiation Oncology
Abstract
A substantial barrier to the single- and multi-institutional aggregation of data to supporting clinical trials, practice quality improvement efforts, and development of big data analytics resource systems is the lack of standardized nomenclatures for expressing dosimetric data. To address this issue, the American Association of Physicists in Medicine (AAPM) Task Group 263 was charged with providing nomenclature guidelines and values in radiation oncology for use in clinical trials, data-pooling initiatives, population-based studies, and routine clinical care by standardizing: (1) structure names across image processing and treatment planning system platforms; (2) nomenclature for dosimetric data (eg, dose–volume histogram [DVH]-based metrics); (3) templates for clinical trial groups and users of an initial subset of software platforms to facilitate adoption of the standards; (4) formalism for nomenclature schema, which can accommodate the addition of other structures defined in the future. A multisociety, multidisciplinary, multinational group of 57 members representing stake holders ranging from large academic centers to community clinics and vendors was assembled, including physicists, physicians, dosimetrists, and vendors. The stakeholder groups represented in the membership included the AAPM, American Society for Radiation Oncology (ASTRO), NRG Oncology, European Society for Radiation Oncology (ESTRO), Radiation Therapy Oncology Group (RTOG), Children’s Oncology Group (COG), Integrating Healthcare Enterprise in Radiation Oncology (IHE-RO), and Digital Imaging and Communications in Medicine working group (DICOM WG); A nomenclature system for target and organ at risk volumes and DVH nomenclature was developed and piloted to demonstrate viability across a range of clinics and within the framework of clinical trials. The final report was approved by AAPM in October 2017. The approval process included review by 8 AAPM committees, with additional review by ASTRO, European Society for Radiation Oncology (ESTRO), and American Association of Medical Dosimetrists (AAMD). This Executive Summary of the report highlights the key recommendations for clinical practice, research, and trials.
Introduction
The radiation oncology community can benefit from standardized nomenclatures applied to targets, normal tissue structures, and treatment planning concepts and metrics. Such conformity will enhance the safety and quality efforts within and between clinics for routine ongoing practice and enable data pooling for outcomes research, registries, and clinical trials. Standardization is a vital precursor to the development of scalable uses of scripting for quality assurance (QA) and treatment plan evaluation (1–3). Increased clarity and consistency through standardizing nomenclatures in these areas would provide broad benefits.
Much has been learned from the groups that have instituted standardized nomenclatures for structures and DVH metrics to facilitate development of outcomes databases, automated analysis of DVH metrics, and interinstitutional data exchanges (1, 4–7). Although some standards for structures have been reported, no single standard has been generally endorsed with multi-institutional and multivendor consensus (4, 5). In addition, the standards that exist have generally not been comprehensive (eg, providing subsets but not the full set of dose–volume metrics, vendor system constraints, generalizability, or radiobiological factors).
The AAPM formed TG-263 to develop a consensus position on nomenclature for use in clinical trials, data-pooling initiatives, population-based studies, and routine clinical care by standardizing:
Structure names across image processing and treatment planning system platforms
Nomenclature for dosimetric data (eg, dose–volume histogram [DVH]-based metrics)
Templates for clinical trial groups and users of an initial subset of software platforms to facilitate adoption of the standards
Formalism for nomenclature schema that can accommodate the addition of other structures defined in the future
The full presentation of the TG report is available at the AAPM web site (available at https://www.aapm.org/pubs/reports/RPT_263.pdf). The present executive summary highlights key points from the report. Regarding the daily clinical operations to treat patients and participate in clinical trials, the practical development of the nomenclature required extensive collaboration with a wide range of members of treatment teams and among members of the American Society for Radiation Oncology (ASTRO), European Society for Radiotherapy and Oncology (ESTRO), American Association of Medical Dosimetrists (AAPM) and other professional societies. The executive summary, presented here in the Red Journal, emphasizes this close and very fruitful collaboration.
Methods and Materials
The TG was composed of a diverse international group of 57 stake holders, including hospital-based physicists (n = 33) and physicians (n = 15), vendor representatives (n = 8), and dosimetrists (n = 1). The TG includes AAPM members (n = 39) and ASTRO members (n = 41), large academic centers (n = 16), community clinics (n = 6), vendors (n = 5), and leaders from NRG (n = 3), IHE-RO (n = 2), and the DICOM (Digital Imaging and Communications in Medicine) Working Group-7 (n = 2). Many TG members were also members of clinical trial groups, including the NRG, Radiation Therapy Oncology Group, Children’s Oncology Group, and Imaging and Radiation Oncology Core and had been involved in creating standardization templates within those groups. The group expanded from the original 20 members as the deliberations became more clearly defined and an enhanced perspective on particular topics was needed (eg, physician input on target naming, vendor input on technical constraints).
Monthly telephone conversations, coupled with iterations on the documentation, were used to address specific issues. When the TG judged the recommendations to be sufficiently well formed, a subset of members volunteered to pilot the nomenclature in their clinics for, at minimum, head and neck patients. The nomenclature was further refined in iterative discussions of the TG from the pilot experience.
The TG wrote its initial draft of the report, which then proceeded through a chain of reviews, revisions, and approvals. In addition to the AAPM groups involved in the conventional approval process (Working Group on Clinical Trials, Quality Assurance and Outcome Improvement Subcommittee, Therapy Physics Committee, Science Council, Professional Council, Executive Committee), the Science Council additionally arranged reviews by ASTRO, European Society for Radiotherapy and Oncology, and American Association of Medical Dosimetrists.
Results
Standardizations are important for supporting a wide range of data use goals for clinical practice, research, and safety (8–23). The primary objective in defining the nomenclature was to reduce variability in naming. Variation is the principle barrier to developing automated solutions for accurate extraction, exchange, and processing of data. Variation in naming occurs over time, between individuals and among institutions and vendors. The second objective for the nomenclature was straightforward adoption into current practice. Discussion of the issues, choices, and compromises involved in arriving at these recommendations can be found in the full version of the TG report.
The guiding principles for nontarget nomenclature are as follows:
All structure names are limited to ≤16 characters to ensure compatibility with most vended systems.
All structure names must resolve to unique values, independent of capitalization. This will ensure that systems with case-insensitive formats will not result in overlapping definitions.
Compound structures are identified using the plural (ie, the name ends with an “s” or “i” as appropriate to the root structure name [eg, lungs, kidneys, hippocampi, LNs (for all lymph nodes), Ribs_L]).
The first character of each structure category is capitalized (eg, Femur_Head, Ears_External).
No spaces are used.
An underscore character is used to separate categorizations (eg, Bowel_Bag).
Spatial categorizations for the primary name are always located at the end of the string following an underscore character (eg, Lung_L, Lung_LUL, Lung_RLL, OpticNrv_PRV03_L).
L for left
R for right
A for anterior
P for posterior
I for inferior
S for superior
RUL, RLL, RML for right upper, lower, and middle lobe
LUL, LLL for left upper and lower lobe
NAdj for nonadjacent
Dist for distal; Prox for proximal
A consistent root structure name is used for all substructures (eg, SeminalVes and SeminalVes_Dist have a consistent root structure name; SeminalVesicle and SemVes_Dist do not have a consistent root structure name).
Standard category roots are used for structures distributed throughout the body
A for artery (eg, A_Aorta, A_Carotid)
V for vein (eg, V_Portal, V_Pulmonary)
LN for lymph node (eg, LN_Ax_L1, LN_IMN)
CN for cranial nerve (eg, CN_IX_L, CN_XII_R)
Glnd for glandular structure (eg, Glnd_Submand)
Bone (eg, Bone_Hyoid, Bone_Pelvic)
Musc for muscle (eg, Musc_Masseter, Musc_Sclmast_L)
Spc for space (eg, Spc_Bowel, Spc_Retrophar_L)
VB for vertebral body
Sinus for sinus (eg, Sinus_Frontal, Sinus_Maxillary)
Planning OAR volumes (PRV) are indicated, with “PRV” following the main structure, separated by an underscore (eg, Brainstem_PRV). Optionally, the uniform expansion used to form the PRV from the main structure in millimeters is indicated with 2 numerals (eg, SpinalCord_PRV05, Brainstem_PRV03), unless the result exceeds the character limit. For example, OpticChiasm_PRV03 is 17 characters and can be truncated to OpticChiasm_PRV3.
Partial structures are designated by appending a tilde (“⁓”) character to the root name (eg, Brain⁓, Lung⁓_L). This designator should be used to ensure a contoured structure is not misinterpreted as a whole organ when such a misinterpretation could have clinical implications (typically parallel organs). A use case example for a partial structure would be a CT scan not long enough to include the whole lung, for which “Lungs⁓” could be used to designate the contoured pair of lungs.
If a custom qualifier string is to be used, it should be placed at the end after the caret (“^”) character (eg, Lungs^Ex).
Establish a primary and a reverse order name for each structure.
Primary name–reading left to right, the structure categorization proceeds from general to specific, with laterality on the end. Thus, an alphabetical sort of structure names will result in a list grouped by organ (eg, Kidney_R, Kidney_Cortex_L, Kidney_Hilum_R). The primary name is recommended as the standard choice.
Reverse order name–reverse order naming reverses the order of the primary name. Some vended systems allow longer strings but have displays that default to show <16 characters. The reverse order name increases the likelihood that sufficient information can be displayed to safely identify the correct structure. For example, R_Hilum_Kidney would display as R_Hilum_Ki if the vendor’s report only showed the first 10 characters. Reverse order name should be limited to situations in which vendor system constraints prevent safe use of the primary name order.
Camel case (a compound word in which each word starts with a capital letter and no space is present between words such as CamelCase) is only used when a structure name implies 2 concepts but the concepts do not appear as distinct categories in common usage (eg, CaudaEquina instead of Cauda_Equina) because several examples of Cauda_xxxxx do not exist. Camel case names for primary and reverse order names are identical.
Structures that are not used for dose evaluation (eg, optimization structures, high- and low-dose regions) should be prefixed with a “z” or underscore (“_”) character such that an alphabetical sort will group them away from structures used for dose evaluation (eg, zPTVopt). The selection of “z” to designate dose evaluation structures is suggested.
Whether to allow for 2 naming values for each structure was considered from a practical perspective. The recommended standard is the primary name. Vendors are encouraged to modify their systems such that the full 16-character length of standard structure names are displayed in applications and reports. Reverse order name values should only be used for those systems unable to support the primary name values until further changes have been made in those systems. As these changes are made and the safety risks introduced by concatenating names are eliminated, usage should converge on the primary name.
Using standard category root names, an alphabetic sort of the primary name structures will group those with similar tolerances. This is especially valuable when structure names might not be commonly used and could be at risk of misinterpreting the structure type (eg, Mesenteric vs A_Mesenteric, Illiac vs A_Illiac, or I vs CN_I). However, a few structures are in routine use for which forcing the use of the category root name could impede adoption (eg, Parotid vs Glnd_Parotid) of the nomenclature. In those few cases, the TG chose to accept the internal inconsistency of forgoing the root name (eg, Parotid) to maintain the overarching objectives of reducing variability in nomenclature and promoting high adoptability into clinical practice.
Structure nomenclature list
A spreadsheet (Fig. 1) was created to facilitate the search for structures. The structures were categorized, described, assigned official values, and linked to the corresponding FMAID. Currently, the nomenclature defines 713 structures. The complete list can be found at the AAPM website for TG-263 (available at: www.aapm.org/reports/TG263_Supplemental/). The list will be a living document with periodic updates. In addition, all guidelines for structure naming and for DVH metrics are included in the document.
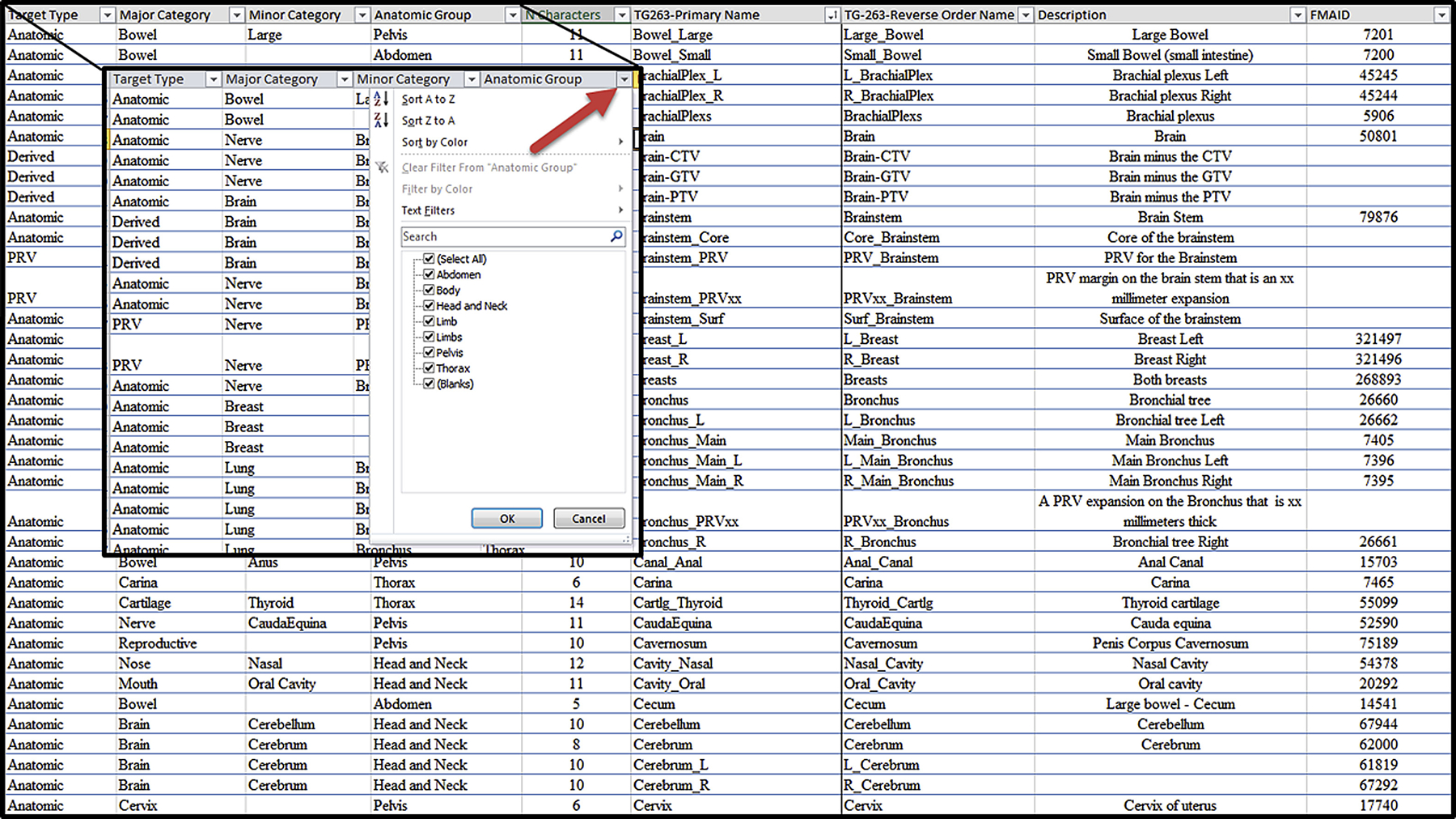
Illustration of a section of the nomenclature list worksheet for nontarget structures. Each column allows sorting and searching by clicking on the down arrow to the right of the heading as shown in the zoomed region.
The spreadsheet has 9 column headings used to aid in finding the names of the structures of interest.
Target type: anatomic, nonanatomic (eg, catheter), derived (eg, Body-PTV)
Major category: general organ category
Minor category: additional distinguishing category
Anatomic group: region of the body where the structure is located
“N” characters: number of characters in the name
TG-263–primary name: preferred naming system
TG-263–reverse order name: alternative naming system
Description: additional description of the structure
FMAID: identification number of the structure in the FMA most closely related
Recommendations for target structure nomenclature
Clinics use a very complex set of concepts in target naming strategies (eg, ICRU and other types, target classifiers for primary and nodal volumes, enumeration of volumes when several structures are present, dose, basis structures, imaging modality used to create).
Clinics did not attempt to represent all concepts but selected those few most important to their process. Within an individual clinic, different naming strategies could be used for different treatment sites and/or physicians.
TG-263 determined that they could not come to a consensus to define a single standard for all use cases and clinics that spanned the numerous concepts for a target name and also met the character string constraints. However, TG-263 did establish a set of guiding principles to specify that if a concept is represented in the target name, where and how it should appear. Therefore, the TG-263 established a set of guiding principles for target nomenclature.
This approach enables construction of computer algorithms to parse the names and to automatically create names based on concepts selected by users. Users choose the supplemental information to incorporate into target names, and these guiding principles will ensure that computer programs can recognize these names for quality and research endeavors. Although these principles accommodate most encountered names, they cannot accommodate all. TG-263 recommends using the caret (“^”) character to designate supplemental information not incorporated in the current guidelines.
Guiding principles for target nomenclature
The first set of characters must be 1 of the allowed target types:
GTV
CTV
ITV
IGTV (internal gross target volume–gross disease with margin for motion)
ICTV (internal clinical target volume–clinical disease with margin for motion)
PTV
PTV!—for low-dose PTVs that exclude overlapping high-dose volumes (see the section “Recommendations for Distinguishing Metrics of Segmented Versus Nonsegmented Target Structures”)
If a target classifier is used, place the target classifier after the target type with no spaces. Allowed target classifiers are as follows:
n: nodal (eg, PTVn)
p: primary (eg, GTVp)
sb: surgical bed (eg, CTVsb) par: parenchyma (eg, GTVpar)
v: venous thrombosis (eg, CTVv) vas: vascular (eg, CTVvas)
If multiple spatially distinct targets are indicated, Arabic numerals are used after the target type plus classifier (eg, PTV1, PTV2, GTVp1, GTVp2).
If designation of the imaging modality and sequential order in the image set require recording for adaptive therapy, the nomenclature follows the type/classifier/enumerator with an underscore and then the image modality type (CT, PT [positron emission tomography], MR, SP [single photon emission computed tomography]) and number of the image in the sequence (eg, PTVp1_CT1PT1, GTV_CT2).
If structure indicators are used, they follow the type/classifier/enumerator/imaging with an underscore prefix and are the values from the approved structure nomenclature list (eg, CTV_A_Aorta, CTV_A_Celiac, GTV_Preop, PTV_Boost, PTV_Eval, PTV_MR2_Prostate).
If dose is indicated, the dose is placed at the end of the target string, prefixed with an underscore character.
The TG strongly recommends using relative dose levels instead of specifying the physical dose
High (eg, PTV_High, CTV_High, GTV_High)
Low (eg, PTV_Low, CTV_Low, GTV_Low)
Mid (eg, PTV_Mid, CTV_Mid, GTV_Mid)
Mid plus 2-digit enumerator: allows specification of >3 relative dose levels (eg, PTV_Low, PTV_Mid01, PTV_Mid02, PTV_Mid03, PTV_High); lower numbers correspond to lower dose values
If numeric values for the physical dose must be used, specification of the numeric value of the dose in units of cGy is strongly recommended (eg, PTV_5040).
If numeric values for the physical dose must be used and these must be specified in units of Gy, “Gy” should be appended to the numeric value of the dose (eg, PTV_50.4Gy). For systems that do not allow the use of a period, the “p” character should be substituted (eg, PTV_50p4Gy).
If the dose indicated must reflect the number of fractions used to reach the total dose, the numeric values of the dose per fraction in cGy, or in Gy with the unit specifier, and number of fractions separated by an “x” character are added at the end (eg, PTV_Liver_2000x3 or PTV_Liver_20Gyx3).
If the structure is cropped back from the external contour for the patient, the quantity of cropping (“-xx” millimeters) is placed at the end of the target string. The cropping length follows the dose indicator, with the amount of cropping indicated by xx millimeters (eg, PTV_Eval_7000–08, PTV-03, CTVp2–05).
If a custom qualifier string is used, the custom qualifier is placed at the end after a caret (“^”) character (eg, PTV^Physician1, GTV_LiverÎCG).
If it is not possible to follow the guidelines and remain within the 16-character limit, preserve the relative ordering but remove the underscore characters, progressing from left to right as needed to meet the limit (eg, PTVLiverR_2000x3). However, this last resort scenario will undermine the use of automated tools.
Two distinct methods are used for sequential treatment of the same target volume (guiding principle 3). Some institutions used sequential numbers as the patient returns for future treatment courses for the same PTV (eg, PTV1 and PTV2 for the original course and PTV3 and PTV4 for lung metastasis treated in a later course). In contrast, other institutions have numbered sequentially for targets treated within the course, independently of historical treatment sessions (eg, PTV1 and PTV2 for the original course and PTV1 and PTV2 for lung metastasis treated in a later course) and using the same nomenclature for repeat irradiation of the same (not spatially distinct) target. TG-263 did not define a recommended sequential numbering method. Practices should ensure their method is self-consistent and guards against the incorrect summing of total doses.
The prescription of doses in units of cGy is common in the United States, is the current recommendation of the ASTRO working group on prescriptions, and is supported by analysis of Radiation Oncology Incident Learning System (RO-ILS) data (9, 10). Prescription in units of Gy is more common in European countries and is also used in some large institutions in the United States. All groups advocating one over the other have cited safety as a primary factor. Although it is highly desirable to specify a single answer in the standard, the most important point for safety and data access is ensuring unambiguous communication. Because it was not possible to identify a single dose unit with wide global adoption, an approach compatible with each was identified.
The use of a relative dose (eg, PTV_High) was the primary recommendation if dose information is conveyed in the target name. This approach has several advantages. First, it is independent of the physical dose units used at various institutions, eliminating the need to specify cGy (eg, PTV_6660) or Gy (eg, PTV_66.6 Gy). Second, it is not uncommon for a prescription to be changed in the course of treating a patient. In that case, if physical dose units were used, the structure name would have to be renamed with the correct dose to convey the correct information (eg, a change from PTV_7560 to PTV_7380). Without this change, the name could convey conflicting information with respect to the current prescription, presenting both logistic and safety issues. Third, when mining dosimetric data, relative dose names greatly improve the speed, accuracy, and composability of queries to extract the needed information. For most disease sites, only 2 or 3 target structure names are needed (eg, PTV_High, PTV_Mid, PTV_Low); thus, extracting the median dose to these structures and number of fractions treated will provide a large amount of information on target structure doses with minimal effort. In contrast, needing to first identify all dose levels from the structure name and then reconstruct the relative dose levels within each plan from the physical doses specified in the name is much more difficult and prone to error.
If physical doses are used, the numeric value should be defined in units of cGy. The use of cGy is consistent with the recommendations from ASTRO and RO-ILS. Enabling unambiguous standardized communication of dose in the name promotes adaptability of the nomenclature in a broad range of national and international clinics. For clinics that currently use Gy for prescriptions, the physical doses in Gy should be communicated explicitly with the addition of “Gy” as a suffix for clarity in communication. This approach uses a similar number of characters for each dose unit, and, when Gy is used, it is consistent with the recommendations for DVH metrics, as described in the next section.
Recommendations for DVH metrics
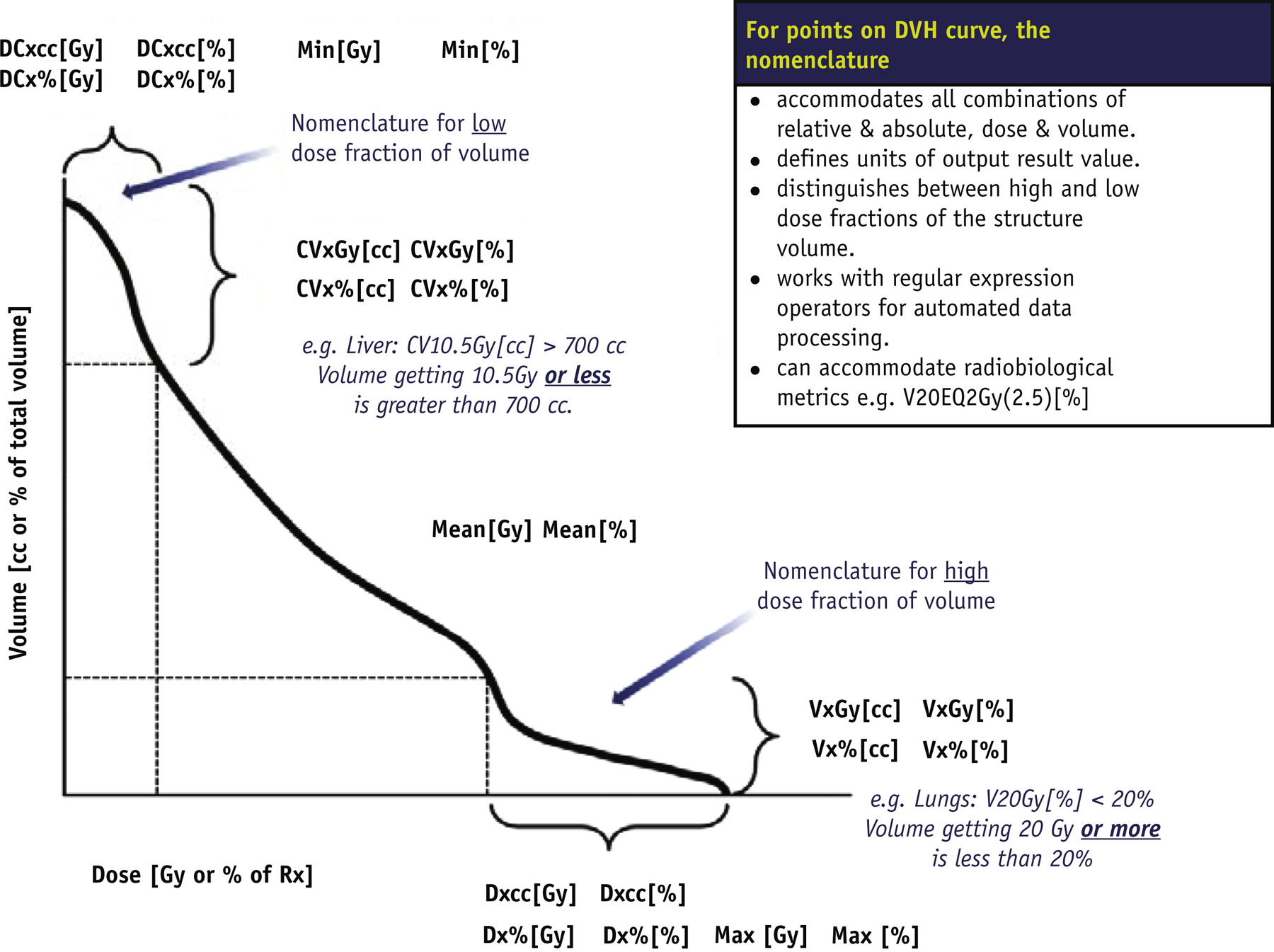
Very few examples of standardized nomenclatures exist for the full set of dose and volume metrics used in practice (1). Providing specificity on exactly what is measured, input parameters, units used for dose and volume, all in a format that can be parsed with regular expression operators, improves ability to use computer algorithms to automate calculation. The ability to incorporate radiobiological metrics and units is also important. Figure 2 illustrates the recommended DVH nomenclature.
Guidelines for DVH metrics
Units or a label for what is measured (output) are specified at the end of the string, enclosed in square brackets.
Dose: Gy or % where the percentage references the dose prescribed to PTV_High structure type
Volume: cc (cm3) or % where the percentage references the volume of the structure
Equivalent 2 Gy: EQD2Gy
Measurement type is specified at the beginning of the string. Units or a label for where on the curve the point is measured (input) are specified.
Vx: volume of the subvolume receiving dose x or greater, with the Dose units or label specified (eg, V20Gy[%], V95%[%], V20Gy[cc])
Dx: minimum dose received by the hottest subvolume x, with volume units or label specified (eg, D0.1cc [Gy], D95%[%])
CVx: volume of the subvolume receiving dose x or less, with dose units or label specified (eg, CV10.5Gy[cc], CV95%[cc])
DCx: maximum dose received by the coolest subvolume x, with volume units or label specified (eg, DC0.1cc[Gy], DC1%[Gy])
If calculation parameters for the metric are required, they are enclosed in parentheses in front of the square brackets defining the output units or label (eg, V50EQD2Gy(2.5)[%]).
Conventional DVH metrics correspond to points receiving a certain dose or more. In the lung, V20Gy[%] is the percentage of the lung volume that receives ≥20 Gy. In contrast, details about the points receiving a certain dose or less use nomenclature with an inserted “C” for “complement” or “cold” to qualify the subvolume. Thus, for liver SBRT (stereotactic body radiation therapy), CV15Gy[cc] is the absolute volume that receives ≤15 Gy. For example, DC700cc[Gy] selects the 700 cc subvolume that receives the lowest overall dose and reports the highest dose in that subvolume.
The nomenclature extends the use specification of input and output units with the addition of the EQD2Gy dose unit to specify the dose delivered in 2-Gy fractions calculated to have the same radiobiological effect with the linear quadratic (LQ) model and a specified a/b value. The calculation parameter values, including a/b, are enclosed in parentheses before the output units. The nomenclature does not currently specify the ordering of parameter values for particular calculations. This approach minimizes the naming constraints for the evolving types of radiobiological calculations, or parameters used, preserving a consistent representation of the involved units and explicit indication parameter values. Designation of an algorithm could also be included as a parameter in the parentheses.
Examples of radiobiological calculations using EQD2Gy follow:
Maximum equivalent 2-Gy dose calculated with an a/b ratio of 4: Max(4)[EQD2Gy]
Equivalent 2-Gy dose encompassing 90% of a target volume, calculated with an α/β ratio of 10: D90%(10) [EQD2Gy]
Percentage of volume of a structure receiving 50 EQD2Gy using an α/β of 3 versus 10: V50EQD2Gy(3) [%] versus V50EQD2Gy(10)[%]
Distinguishing the use of the LQ versus the LQ-linear (LQL) model in calculating the 2-Gy equivalent dose encompassing 95% of a structure when an α/β of 10 is used: D95%(10,LQ)[EQD2Gy] versus D95%(10,LQL) [EQD2Gy]
Research settings use a wide range of radiobiological metrics. Examples include tumor control probability, normal tissue complication probability (NTCP), and biologically effective dose (BED). These are not typically encountered in clinical settings at present. Models continue to evolve, defining new types and parameters. Approaches currently in use at several member institutions were compatible with the guideline recommendations for enclosing calculation parameters in parentheses [eg, NTCP(LQL, α/β = 2.5, TD50 = 40, n = 1.0, m = 0.13), NTCP(40, 1.0, 0.13), BED(α/β = 10), BED(10)]. The TG did not make specific nomenclature recommendations for these radiobiological metric types and parameters.
Recommendations for Distinguishing Metrics of Segmented Versus Nonsegmented Target Structures
The reported DVH metrics for multiple PTVs treated to differing dose levels should define whether the lower dose PTVs exclude (segmented) or include (nonsegmented) the higher dose PTVs. For example, a low-dose nodal volume could be treated to 5000 cGy (PTV_5000) and a boost volume within that nodal volume could be treated to 7000 cGy (PTV_7000).
Both segmented and nonsegmented volumes can be valuable for dose evaluation. The concern is that the clinical PTVs used to evaluate the plan might not be clearly delineated as segmented or nonsegmented PTVs in the nomenclature. This is illustrated in Figure 3. For nonsegmented low-dose PTVs, the DVH typically shows a “foot” of the overlap with the high-dose PTV. Segmented low-dose PTVs have a long high-dose tail. The nomenclature needs to clearly delineate between segmented and nonsegmented PTVs for pooling data. Either approach can work; however, if the standards vary among institutions, metrics such as PTV_5000: V115% [%] would be significantly different depending on the approach. For example, if the PTV_5000 is not segmented and contains PTV_7000, V115%[%] would necessarily be high, reflecting the ratio of the volumes. In contrast, for a segmented PTV_5000, the V115%[%] would be significantly lower.
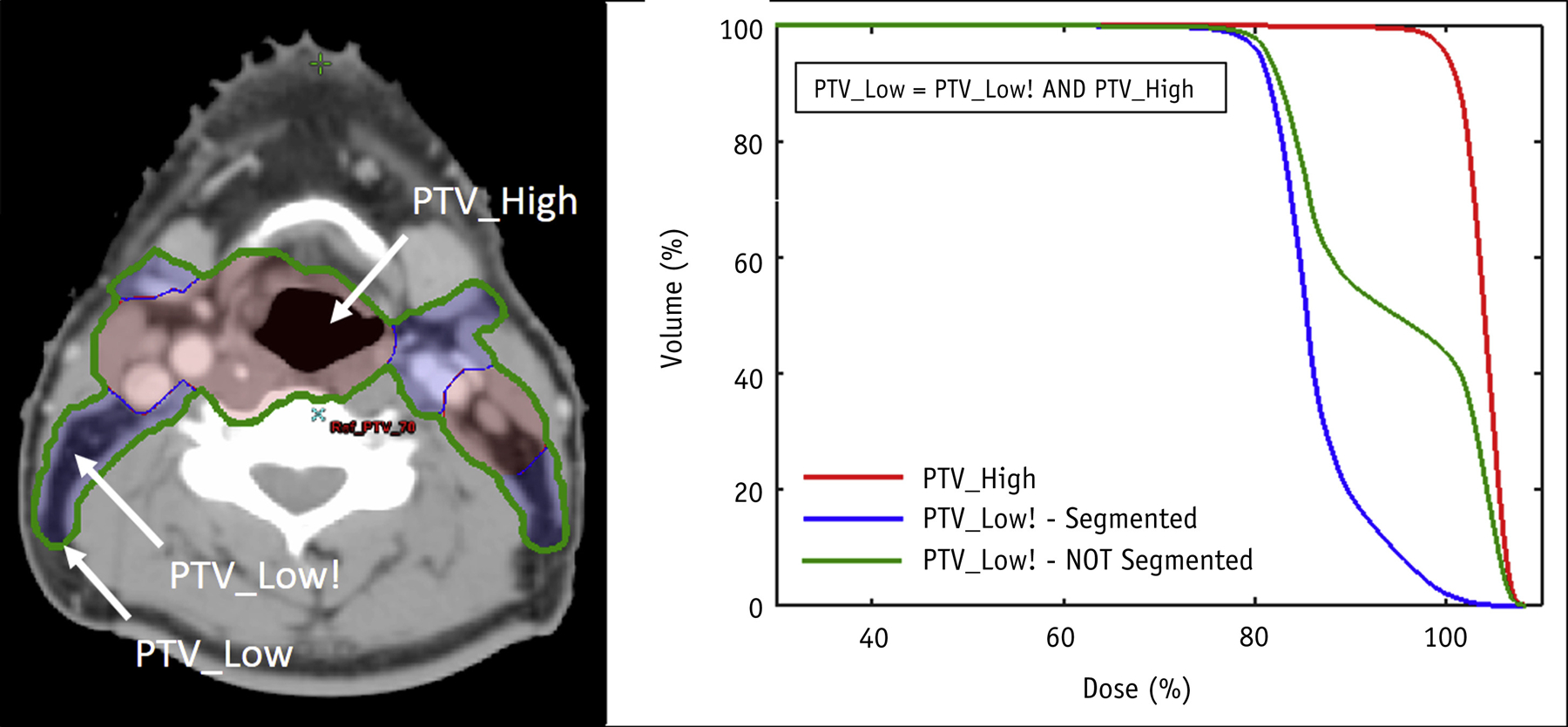
Illustration of dose–volume histogram differences when using segmented planning target volume (PTV) definitions, where the high-dose PTV (PTV_High, red curve) is not included as part of a lower dose PTV (PTV_Low, blue curve) compared with a nonsegmented approach, where the high-dose PTV is included in the lower dose PTV (PTV_Low, green curve). In this example, the volume of PTV_High is 55% of the volume of the nonsegmented PTV_Low.
Because nonsegmented PTVs retain information on overlaps relevant to dose evaluation but segmented PTVs do not, many institutions typically use nonsegmented volumes. To retain the ability to use both approaches, TG-263 recommended that the default assumption is nonsegmented target volumes. If a segmented volume is used (ie, exclusion of overlap with high-dose subvolumes), its target type should include an exclamation point (“!”) character suffix to clarify (eg, GTV!, CTV!, PTV!). This should be exceedingly rare for GTV and CTV structures.
Discussion
The combined efforts of the multistakeholder TG produced a set of nomenclature recommendations to reduce variability in clinical practice and clinical trials, paving the way for the development of automated applications to improve data aggregation and safety. The present executive summary highlights key points of the nomenclature. Additional topics presented in the full report include background information on related nomenclatures, ontologies, DICOM parameters and character constraints, color specification, pilot study design and results, recommendations for implementation, recommendations to clinical trial groups, and recommendations to vendors.
Summary of key take home points
Standardized nomenclatures add value to the radiation oncology by providing a basis for improved communication and the ability to develop automated solutions for data extraction and QA to improve clinical workflow, safety, and research.
The nomenclature was developed through the combined effort of many clinics, vendors, and clinical trial groups (eg, NRG, Radiation Therapy Oncology Group) to define a viable consensus recommendation. The nomenclature has already been put into routine use in many clinics, as a part of clinical trials, and in vendor software, demonstrating that it is a viable solution.
The nomenclature was defined to work within the storage and display limits of a range of vended systems to convey information on structure types and laterality.
Guidelines for target structure naming were created to allow a range of information to be conveyed using a standardized syntax and allowing automated parsing of the information from the name.
When dose is used as a part of target naming, relative dose levels are recommended (eg, PTV_High, PTV_Low). If the physical dose is required, units of cGy are preferred, aligning with the current recommendations of the ASTRO group-defined guidelines for prescriptions and RO-ILS.
Guidelines for nontarget structures and specific values defined for >700 structures were created, including identification codes for corresponding FMA structures.
A DVH nomenclature detailing the input and output units for high-dose and low-dose metrics and radiobiological metrics was recommended that was designed to use regular expressions to automate the parsing parameters needed for automated calculations.
Nonsegmented target structures were recommended for the default standard for contouring. However, target structure nomenclature guidelines define a method to identify segmented structures when preferred.
The nomenclature was piloted in clinic, vendor, and trials groups to prove the viability of the recommendations before release.
Vendor participation was important in nomenclature development and is beneficial to facilitate implementation in those vended systems.
Acknowledgments
The contributions of the many reviewers who donated their time, effort, and expertise to reviewing the task group report played a vital role in helping the group ensure that the recommendations emerging were practical and clinically viable as a standard to be broadly applied. We are very grateful for their contribution.
Footnotes
Conflict of interest:
Dr Molineu reports grants from the National Cancer Institute, during the conduct of the study. Dr Matuszak reports grants from Varian Medical Systems, outside the submitted work. Dr Napolitano reports other support from IBA Dosimetry, outside the submitted work. Ms Lansing is an employee of Eleckta Corporation. Dr Bosch reports grants from the US National Institutes of Health, during the conduct of the study. Dr Bosch reports grants from the US National Institutes of Health, during the conduct of the study.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.ijrobp.2017.12.013
Read article for free, from open access legal sources, via Unpaywall:
http://www.redjournal.org/article/S0360301617342323/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.ijrobp.2017.12.013
Article citations
Dummy run for planning of isotoxic dose-escalated radiation therapy for glioblastoma used in the PRIDE trial (NOA-28; ARO-2024-01; AG-NRO-06).
Clin Transl Radiat Oncol, 47:100790, 04 May 2024
Cited by: 0 articles | PMID: 38765202 | PMCID: PMC11101689
Perceptions on and roadblocks to implementation of standardized nomenclature in radiation oncology: A survey from TG-263U1.
J Appl Clin Med Phys, 25(6):e14359, 30 Apr 2024
Cited by: 1 article | PMID: 38689502
Interdisciplinary Collaboration in Head and Neck Cancer Care: Optimizing Oral Health Management for Patients Undergoing Radiation Therapy.
Curr Oncol, 31(4):2092-2108, 07 Apr 2024
Cited by: 1 article | PMID: 38668058 | PMCID: PMC11049200
Review Free full text in Europe PMC
Uncovering the armpit of SBRT: An institutional experience with stereotactic radiation of axillary metastases.
Clin Transl Radiat Oncol, 45:100730, 17 Jan 2024
Cited by: 0 articles | PMID: 38317679 | PMCID: PMC10839264
Clinical Evaluation of Deep Learning for Tumor Delineation on 18F-FDG PET/CT of Head and Neck Cancer.
J Nucl Med, jnumed.123.266574, 22 Feb 2024
Cited by: 0 articles | PMID: 38388516 | PMCID: PMC10995525
Go to all (69) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Standardizing naming conventions in radiation oncology.
Int J Radiat Oncol Biol Phys, 83(4):1344-1349, 13 Jan 2012
Cited by: 45 articles | PMID: 22245204 | PMCID: PMC4306340
Perceptions on and roadblocks to implementation of standardized nomenclature in radiation oncology: A survey from TG-263U1.
J Appl Clin Med Phys, 25(6):e14359, 30 Apr 2024
Cited by: 1 article | PMID: 38689502
Open RT Structures: A Solution for TG-263 Accessibility.
Int J Radiat Oncol Biol Phys, 118(3):859-863, 29 Sep 2023
Cited by: 1 article | PMID: 37778423
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: P30 CA008748
NIGMS NIH HHS (1)
Grant ID: U54 GM104942