J Immunol Res. 2018; 2018: 2349045.
Dysregulated Functions of Lung Macrophage Populations in COPD
,
 1
1
,
1
,
1
,
1
and
 1
,
2
1
,
2
Theodore S. Kapellos
1Genomics & Immunoregulation, Life and Medical Sciences Institute (LIMES), Carl-Troll-Str. 31, 53115 Bonn, Germany
Kevin Bassler
1Genomics & Immunoregulation, Life and Medical Sciences Institute (LIMES), Carl-Troll-Str. 31, 53115 Bonn, Germany
Anna C. Aschenbrenner
1Genomics & Immunoregulation, Life and Medical Sciences Institute (LIMES), Carl-Troll-Str. 31, 53115 Bonn, Germany
Wataru Fujii
1Genomics & Immunoregulation, Life and Medical Sciences Institute (LIMES), Carl-Troll-Str. 31, 53115 Bonn, Germany
Joachim L. Schultze
1Genomics & Immunoregulation, Life and Medical Sciences Institute (LIMES), Carl-Troll-Str. 31, 53115 Bonn, Germany
2Platform for Single Cell Genomics and Epigenomics, German Center for Neurodegenerative Diseases and University of Bonn, Sigmund-Freud-Str. 27, 53175 Bonn, Germany
1Genomics & Immunoregulation, Life and Medical Sciences Institute (LIMES), Carl-Troll-Str. 31, 53115 Bonn, Germany
2Platform for Single Cell Genomics and Epigenomics, German Center for Neurodegenerative Diseases and University of Bonn, Sigmund-Freud-Str. 27, 53175 Bonn, Germany

Corresponding author.
Academic Editor: Ethan M. Shevach
Received 2017 Jul 30; Accepted 2017 Nov 29.
This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article has been
cited by other articles in PMC.
Abstract
Chronic obstructive pulmonary disease (COPD) is a diverse respiratory disease characterised by bronchiolitis, small airway obstruction, and emphysema. Innate immune cells play a pivotal role in the disease's progression, and in particular, lung macrophages exploit their prevalence and strategic localisation to orchestrate immune responses. To date, alveolar and interstitial resident macrophages as well as blood monocytes have been described in the lungs of patients with COPD contributing to disease pathology by changes in their functional repertoire. In this review, we summarise recent evidence from human studies and work with animal models of COPD with regard to altered functions of each of these myeloid cell populations. We primarily focus on the dysregulated capacity of alveolar macrophages to secrete proinflammatory mediators and proteases, induce oxidative stress, engulf microbes and apoptotic cells, and express surface and intracellular markers in patients with COPD. In addition, we discuss the differences in the responses between alveolar macrophages and interstitial macrophages/monocytes in the disease and propose how the field should advance to better understand the implications of lung macrophage functions in COPD.
1. Lung Macrophage Populations in Mice and Humans
The lung is constantly exposed to the host's outer environment; therefore, constitutively active mechanisms are required to monitor for irritants and infections with pathogens. This pivotal sentinel function is assumed by lung-resident immune cell populations including macrophages, dendritic cells (DCs), and airway epithelial cells [1]. To date, three major myeloid cell populations have been identified in the lung which differ in their exact localisation in the tissue and their developmental origin (): resident alveolar macrophages (AMs), resident interstitial macrophages (IMs), and blood monocytes [2–4].
Murine and human lung macrophage populations under steady-state conditions. AMs reside at the airspaces of the lung, while IMs localise in the interstitial space between the alveoli and blood vessels. In both the murine and human lungs, there is also a monocyte population which enters the tissue from blood vessels. AMs are the biggest of all three lung macrophage populations, are potent phagocytes, and secrete a range of proinflammatory mediators. IMs are smaller than AMs but display comparable phagocytic capacity and ability to produce soluble factors. They are believed to serve as an intermediate step in monocyte differentiation towards AMs and demonstrate proliferative potential. Finally, monocytes are sensitive to migratory gradients and have been shown to exhibit proinflammatory mediator capacity, but no antigen presentation. The currently acceptable nomenclatures for AMs, IMs, and monocytes in mice (Mm) and humans (Hs) are indicated next to each population.
AMs reside in the airspaces of the lung, whereas IMs are found in the interstitial space between the alveoli and blood vessels. Morphological observation of these two populations indicated that AMs are larger in size than IMs [5]. In addition, phenotypic characterisation of AMs and IMs in mice revealed differences in the expression levels of MHC class-II, CD11b, CD14, CD45, CD54, CD68, CD71, CD204, CD206, and Siglec-F [5–9]. Altogether, lung-resident macrophages have been characterized as CD11c+CD11blo cells and can be distinguished from recruited cells during endotoxin or viral-induced inflammation by the level of CD11b expression [10]. In humans, AMs are described as CD45+CD206+CD14loCD71+CD169+ cells, whereas IMs are reported as CD45+CD206+CD14hiCD71−CD169− cells [11]. However, recently a study suggested high expression of the mannose receptor (CD206) in both macrophage populations and revealed two AM subpopulations with differential expression of the hemoglobin-haptoglobin complex scavenger receptor CD163 [12]. Lastly, Desch et al. found that human AMs (CD206+CD14loHLA-DR+CD64+CD141+ cells) could be distinguished from lung tissue monocytes based on CD14 and CD16 surface expression [13].
Functionally, although a small fraction of AMs was shown to be present in lymph nodes in S. pneumoniae-infected mice [14], IMs are considered to be classical modulators of adaptive immunity in human and murine lungs [7, 15–18]. In humans and rodents, AMs have been reported to remove surfactants and debris [19], suppress adaptive immunity [20, 21], and regulate neutrophil and monocyte recruitment to the lung [22–24]. With regard to other typical macrophage functions, both populations display high phagocytic capacity [5, 25], but AMs are considered to be more potent phagocytes [17, 26–28] and they were shown to secrete proinflammatory mediators and reactive oxygen species (ROS) upon activation in animal studies [17, 27, 29, 30].
Research on both human and animal AMs challenged the homogeneity of this population [31, 32]. Instead, density-gradient centrifugation splits them into distinct subpopulations with differences in the expression of surface markers and intracellular enzymes as well as tumour lysis, migration, cytotoxicity, phagocytosis, lymphoproliferative response augmentation, soluble mediator release, and procoagulant activity [33–42].
Under steady-state conditions, the replenishment of AMs in humans and mice occurs mainly via self-renewal as recently demonstrated in long-term lung transplant, parabiosis, and fate-mapping studies [43–45]. During lung inflammation, a proportion of AMs dies by apoptosis and the cells are replenished in part by local proliferation of local stem cells, but also via the recruitment of blood mononuclear phagocytes [46–48]. IMs acquire proinflammatory markers upon activation, such as CD40, CD80, and CD86, and their numbers are increased in mice [6]. Between the two populations, AMs secrete more TNF-α, but less IL-6, IL-1ra, and IL-10 than IMs in rats [49]. Furthermore, in humans, the two populations exhibit differential sensitivity to pathogen recognition receptor (PRR) activation with IMs being less sensitive to TLR9 priming [5].
IMs are not a homogeneous population either, and in the rat lung interstitium, they are currently believed to be contaminated with up to 20% AMs [50]. Similar to AMs, several density-defined populations have been identified exhibiting differential prostaglandin secretion, migration, and phagocytosis capabilities [51–53]. It has long been considered that IMs are an intermediate step in maturation of infiltrating blood monocytes towards AMs [54, 55] because they display blunt lamellipodia and fewer lamellar inclusions than AMs and are morphologically more closely related to blood monocytes [4, 56, 57]. Moreover, in mice, they seem to proliferate more than AMs [17]. However, considering more recent findings in macrophage ontogeny and the possibility to measure hundreds to thousands of genes at the single cell level, these observations need to be revisited.
Monocytes are divided into subpopulations in both humans and mice (reviewed in [58]). Fate-mapping experiments in mice unraveled a CD115+CD11b+Ly-6ChiCCR2+ and a CD115+CD11b+Ly-6Clo monocyte population [59, 60]. Ly-6Clo monocytes express high levels of the fractalkine receptor CX3CR1, and they were shown to crawl inside blood vessels via lymphocyte function-associated antigen 1 interactions with the endothelial lining [60, 61]. Upon activation with an inflammatory stimulus, they rapidly respond by secreting TNF-α [62]. In contrast, Ly-6ChiCCR2+CX3CR1−GR-1+ monocytes are actively recruited to inflamed tissues where they can differentiate into so-called inflammatory DCs or different flavours of macrophages [60, 63–65]. This subset was shown to express high levels of chemokine receptors, complement peptides, and annexins, while Ly-6Clo monocytes express more MHC class-II, growth factors, integrins, and scavenger receptors [66, 67].
In analogy to mice, human monocytes are divided into different subsets including CD14++CD16− (classical), CD14+CD16+ (intermediate), and CD14−CD16+ (nonclassical) [58]. All subsets are CD206−CD64+ [13] and express CX3CR1 and CXCR4 (CD16+ monocytes express CX3CR1 at higher levels which allows them to adhere firmly to vessel walls [58]). Classical monocytes also express several CC chemokine receptors [58, 60] and are characterised by an antimicrobial phenotype [68]. Intermediate monocytes express genes related to antigen processing and presentation, transendothelial migration, and angiogenesis and secrete higher amounts of cytokines and ROS than other subsets [68, 69]. Human classical monocytes resemble murine Ly-6Chi monocytes, whereas nonclassical monocytes were described to be the counterparts of Ly-6Clo monocytes (reviewed in [64]). The human blood monocyte population structure was recently challenged by Villani et al. who, by application of single cell RNA sequencing, suggested that peripheral blood monocytes can be further divided in four subsets [70]. Whether this also holds true for lung monocytes awaits further investigation.
2. Chronic Obstructive Pulmonary Disease (COPD): Epidemiology, Pathology, and the Role of the Immune System
COPD is a chronic disease of the lower respiratory tract and is characterised by irreversible airway obstruction, chronic bronchitis, and loss of alveolar parenchyma (emphysema) [71]. It affects almost equally men and women, has its onset in midlife, and progresses slowly during adulthood [72] resulting in airway obstruction by mucus exudates and lung tissue remodelling [71]. Patients with COPD are diagnosed as stage 1 (mild) to 4 (very severe) based on spirometric grading as well as group A to D based on clinical assessment of symptoms and exacerbation risk according to GOLD classification [73]. Besides the well-documented increase in patients' disability-adjusted life years, COPD is also a huge economic burden for countries due to its chronic nature, the exacerbations which lead to patient hospitalisation and the lack of effective drugs [74–76].
COPD ranked sixth globally as a leading cause of death in 1990 and is projected to rank third by 2020 accounting for 7% of total deaths worldwide [73, 77, 78]. There are several causative factors for the disease (reviewed in [79, 80]) including environmental factors, such as smoking (which is now accepted as the main causal factor of the disease), the use of biomass fuel, occupational exposure to toxic gases or dust, infections, outdoor pollution, genetic susceptibility as exemplified by the deficiency of α1-antitrypsin (reviewed in [81]), and accelerated lung ageing [82, 83].
COPD is thought to be initiated when inhaled irritants activate innate immunity either directly by triggering common PRRs on immune and bronchial epithelial cells or indirectly by inducing the release of danger signals by epithelial and endothelial cells [84–86]. In fact, the subsequent recruitment of blood leukocytes and the destruction of lung tissue are TLR-dependent and macrophage activation occurs in an inflammasome-dependent manner [87]. Patients with COPD present with elevated levels of a broad range of proinflammatory mediators in their bronchial lavage, such as TNF-α, IL-8, CCL2, CCL3, LTB4, myeloperoxidase, and eosinophilic cationic protein among others [88–94]. In parallel, the vasculature upregulates surface adhesion molecules [95] and becomes permeable to attract blood neutrophils, monocytes, and eosinophils to the lung. Secretion of the tissue remodelling cytokine TGF-β by epithelial cells has also been reported to relate to small airway obstruction in COPD [96].
Neutrophil percentages in COPD correlate with deterioration of lung function and airway obstruction [97] and, together with macrophages [98], they contribute to disease pathology via the production of extracellular matrix- (ECM-) degrading enzymes [99]. Disintegrated alveolar wall components can be readily detected in the biological fluids of patients with COPD and are significantly higher than in healthy smokers [100]. Neutrophil elastase (NE) and metalloproteinases (MMPs) cause lung tissue destruction and trigger mucus secretion which obstructs small airways [101]. The imbalance between proteases and protease inhibitors in the lungs of patients with COPD causes enhanced chemotactic factor secretion by macrophages and further amplification of neutrophil recruitment [102].
In the healthy lung, DC sample inhaled exogenous material or apoptotic cells to induce immune tolerance or initiate appropriate immune responses [1]. In COPD, DCs accumulate in the lung in an IL-1α-dependent manner following a CCL20-CCR6 axis [103, 104]. Recent reports have suggested that the numbers of the various DC subsets are differentially altered in the several lung compartments. For example, Langerhans-type DCs have been observed selectively in small airways [105], whereas the numbers of bronchial mucosal DCs in the epithelium as well as the migratory CD83+ and CCR7+ DC subsets are reduced in patients with COPD [106, 107]. The dysregulated localisation of these immune cells comes together with altered immune responses regulated by the different subsets [108]; cigarette smoke and the lung inflammatory milieu decrease lung myeloid DC maturation [109, 110] and cause an imbalance to the costimulatory status of these cells [111]. In contrast, CD1c+ DCs favour tolerogenic signalling and the induction of regulatory T cells [112].
DC-mediated CD4+ T cell activation is predominantly skewed to a TH1 phenotype [113], although TH17 cells have also been found in the lungs of patients with COPD [114, 115]. However, in the epithelium, submucosa, and adventitia of peripheral airways of patients with COPD, CXCR3-expressing CD8+ cells are the predominant T cell subtype [116]. CD8+ lymphocytes contribute to tissue injury and cell death in the lung via the release of proteolytic enzymes, such as perforin and granzymes [117–120]. Finally, the numbers of regulatory T cells have been demonstrated to be in decline in patients with COPD in comparison with healthy smokers which highlights another causality factor for the chronicity of the disease [121, 122]. Regarding the factors responsible for the increase in T cell numbers, Di Stefano et al. showed that IL-27 secretion by CD68+ cells in the BAL of patients with COPD may contribute to IFN-γ and granzyme B secretion by CD8+ lymphocytes as well as the induction of regulatory T cells [123]. However, more studies are needed to clarify the role of T cells as part of an efficient acute or a dysregulated chronic response mounted by alterations in innate immunity.
In 2006, the presence of B cells was also described in lymphoid follicles in small airways and lung parenchyma of patients with COPD and animal models [124]. Supporting evidence came from the detection of elevated levels of B cell-activating factor in lymphoid follicles which inversely correlated with lung function [125]. Although the nature of the antigens that activate B cells is not fully known, it has been speculated that they range from cigarette smoke irritants [126] to cell death and ECM degradation by-products, microbial components, and autoantigens [127].
Finally, a frequent manifestation of COPD is the colonisation of the patients' lungs by bacteria and viruses (likely due to impaired phagocytosis by AMs [126]) which cause exacerbations diminishing the patients' quality of life [128, 129]. H. influenzae, S. pneumoniae, and M. catarrhalis are most usually detected in patients with frequent exacerbations, while P. aeruginosa infections account for exacerbations in patients with severe COPD [130–132]. Furthermore, in recent years, the role of viral infections in the worsening of patients' health has begun to be appreciated and research has focused on the identification of the immune cells and mechanisms that contribute to the loss of lung function. Rhinoviruses [133], picornavirus [134], adenoviruses, the respiratory syncytial virus, and influenza virus are the most common viruses found in the sputum of patients with COPD and are responsible for about half of all exacerbations observed (reviewed in [135]). Infections augment the innate immune responses and lung tissue remodelling in mice [136], while human patients present with dysregulated neutrophil and T cell mobilisation [89, 137], increased proinflammatory mediator levels [138, 139], and antibacterial humoral responses [140].
3. Why the Functions of Lung Macrophage Populations in COPD Warrant Further Investigation
The numbers of lung-resident macrophages in the lung have been reported to be dramatically increased in COPD due to the recruitment of blood leukocytes from the periphery [141, 142]. Macrophages are plastic cells and respond in several ways to accommodate changes in their microenvironment. For example, AMs from smokers present with increased expression of cytokines and chemokines, growth factors, proteases, antioxidant proteins, adhesion molecules, transcription regulators, and signalling pathway genes, whereas they reduce expression of genes related to neutrophil activation, serine protease inhibitors, and macrophage differentiation genes [143]. Consequently, in the constantly changing microenvironment of the COPD lung, resident macrophages will respond accordingly and shape their effector functions to orchestrate the immune responses. Hence, the study of the functions of lung macrophage populations as well as their interplay with other immune cells and the lung stroma has the potential to enhance our understanding of COPD pathology and provide with novel biomarkers and therapeutic targets.
4. AMs in COPD
Over the last decades, numerous studies have accumulated knowledge about the role and functions of AMs in COPD. Major aspects of change in cellular functions concern the secretion of proinflammatory mediators, the induction of oxidative stress, the deregulation of the protease-protease inhibitor balance, and the impairment of pathogen phagocytosis as well as changes in gene expression which we highlight next ( and ). Many of these studies have been performed in the pregenomic era and most of them prior to the era of single cell genomics. Therefore, as for every other field in life sciences, some of the previous findings might be challenged once we have applied cutting-edge technologies to better understand the basic unit of life—the cell—and its changed functionality in complex diseases like COPD. Nevertheless, we review the current knowledge which has often been obtained only at the population level, but not at the single cell level yet.
Lung macrophage population functions in COPD. AMs exhibit alterations in their physiological responses in COPD; the secretion of proinflammatory cytokines and chemokines is dysregulated (1). The cells undergo oxidative stress and secrete ROS and nitrite species into the lung micro-environment (2), they store intracellularly large amounts of iron (2), and they overexpress and release proteases which cause alveolar tissue destruction (3). In contrast, processes, such as phagocytosis of microbes (4) and apoptotic neutrophils or epithelial cells (5), are downregulated in AMs from patients with COPD, an observation which could explain the frequent colonisation of the lungs with bacteria and viruses in exacerbations. In the meantime, monocytes are recruited from blood vessels following chemokine gradients and contribute to disease pathology via the secretion of proinflammatory mediators and proteases. It is also believed that monocytes differentiate into macrophages via an intermediate step of IMs which morphologically and functionally resemble monocytes.
Table 1
Molecules differentially expressed by AMs from animals or patients with COPD compared to healthy controls.
| Molecule family | Encoded proteins | References |
|---|
| Cytokines | TNF-α ↓, IL-1B ↑↓, IL-6 ↓, IL-10 ↓, IL-12 ↑, Tnf-α ↓, Il-6 ↓ | [126, 145, 147, 148, 150–152] |
| Chemokines | IL-8 ↓, CCL2 ↑, CCL5 ↓, CCL7 ↑, CCL13 ↑, CCL22 ↑, Cxcl10 ↓, CXCL9 ↓, CXCL10 ↓, CXCL11 ↓ | [126, 145, 147–149, 151–154, 158, 165] |
| Chemokine receptors | CCR2 ↑, CCR5 ↑ | [153, 154] |
| Prostaglandin metabolism | PTGS1 ↑, PTGS2 ↑ | [156] |
| Oxidative stress | GSH ↓, Gsh ↓, iNOS ↑, HO-1 ↓ | [147, 150, 155, 165, 167] |
| Iron metabolism | Hemosiderin ↑, transferrin ↑, transferrin receptor ↓, ferritin ↑ | [172–175, 219] |
| Proteinases | MMP-1 ↑, MMP-2 ↑, MMP-7 ↑, MMP-9 ↑ (SNPs), MMP-12 ↑, matriptase ↑ | [154, 188–194, 196] |
| Neutrophil proteases and inhibitors |
α
1-Antitrypsin | [185] |
| Chitinolytic activity | CHIT1 ↑, YKL-40 ↑ | [199, 200] |
| Recognition markers | CD31 ↓, CD44 ↓, CD91 ↓, CR-3 ↑, CR-4 ↑, DC-SIGN ↑, MARCO ↓ | [150, 219, 226] |
| Cytoskeletal rearrangements | RAC1 ↓, VAV1 ↓, RhoA ↑ | [216, 229] |
| Mitochondrial stress | MCL-1 ↑ | [230] |
| Integrins, scavenger receptors, and adhesion molecules | CD11a ↓, CD11c ↑, CD163 ↑, CD204 ↑, CD206 ↑, MSR-1 (SNPs), MERTK ↑ | [220, 227, 234, 235] |
| Antigen presentation molecules | MHC-I ↓, MHC-II ↓, HLA-DR ↓, CD80 ↓ | [150, 233] |
| Fc gamma receptors, PRRs | FcγR1 ↑, CD16 ↓, TLR2 ↓, TLR3 ↓, TLR4 ↓, TLR5 ↑, TLR9 (SNPs) | [126, 148, 150, 158, 165, 206, 233, 234, 236–238] |
4.1. Altered Secretion of Proinflammatory Mediators
AMs from patients with COPD present with alterations in the secretion of cytokines and chemokines. In particular, the levels of TNF-α, IL-1β, IL-6, IL-10, IL-12, CCL2, CCL5, CCL7, CCL13, CCL22, IL-8, CXCL9, and CXCL10 in AM secretions from smokers were significantly different from healthy subjects [126, 144–152]. Similarly, the levels of the chemokine receptors CCR2 and CCR5 were found to be increased [153, 154]. Moreover, macrophages primed with endotoxin and cigarette smoke presented with delayed IL-1β and IL-6 secretion in comparison with control endotoxin-treated cells and a subsequent increase in IL-8 levels [155]. Finally, sputum macrophages from patients with COPD were found to express more prostaglandin H synthases 1 and 2 than unaffected control subjects [156].
TLR signalling is pivotal for proinflammatory mediator secretion by macrophages in COPD as exemplified by the TLR4-dependent cigarette smoke-mediated activation of human macrophages [157]. Downstream of TLR activation, lung macrophages from patients with COPD also exhibit dysregulated signalling including p38, ERK1/2, JNK and IRAK-1 phosphorylation, IκBα expression, and NF-κB p65 activation compared to healthy individuals [145, 147, 155]. Finally, the importance of TLR signalling for macrophage proinflammatory mediator secretion in COPD is also illustrated by the downregulation of the chemokines CXCL9, CXCL10, and CXCL11 [147, 154, 158] as a result of the attenuation of TLR3 activation [158]. While all these findings are very informative, we still do not have an integrative, systemic, and causal model of the main regulatory mechanisms operative in AMs of patients with COPD.
Therefore, more light needs to be shed on the molecular programmers that drive these functional differences and conclude whether these are observed in a fraction of the AM population. To this end, microRNAs have been involved in the regulation of proinflammatory cytokine release by AMs [159], whereas recent investigation into the epigenetic networks active in macrophage populations of patients with COPD and healthy smokers revealed that the histone deacetylases HDAC2 and HDAC3 are downregulated in comparison with healthy individuals and correlate negatively with disease severity [160, 161]. Similarly, Yang et al. showed that oxidative stress induces posttranslational modifications on HDAC2 which are responsible for the loss of function of this enzyme's activity [162]. Taken together, it seems plausible to hypothesise that defects in the transcriptional and epigenetic regulation of proinflammatory genes in COPD cause dysregulated TLR signalling and effector biomolecule secretion by AMs.
4.2. Induced Oxidative Stress
Inhaled cigarette smoke and airborne pollutants induce oxidative stress in human lungs. In more detail, cigarette smoke contains approximately 4000 chemicals including oxidants which impact lung physiology [163, 164]. On the contrary, the antioxidant protein glutathione (GSH) is heavily suppressed [150, 165] in macrophages by the actions of aldehydes in cigarette smoke [166] and biomolecules are modified (e.g., protein carbonylation) [147] leading to deleterious effects on living cells.
In response, AMs from patients with COPD have been demonstrated to express the nitrite synthase gene iNOS, but less heme oxygenase 1 (HO-1) than healthy smokers [167]. As mentioned above, other inflammation-related molecules, such as the histone deacetylases HDAC2 and SIRT1, are downregulated in AMs in an oxidative stress-dependent manner [165, 168, 169]. Eventually, cigarette smoke-induced oxidative stress and subsequent downstream gene expression changes in AMs result in Bak/Bax and cytochrome c-dependent apoptosis [170] increasing the cell debris pool that needs to be removed from the lung tissue to prevent secondary inflammation.
Finally, iron metabolism is dysregulated in the lungs of patients with COPD. Iron regulatory protein 2 and hemosiderin overexpression cause cellular and mitochondrial deposition of iron in alveolar tissue and resident macrophages which is associated with neutrophilia and infective exacerbations [171, 172]. Indeed, a recent report showcased the enhanced nutrient uptake and storage in AMs from patients with COPD. Philippot et al. found that these cells present with increased transferrin and ferritin expression important for iron uptake and storage [173]. Iron-loaded AMs from smokers also secrete higher amounts of ferritin than nonsmokers [174, 175] which could catalyse oxidative stress reactions in the alveolar tissue.
It has become apparent that exacerbated oxidative stress in AMs of patients with COPD impacts on other physiological pathways. For instance, oxidation of phospholipids in AMs impairs bacterial intracellular killing in mice [176]. To date, investigation of such concepts with available analytical tools is challenging. On the contrary, whole transcriptome analysis approaches complemented by bioinformatic coexpression network analysis would allow to link the expression patterns of dysregulated oxidative stress genes to the rest of the transcriptome in order to uncover overlooked interconnected biological pathways.
4.3. Deregulation of the Protease-Protease Inhibitor Balance
COPD progression correlates with the persistent activation of AMs and changes in the balance of secreted proteases and protease inhibitors (). The importance of these molecules was illustrated in an experimental model of COPD where macrophage infiltration and the expression of proinflammatory mediators were induced in response to released mast cell-tryptases [177, 178].
ECM degradation enzymes, such as MMPs and cathepsins, are produced by macrophages and result in elastolysis and alveolar tissue damage [179–182]. Furthermore, these proteases have the potential to cleave small proteins and expose chemotactic fragments or they act as chemoattractants themselves and perpetuate macrophage accumulation in the lungs [183, 184]. On the contrary, cigarette smoking has been shown to induce the functional inactivation of α1-antitrypsin, a NE inhibitor, which leaves smokers vulnerable to lung tissue destruction [185].
Monocytes and AMs are potent producers of several proteases; MMPs including MMP-1, MMP-2, MMP-7, MMP-9, and MMP-12, and cathepsins, such as K, L, B, and S, [180, 181, 184, 186, 187] and study have documented the overexpression of MMP-1, MMP-2, MMP-7, MMP-9, and MMP-12 in the lungs of smokers compared to healthy individuals [154, 188–194].
In patients with COPD, the expression of MMP-9 by AMs was shown to coincide with that of tissue inhibitor of metalloproteinases 1. The balance of these two mediators can be detrimental for the level of tissue damage in COPD lung and is controlled by the anti-inflammatory cytokine IL-10 [195]. Additional evidence for the current consensus of protease-protease inhibitor deregulation in macrophages from patients with COPD was provided by the fact that human patients with the most common α1-antitrypsin mutation have greater proteolytic activity partially due to higher expression levels of the membrane-bound serine protease matriptase [196].
Furthermore, patients with COPD have more MMP-12-positive macrophages than healthy individuals in their lungs [193]. Macrophages are the main source of MMP-12 in the lungs of emphysematous mice [113, 182], and this MMP was shown to be important for connective tissue breakdown and neutrophil recruitment [99]. The mechanism MMP-12 utilises to promote inflammation was shown to involve the cleavage of the TNF precursor on the surface of macrophages and its release to the lung microenvironment [197].
Lastly, a perhaps not so well-documented function of AMs in COPD is their chitinolytic activity. Chitinases are released in the bronchoalveolar fluid of patients and are overexpressed by AMs from patients with COPD [198]. The presence of chitinase 1 and YKL-40, a chitin-binding protein, was found to correlate with airway obstruction and emphysema and to promote the production of proinflammatory mediators, such as cytokines, chemokines, and proteases by AMs from patients with COPD [198–200]. To date, we do not fully understand whether the upregulation in the expression of chitinases by AMs is a specific immune response against fungal opportunistic infection of patients with COPD and this warrants further investigation.
Given the significance of the protease activation pathway in irreversible tissue damage, it is necessary to understand how protease and protease inhibitor production is regulated in AMs aiming to fully characterise potentially defective molecular pathways that are responsible for the imbalance in the release of these mediators. Moreover, the literature is often contradicting with regard to the identity of protease members expressed by AMs. Currently available genomic techniques could settle the discrepancy noticed between older and more recent reports and show whether genetic polymorphisms account for the deregulation of protease-protease inhibitor imbalance in AMs.
4.4. Impaired Pathogen Phagocytosis
Due to their strategic localisation at the host-environment interface, AMs are key players in sensing microbes and irritants and initiating the phagocytosis process in order to remove and destroy them. Macrophage phagocytosis in patients with COPD has been extensively studied in humans and animal models, and our current understanding is that AMs present with a phagocytosis defect when treated with air pollutants () [201, 202].
AMs from patients with COPD and cigarette smoke-treated animals have been reported to display impaired phagocytosis of pathogens, such as H. influenzae [203–207], C. albicans [208, 209], E. coli, M. catarrhalis [206, 207], and S. pneumoniae [205, 206, 210] compared to controls. Interestingly, defective phagocytosis of latex particles has only been described for murine AMs which implies that data generated from different species should be taken with caution [211]. It is not entirely clear whether the inability of macrophages to efficiently uptake foreign material is tissue-specific or whether it is the result of a global genetic defect. For instance, in some studies, monocyte-mediated phagocytosis was comparable with that of AMs [204], whereas in others monocytes from patients with COPD demonstrated dysregulated phagocytic abilities [212], especially when the subjects were diagnosed with acute bronchopneumonia [213]. Therefore, further work is needed to determine whether the suppressed macrophage phagocytic capacity in patients with COPD is governed by lung-specific factors.
Besides phagocytosis of external stimuli, macrophages are also responsible for the clearance of accumulating apoptotic cells to avoid the release of toxic intracellular substances which can cause secondary inflammation and inhibit tissue repair [214]. This process, coined efferocytosis, has been suggested by some studies to be compromised in AMs from patients with COPD when coincubated with apoptotic neutrophils [215, 216], eosinophils [217], or epithelial cells [150, 218, 219]. Moreover, AMs from cigarette smokers upregulate the apoptotic cell removal tyrosine kinase MERTK, arguably in a compensation mechanism to restore endogenous efferocytosis levels [220]. Interestingly, macrophage efferocytosis index was reversed in AMs from animals and patients with COPD treated with native α1-antitrypsin implying a relationship between the protease-protease inhibitor balance and apoptotic cell engulfment [221]. Moreover, mechanistic data provided by a number of groups support the idea that an increased expression of genes of the sphingosine-1 phosphate system can explain the defective efferocytic responses of AMs [222–225], although it is currently unclear whether other lipid metabolism pathways also play a role.
Studies designed to provide an insight into the molecular mechanisms that account for the suppressed AM efferocytosis showed that the expression of recognition receptors, such as CD31, CD44, CD91 [219], CR-3, CR-4, FcγR1, MARCO, and DC-SIGN, was significantly changed in AMs from patients with COPD [150, 226]. However, the expression of recognition molecules was found to be similar between smokers and patients with COPD in other reports contradicting the original findings [205]. In another report, the expression of the macrophage scavenger receptor 1 in monocyte-derived macrophages was associated with genetic variants which also controlled in vitro cell adhesion and survival in culture [227]. Finally, conflicting data have been published concerning the involvement of p38, ERK1/2, PI3K, ROCK, and p65 kinases and cytoskeletal changes in AM phagocytosis in COPD [147, 228].
Recently, Richens et al. showed that Rac1 activation inhibits RhoA kinase resulting in actin rearrangement and lamellipodia protrusion [229], while Minematsu et al. confirmed that RAC1 and VAV1 kinase levels are reduced in cigarette smoke-treated macrophages [216]. Therefore, it is possible that the compromised phagocytic/efferocytic capacity of macrophages in COPD can be partially explained by impaired effector kinase signalling. Finally, Bewley et al. recently showed that the defective intracellular pathogen killing exhibited by AMs from patients with COPD is caused by a MCL-1-mediated failure to increase mitochondrial ROS production [230]. Collectively, while enormous progress has been made in understanding the molecular mechanisms of altered phagocytosis in COPD, we still do not have an integrated model of the pathophysiological changes operative in AMs in this disease.
4.5. Surface and Intracellular Marker Expression
To date, the assessment of AM surface marker expression in patients with COPD has focused on classical M1/M2 markers [231, 232], while our own work clearly indicated that this outdated classification cannot be applied to macrophages in COPD [144]. AMs from patients with COPD express less costimulatory molecules, such as the T cell activation and survival signalling molecule CD80, major histocompatibility antigens [150, 233], Fcγ receptors and integrins on their surface [234], more CD163, and carbohydrate and lipid scavenger receptors, such as CD206 and CD204 than non-COPD smokers and nonsmokers [235].
Similarly, as already indicated above, the expression of surface PRRs is modulated in patients with COPD; TLR2, TLR4, and TLR5 are expressed at lower levels in macrophages from patients with COPD [126, 148, 236, 237]. However, there is contradicting evidence regarding the regulation of TLR2 expression which suggests that more work is needed to delineate whether this PRR and subsequent downstream signalling pathways play a role in the functional differences observed between macrophages from healthy individuals and patients with COPD. In contrast to the aforementioned receptors, TLR3 expression as well as downstream effector molecules, such as IL-8 and MMP-9, are overexpressed in macrophages in COPD [238]. Furthermore, polymorphisms in certain PRRs, such as TLR9, are associated with the compromised proinflammatory mediator secretion described above [206]. Lastly, patients with COPD have more CD163+ macrophages in their lungs [239] which is most likely the consequence of lung microenvironment imprinting, as incubation of a human macrophage cell line with sputum from patients with acute exacerbation of COPD induced the expression of other anti-inflammatory genes, such as CD206 and arginase in vitro [240].
5. IMs and Monocytes in COPD
The literature has mainly focused on the role that AMs play in COPD. However, not much is known about the functions of IMs in the lung or monocytes in the blood ( and ). In mice, inhaled smoke causes an accumulation of CX3CR1+ monocytes and lung macrophages which associate with lung inflammation [241]. Monocytes infiltrate the lung and were shown to replace the dying resident macrophages [242]. In particular, CX3CR1−GR-1hi monocytes undergo a differentiation step into CX3CR1+GR-1lo cells before subsequently differentiating into lung macrophages after an inflammatory insult or the depletion of lung-resident macrophages [243]. Whether this is also the case for humans remains an open question.
Table 2
Molecules differentially expressed by monocytes or IMs from animals or patients with COPD compared to healthy controls.
| Molecule family | Encoded proteins | References |
|---|
| Cytokines | TNF-α ↓, IL-6 ↑ | [146, 245] |
| Chemokines | CCL2 ↑, IL-8 ↓ | [146, 252] |
| Chemokine receptors | CCR2 ↑ | [253] |
| Metalloproteinases | MMP-9 ↓, Mmp-12 ↑ | [146, 251] |
| Antigen presentation molecules | CD86 ↓ | [252] |
| Integrins, PRRs | CD11b ↓, CD14 ↓, CD54 ↓ | [146, 252] |
| MicroRNAs | miR-24-3p ↑, miR-93-5p ↑, miR-320a ↑, miR-320b ↑, miR1273g-3p ↓ | [254] |
Monocytes are believed to develop into lung parenchyma macrophages which in mice have been identified as CX3CR1hiCD11b+CD11chiMHC-IIhi macrophages and express TNF-α and IL-6 [244]. More evidence for the presence of monocytes in the human lung during inflammatory diseases came from the characterisation of a CD14+HLA-DR+ macrophage population in the sputum of patients with COPD capable to produce high levels of TNF-α [245]. In the lung, recruited monocytes have been shown to modulate neutrophil infiltration via the secretion of proinflammatory mediators [246].
Similar to AMs, monocyte activation in patients with COPD presents with gene expression signatures related to apoptosis, protease function, proliferation and differentiation, glycerol metabolism, and cytosolic transport as shown by a microarray study [247]. As a result of their activation state, monocytes display more prominent migration towards CCL5, CXCL1, CXCL7, or CXCR3 chemokine gradients [248, 249], production of IL-6 and CCL2, but less IL-8, MMP-9, and CD54 compared to controls [146]. In contrast, the literature on phagocytosis by monocytes from healthy individuals and patients with COPD is contradictory [205, 250]. With regard to MMP production, Pérez-Rial et al. showed that the recruited monocytes are responsible for the overall increase of macrophage numbers in a murine model of COPD [251]. Interestingly, monocyte/macrophage responses depend a lot on the causative agent of COPD as exemplified in a diesel exhaust particle-induced study where monocytes exhibited less CXCL8 and phagocytic responses due to dampened CD11b, CD14, and CD86 surface expression [252], while they overexpress CCR2 in smokers [253].
There have been various mechanistic lines of evidence to explain the augmented proinflammatory phenotype of monocytes; Dang et al., for example, found that miRNA expression, such as miR-24-3p and miR-93-5p, correlates with dysregulated downstream TLR and NOD-like receptor signalling proteins, such as IκBα [254]. On top of that, altered epigenetic cues as exemplified by the downregulation of HDAC levels cause an upregulation in proinflammatory gene expression and NF-κB-mediated inflammation [160, 255].
6. Concluding Remarks
COPD affects around 328 million people worldwide, and it is projected to rank within the top four most fatal diseases by 2030 [77, 256]. Moreover, the chronic nature of the disease and the frequently observed exacerbations and comorbidities have major consequences on patients' lives and countries' economic status [256]. It is therefore important to advance our knowledge of immune system manifestations in COPD and uncover the molecular pathways responsible for the cross talk between immune cells and the lung stroma in order to provide the clinic with prognosis/diagnosis biomarkers and the pharmaceutical industry with novel testable genes/pathways for future drug development screenings.
Already in 1979, it had been suggested that the macrophage population, which comprises of lung-resident macrophages and blood monocytes, constitutes more than 97% of all cells in the human bronchoalveolar lavage [257], while two decades later, the severity in COPD was linked to the presence of macrophages, neutrophils, NK cells, and activated epithelial cells in the lung [258]. However, due to the lack of specific markers and respective technologies at that time, no further subset specifications or functional subdivision could be performed and these studies remained incomplete. This is also true for studies which suggested correlation between COPD severity or small airway infiltration and macrophages [259–261] and reports which demonstrated less apoptosis and more proliferation in AMs from smokers [262]. Taken together, many of the findings concerning the role of certain immune cells and their relation to disease state, severity, and outcome have been obtained more than two decades ago. While still of value, these findings are challenged by very recent findings concerning cellular classification and function of immune cells in general.
With regard to lung macrophage populations, the efforts to better appreciate their role in COPD remain elusive. AMs are the only lung-resident macrophage population that has been extensively investigated in the past, whereas IMs have long been considered solely as an intermediate step in monocyte differentiation mainly due to limitations associated with their harvest from human subjects. The field is missing out on valuable information about potentially existing homogeneous macrophage subsets with distinct phenotypes associated with a pathological feature or clinical subgroup of COPD. In addition, the molecular mechanisms that dictate the functions of lung macrophage populations remain poorly characterised; for example, although there is evidence that the dysfunctions of lung macrophages in COPD are regulated epigenetically, an unbiased evaluation of the interplay between transcription factors and epigenetic networks active in lung macrophages in COPD is currently lacking.
To this end, latest advances in the fields of Immunogenomics and Systems Biology have been very encouraging and can help address these open questions (). The deconvolution of the lung macrophage structure with high-dimensional single cell technologies, such as RNA sequencing, could identify lung-resident macrophage subpopulations with unique transcriptomes that reflect the niche, activation state, or interactions with other immune cells at the time of harvest [232]. Subset-specific genes could then be associated with a COPD subgroup and be validated with mass cytometry. Such an approach could stratify COPD patient cohorts according to new biomarkers and replace currently used symptom-based readouts [263].
Future directions in COPD lung macrophage population research. Recent advances in Immunogenetics and Structural Biology make it possible to evaluate the heterogeneity of lung macrophage populations. In particular, single cell RNA sequencing can identify homogeneous macrophage subsets with distinct transcriptomes and functions. Mass cytometry can complement and validate initial findings establishing prognosis/diagnosis biomarkers for human patients with COPD. Moreover, analysis of the nuclear heterochromatin state with ATAC sequencing and subsequent validation with ChIP-sequencing can shed light on the epigenetic regulation of lung macrophage populations and highlight the molecular mechanisms responsible for their functions in vivo. Lastly, the role of AMs, IMs, and lung monocytes warrants further investigation in order to better understand the contributions of each macrophage population to COPD progression and severity. Transcriptome analysis will determine whether these populations are distinct or part of a differentiation continuum from the monocyte to the AM phenotype and will associate gene expression with unique biological processes.
Furthermore, the early discovery of HDAC downregulation in patients with COPD should be followed up by complementary assay for transposase-accessible chromatin (ATAC) sequencing to predict complex networks of histone-modifying enzymes and transcription factors that direct transcription in lung macrophages and link them to certain genes/biological functions [232]. Subsequent chromatin immunoprecipitation (ChIP) sequencing would validate these targets and lead to new hypothesis generation and potentially novel therapeutic interventions.
To conclude, there are many exciting research avenues to be followed, now supported by genetic and computational approaches made available in the last decade. The high level of macrophage plasticity in vivo implies that there are complex stimulatory and regulatory molecular circuits that act simultaneously and result in their physiological dynamics. Hence, to better understand the role lung macrophages play in COPD, we will need to take advantage of these novel tools and revisit older findings.
Acknowledgments
This work was supported by the German Research Foundation to Joachim L. Schultze (SFB704, Excellence Cluster ImmunoSensation).
Abbreviations
| AM: | Alveolar macrophage |
| ATAC: | Assay for transposase-accessible chromatin |
| COPD: | Chronic obstructive pulmonary disease |
| ChIP: | Chromatin immunoprecipitation |
| DC: | Dendritic cell |
| ECM: | Extracellular matrix |
| GSH: | Glutathione |
| HO-1: | Heme oxygenase 1 |
| IM: | Interstitial macrophage |
| MMP: | Metalloproteinase |
| NE: | Neutrophil elastase |
| PRR: | Pathogen recognition receptor |
| ROS: | Reactive oxygen species. |
Conflicts of Interest
The authors declare no conflicts of interest. Joachim L. Schultze is a member of the Excellence Cluster ImmunoSensation.
References
1.
Kopf M., Schneider C., Nobs S. P. The development and function of lung-resident macrophages and dendritic cells. Nature Immunology. 2014;16(1):36–44. 10.1038/ni.3052. [Abstract] [CrossRef] [Google Scholar]3.
Crowell R. E., Heaphy E., Valdez Y. E., Mold C., Lehnert B. E. Alveolar nd interstitial macrophage populations in the murine lung. Experimental Lung Research. 1992;18(4):435–446. 10.3109/01902149209064338. [Abstract] [CrossRef] [Google Scholar]5.
Hoppstädter J., Diesel B., Zarbock R., et al. Differential cell reaction upon Toll-like receptor 4 and 9 activation in human alveolar and lung interstitial macrophages. Respiratory Research. 2010;11(1):p. 124. 10.1186/1465-9921-11-124. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]6.
Misharin A. V., Morales-Nebreda L., Mutlu G. M., Budinger G. R. S., Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. American Journal of Respiratory Cell and Molecular Biology. 2013;49(4):503–510. 10.1165/rcmb.2013-0086MA. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]7.
Zaynagetdinov R., Sherrill T. P., Kendall P. L., et al. Identification of myeloid cell subsets in murine lungs using flow cytometry. American Journal of Respiratory Cell and Molecular Biology. 2013;49(2):180–189. 10.1165/rcmb.2012-0366MA. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]9.
Johansson A., Lundborg M., Sköld C. M., et al. Functional, morphological, and phenotypical differences between rat alveolar and interstitial macrophages. American Journal of Respiratory Cell and Molecular Biology. 1997;16(5):582–588. 10.1165/ajrcmb.16.5.9160840. [Abstract] [CrossRef] [Google Scholar]10.
Duan M., Li W. C., Vlahos R., Maxwell M. J., Anderson G. P., Hibbs M. L. Distinct macrophage subpopulations characterize acute infection and chronic inflammatory lung disease. The Journal of Immunology. 2012;189(2):946–955. 10.4049/jimmunol.1200660. [Abstract] [CrossRef] [Google Scholar]11.
Yu Y.-R. A., Hotten D. F., Malakhau Y., et al. Flow cytometric analysis of myeloid cells in human blood, bronchoalveolar lavage, and lung tissues. American Journal of Respiratory Cell and Molecular Biology. 2016;54(1):13–24. 10.1165/rcmb.2015-0146OC. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]12.
Bharat A., Bhorade S. M., Morales-Nebreda L., et al. Flow cytometry reveals similarities between lung macrophages in humans and mice. American Journal of Respiratory Cell and Molecular Biology. 2016;54(1):147–149. 10.1165/rcmb.2015-0147LE. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]13.
Desch A. N., Gibbings S. L., Goyal R., et al. Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung-draining lymph nodes. American Journal of Respiratory and Critical Care Medicine. 2016;193(6):614–626. 10.1164/rccm.201507-1376OC. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]15.
Toews G. B., Vial W. C., Dunn M. M., et al. The accessory cell function of human alveolar macrophages in specific T cell proliferation. The Journal of Immunology. 1984;132(1):181–186. [Abstract] [Google Scholar]16.
Lyons C. R., Ball E. J., Toews G. B., Weissler J. C., Stastny P., Lipscomb M. F. Inability of human alveolar macrophages to stimulate resting T cells correlates with decreased antigen-specific T cell-macrophage binding. The Journal of Immunology. 1986;137(4):1173–1180. [Abstract] [Google Scholar]17.
Franke-Ullmann G., Pförtner C., Walter P., Steinmüller C., Lohmann-Matthes M. L., Kobzik L. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. The Journal of Immunology. 1996;157(7):3097–3104. [Abstract] [Google Scholar]18.
Bedoret D., Wallemacq H., Marichal T., et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. Journal of Clinical Investigation. 2009;119(12):3723–3738. 10.1172/JCI39717. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]19.
Green G. M. The J. Burns Amberson lecture—in defense of the lung. American Review of Respiratory Disease. 1970;102(5):691–703. 10.1164/arrd.1970.102.5.691. [Abstract] [CrossRef] [Google Scholar]20.
Blumenthal R. L., Campbell D. E., Hwang P., DeKruyff R. H., Frankel L. R., Umetsu D. T. Human alveolar macrophages induce functional inactivation in antigen-specific CD4 T cells. Journal of Allergy and Clinical Immunology. 2001;107(2):258–264. 10.1067/mai.2001.112845. [Abstract] [CrossRef] [Google Scholar]21.
Thepen T., Van Rooijen N., Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. Journal of Experimental Medicine. 1989;170(2):499–509. 10.1084/jem.170.2.499. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]22.
Maus U., Koay M. A., Delbeck T., et al. Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2002;282(6):L1245–L1252. 10.1152/ajplung.00453.2001. [Abstract] [CrossRef] [Google Scholar]23.
Hashimoto S., Pittet J. F., Hong K., et al. Depletion of alveolar macrophages decreases neutrophil chemotaxis to pseudomonas airspace infections. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1996;270, 5, Part 1:L819–L828. [Abstract] [Google Scholar]24.
Beck-Schimmer B., Schwendener R., Pasch T., Reyes L., Booy C., Schimmer R. C. Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Respiratory Research. 2005;6(1):p. 61. 10.1186/1465-9921-6-61. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]25.
Hoidal J. R., Schmeling D., Peterson P. K. Phagocytosis, bacterial killing, and metabolism by purified human lung phagocytes. The Journal of Infectious Diseases. 1981;144(1):61–71. 10.1093/infdis/144.1.61. [Abstract] [CrossRef] [Google Scholar]26.
Gautier E. L., Shay T., Miller J., et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature Immunology. 2012;13(11):1118–1128. 10.1038/ni.2419. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]27.
Lagranderie M., Nahori M. A., Balazuc A. M., et al. Dendritic cells recruited to the lung shortly after intranasal delivery of Mycobacterium bovis BCG drive the primary immune response towards a type 1 cytokine production. Immunology. 2003;108(3):352–364. 10.1046/j.1365-2567.2003.01609.x. [Abstract] [CrossRef] [Google Scholar]28.
Fathi M., Johansson A., Lundborg M., Orre L., Sköld C. M., Camner P. Functional and morphological differences between human alveolar and interstitial macrophages. Experimental and Molecular Pathology. 2001;70(2):77–82. 10.1006/exmp.2000.2344. [Abstract] [CrossRef] [Google Scholar]29.
Prokhorova S., Lavnikova N., Laskin D. L. Functional characterization of interstitial macrophages and subpopulations of alveolar macrophages from rat lung. Journal of Leukocyte Biology. 1994;55(2):141–146. [Abstract] [Google Scholar]30.
Liu H.-W., Anand A., Bloch K., Christiani D., Kradin R. Expression of inducible nitric oxide synthase by macrophages in rat lung. American Journal of Respiratory and Critical Care Medicine. 1997;156(1):223–228. 10.1164/ajrccm.156.1.9609140. [Abstract] [CrossRef] [Google Scholar]31.
Spiteri M. A., Clarke S. W., Poulter L. W. Isolation of phenotypically and functionally distinct macrophage subpopulations from human bronchoalveolar lavage. European Respiratory Journal. 1992;5(6):717–726. [Abstract] [Google Scholar]32.
Chandler D. B., Fuller W. C., Jackson R. M., Fulmer J. D. Fractionation of rat alveolar macrophages by isopycnic centrifugation: morphological, cytochemical, biochemical, and functional properties. Journal of Leukocyte Biology. 1986;39(4):371–383. [Abstract] [Google Scholar]33.
Haugen T. S., Nakstad B., Lyberg T. Heterogeneity of procoagulant activity and cytokine release in subpopulations of alveolar macrophages and monocytes. Inflammation. 1999;23(1):15–23. 10.1023/A:1020283316002. [Abstract] [CrossRef] [Google Scholar]34.
Zwilling B. S., Campolito L. B., Reiches N. A. Alveolar macrophage subpopulations identified by differential centrifugation on a discontinuous albumin density gradient. American Review of Respiratory Disease. 1982;125(4):448–452. 10.1164/arrd.1982.125.4.448. [Abstract] [CrossRef] [Google Scholar]35.
Holian A., Dauber J. H., Diamond M. S., Daniele R. P. Separation of bronchoalveolar cells from the guinea pig on continuous gradients of Percoll: functional properties of fractionated lung macrophages. Journal of the Reticuloendothelial Society. 1983;33(2):157–164. [Abstract] [Google Scholar]36.
Calhoun W. J., Salisbury S. M. Heterogeneity in cell recovery and superoxide production in buoyant, density-defined subpopulations of human alveolar macrophages from healthy volunteers and sarcoidosis patients. The Journal of Laboratory and Clinical Medicine. 1989;114(6):682–690. [Abstract] [Google Scholar]37.
Shellito J., Kaltreider H. B. Heterogeneity of immunologic function among subfractions of normal rat alveolar macrophages: II. Activation as a determinant of functional activity. American Review of Respiratory Disease. 1985;131(5):678–683. 10.1164/arrd.1985.131.5.678. [Abstract] [CrossRef] [Google Scholar]38.
Shellito J., Kaltreider H. B. Heterogeneity of immunologic function among subfractions of normal rat alveolar macrophages. American Review of Respiratory Disease. 1984;129(5):747–753. 10.1164/arrd.1984.129.5.747. [Abstract] [CrossRef] [Google Scholar]39.
Murphy M. A., Herscowitz H. B. Heterogeneity among alveolar macrophages in humoral and cell-mediated immune responses: separation of functional subpopulations by density gradient centrifugation on Percoll. Journal of Leukocyte Biology. 1984;35(1):39–54. [Abstract] [Google Scholar]40.
Gant V. A., Hamblin A. S. Human bronchoalveolar macrophage heterogeneity demonstrated by histochemistry, surface markers and phagocytosis. Clinical & experimental immunology. 1985;60(3):539–545. [Abstract] [Google Scholar]41.
Sitrin R. G., Brubaker P. G., Shellito J. E., Kaltreider H. B. The distribution of procoagulant and plasminogen activator activities among density fractions of normal rabbit alveolar macrophages. American Review of Respiratory Disease. 1986;133(3):468–472. 10.1164/arrd.1986.133.3.468. [Abstract] [CrossRef] [Google Scholar]42.
Sakai K., Moriya H., Ueyama A., Kishino Y. Morphological heterogeneity among fractionated alveolar macrophages in their release of lysosomal enzymes. Cellular and Molecular Biology. 1991;37(1):85–94. [Abstract] [Google Scholar]43.
Nayak D. K., Zhou F., Xu M., et al. Long-term persistence of donor alveolar macrophages in human lung transplant recipients that influences donor-specific immune responses. American Journal of Transplantation. 2016;16(8):2300–2311. 10.1111/ajt.13819. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]44.
Eguíluz-Gracia I., Schultz H. H. L., Sikkeland L. I. B., et al. Long-term persistence of human donor alveolar macrophages in lung transplant recipients. Thorax. 2016;71(11):1006–1011. 10.1136/thoraxjnl-2016-208292. [Abstract] [CrossRef] [Google Scholar]45.
Hashimoto D., Chow A., Noizat C., et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. 10.1016/j.immuni.2013.04.004. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]46.
Maus U. A., Janzen S., Wall G., et al. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. American Journal of Respiratory Cell and Molecular Biology. 2006;35(2):227–235. 10.1165/rcmb.2005-0241OC. [Abstract] [CrossRef] [Google Scholar]47.
Tarling J. D., Lin H. S., Hsu S. Self-renewal of pulmonary alveolar macrophages: evidence from radiation chimera studies. Journal of Leukocyte Biology. 1987;42(5):443–446. [Abstract] [Google Scholar]48.
Thomas E., Ramberg R., Sale G., Sparkes R., Golde D. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science. 1976;192(4243):1016–1018. 10.1126/science.775638. [Abstract] [CrossRef] [Google Scholar]49.
Steinmüller C., Franke-Ullmann G., Lohmann-Matthes M. L., Emmendörffer A. Local activation of nonspecific defense against a respiratory model infection by application of interferon- γ: comparison between rat alveolar and interstitial lung macrophages. American Journal of Respiratory Cell and Molecular Biology. 2000;22(4):481–490. 10.1165/ajrcmb.22.4.3336. [Abstract] [CrossRef] [Google Scholar]50.
Lavnikova N., Prokhorova S., Helyar L., Laskin D. L. Isolation and partial characterization of subpopulations of alveolar macrophages, granulocytes, and highly enriched interstitial macrophages from rat lung. American Journal of Respiratory Cell and Molecular Biology. 1993;8(4):384–392. 10.1165/ajrcmb/8.4.384. [Abstract] [CrossRef] [Google Scholar]51.
Chandler D. B., Brannen A. L. Interstitial macrophage subpopulations: responsiveness to chemotactic stimuli. Tissue and Cell. 1990;22(4):427–434. 10.1016/0040-8166(90)90072-H. [Abstract] [CrossRef] [Google Scholar]52.
Chandler D. B., Bayles G., Fuller W. C. Prostaglandin synthesis and release by subpopulations of rat interstitial macrophages. American Review of Respiratory Disease. 1988;138(4):901–907. 10.1164/ajrccm/138.4.901. [Abstract] [CrossRef] [Google Scholar]53.
Chandler D. B., Kennedy J. I., Fulmer J. D. Studies of membrane receptors, phagocytosis, and morphology of subpopulations of rat lung interstitial macrophages. American Review of Respiratory Disease. 1986;134(3):542–547. 10.1164/arrd.1986.134.3.542. [Abstract] [CrossRef] [Google Scholar]54.
Holt P. G., Warner L. A., Papadimitriou J. M. Alveolar macrophages: functional heterogeneity within macrophage populations from rat lung. Australian Journal of Experimental Biology and Medical Science. 1982;60(6):607–618. 10.1038/icb.1982.63. [Abstract] [CrossRef] [Google Scholar]55.
Bilyk N., Mackenzie J. S., Papadimitriou J. M., Holt P. G. Functional studies on macrophage populations in the airways and the lung wall of SPF mice in the steady-state and during respiratory virus infection. Immunology. 1988;65(3):417–425. [Abstract] [Google Scholar]56.
Sebring R. J., Lehnert B. E. Morphometric comparisons of rat alveolar macrophages, pulmonary interstitial macrophages, and blood monocytes. Experimental Lung Research. 1992;18(4):479–496. 10.3109/01902149209064341. [Abstract] [CrossRef] [Google Scholar]57.
Warren J. S., Kunkel R. G., Johnson K. J., Ward P. A. Comparative O2-. responses of lung macrophages and blood phagocytic cells in the rat. Possible relevance to IgA immune complex induced lung injury. Laboratory Investigation. 1987;57(3):311–320. [Abstract] [Google Scholar]58.
Ziegler-Heitbrock L., Ancuta P., Crowe S., et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–e80. 10.1182/blood-2010-02-258558. [Abstract] [CrossRef] [Google Scholar]60.
Geissmann F., Jung S., Littman D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. 10.1016/S1074-7613(03)00174-2. [Abstract] [CrossRef] [Google Scholar]61.
Carlin L. M., Stamatiades E. G., Auffray C., et al.
Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153(2):362–375. 10.1016/j.cell.2013.03.010. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]62.
Auffray C., Fogg D., Garfa M., et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–670. 10.1126/science.1142883. [Abstract] [CrossRef] [Google Scholar]63.
Segura E., Touzot M., Bohineust A., et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38(2):336–348. 10.1016/j.immuni.2012.10.018. [Abstract] [CrossRef] [Google Scholar]65.
Jakubzick C. V., Randolph G. J., Henson P. M. Monocyte differentiation and antigen-presenting functions. Nature Reviews Immunology. 2017;17(6):349–362. 10.1038/nri.2017.28. [Abstract] [CrossRef] [Google Scholar]67.
Hettinger J., Richards D. M., Hansson J., et al. Origin of monocytes and macrophages in a committed progenitor. Nature Immunology. 2013;14(8):821–830. 10.1038/ni.2638. [Abstract] [CrossRef] [Google Scholar]68.
Zawada A. M., Rogacev K. S., Rotter B., et al. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118(12):e50–e61. 10.1182/blood-2011-01-326827. [Abstract] [CrossRef] [Google Scholar]69.
Belge K.-U., Dayyani F., Horelt A., et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. The Journal of Immunology. 2002;168(7):3536–3542. 10.4049/jimmunol.168.7.3536. [Abstract] [CrossRef] [Google Scholar]70.
Villani A.-C., Satija R., Reynolds G., et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356(6335, article eaah4573) 10.1126/science.aah4573. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]71.
Hogg J. C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–721. 10.1016/S0140-6736(04)16900-6. [Abstract] [CrossRef] [Google Scholar]72.
Gershon A. S., Warner L., Cascagnette P., Victor J. C., To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378(9795):991–996. 10.1016/S0140-6736(11)60990-2. [Abstract] [CrossRef] [Google Scholar]74.
Kim C., Yoo K. H., Rhee C. K., et al. Health care use and economic burden of patients with diagnosed chronic obstructive pulmonary disease in Korea. The International Journal of Tuberculosis and Lung Disease. 2014;18(6):737–743. 10.5588/ijtld.13.0634. [Abstract] [CrossRef] [Google Scholar]75.
Kelvin Teo W.-S., Tan W.-S., Chong W.-F., et al. Economic burden of chronic obstructive pulmonary disease. Respirology. 2012;17(1):120–126. 10.1111/j.1440-1843.2011.02073.x. [Abstract] [CrossRef] [Google Scholar]76.
Lou P., Zhu Y., Chen P., et al. Vulnerability, beliefs, treatments and economic burden of chronic obstructive pulmonary disease in rural areas in China: a cross-sectional study. BMC Public Health. 2012;12(1):p. 287. 10.1186/1471-2458-12-287. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]78.
Murray C. J. L., Lopez A. D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. 10.1016/S0140-6736(96)07492-2. [Abstract] [CrossRef] [Google Scholar]79.
Lopez-Campos J. L., Tan W., Soriano J. B. Global burden of COPD. Respirology. 2016;21(1):14–23. 10.1111/resp.12660. [Abstract] [CrossRef] [Google Scholar]80.
Mannino D. M., Buist A. S. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. 10.1016/S0140-6736(07)61380-4. [Abstract] [CrossRef] [Google Scholar]81.
Petrescu D., Biciusca F., Voican V., Petrescu C., Ciobanu I. C., Tudorascu D. The clinical implications of the Alpha 1- antitrypsin deficiency. Current Health Sciences Journal. 2013;39(3) [Google Scholar]82.
Mercado N., Ito K., Barnes P. J. Accelerated ageing of the lung in COPD: new concepts. Thorax. 2015;70(5):482–489. 10.1136/thoraxjnl-2014-206084. [Abstract] [CrossRef] [Google Scholar]83.
Amsellem V., Gary-Bobo G., Marcos E., et al. Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2011;184(12):1358–1366. 10.1164/rccm.201105-0802OC. [Abstract] [CrossRef] [Google Scholar]85.
Nadigel J., Préfontaine D., Baglole C. J., et al. Cigarette smoke increases TLR4 and TLR9 expression and induces cytokine production from CD8+ T cells in chronic obstructive pulmonary disease. Respiratory Research. 2011;12(1):p. 149. 10.1186/1465-9921-12-149. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]86.
Gao W., Li L., Wang Y., et al. Bronchial epithelial cells: the key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology. 2015;20(5):722–729. 10.1111/resp.12542. [Abstract] [CrossRef] [Google Scholar]87.
Doz E., Noulin N., Boichot E., et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. The Journal of Immunology. 2008;180(2):1169–1178. 10.4049/jimmunol.180.2.1169. [Abstract] [CrossRef] [Google Scholar]88.
Mio T., Romberger D. J., Thompson A. B., Robbins R. A., Heires A., Rennard S. I. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. American Journal of Respiratory and Critical Care Medicine. 1997;155(5):1770–1776. 10.1164/ajrccm.155.5.9154890. [Abstract] [CrossRef] [Google Scholar]89.
Pesci A., Balbi B., Majori M., et al. Inflammatory cells and mediators in bronchial lavage of patients with chronic obstructive pulmonary disease. European Respiratory Journal. 1998;12(2):380–386. 10.1183/09031936.98.12020380. [Abstract] [CrossRef] [Google Scholar]90.
Keatings V. M., Collins P. D., Scott D. M., Barnes P. J. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. American Journal of Respiratory and Critical Care Medicine. 1996;153(2):530–534. 10.1164/ajrccm.153.2.8564092. [Abstract] [CrossRef] [Google Scholar]91.
Corhay J.-L., Henket M., Nguyen D., Duysinx B., Sele J., Louis R. Leukotriene B4 contributes to exhaled breath condensate and sputum neutrophil chemotaxis in COPD. Chest. 2009;136(4):1047–1054. 10.1378/chest.08-2782. [Abstract] [CrossRef] [Google Scholar]92.
Traves S. L., Culpitt S. V., Russell R. E., Barnes P. J., Donnelly L. E. Increased levels of the chemokines GROα and MCP-1 in sputum samples from patients with COPD. Thorax. 2002;57(7):590–595. 10.1136/thorax.57.7.590. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]93.
Morrison D., Rahman I., Lannan S., MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. American Journal of Respiratory and Critical Care Medicine. 1999;159(2):473–479. 10.1164/ajrccm.159.2.9804080. [Abstract] [CrossRef] [Google Scholar]95.
Di Stefano A., Maestrelli P., Roggeri A., et al. Upregulation of adhesion molecules in the bronchial mucosa of subjects with chronic obstructive bronchitis. American Journal of Respiratory and Critical Care Medicine. 1994;149(3):803–810. 10.1164/ajrccm.149.3.7509705. [Abstract] [CrossRef] [Google Scholar]96.
Takizawa H., Tanaka M., Takami K., et al. Increased expression of transforming growth factor- β 1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD) American Journal of Respiratory and Critical Care Medicine. 2001;163(6):1476–1483. 10.1164/ajrccm.163.6.9908135. [Abstract] [CrossRef] [Google Scholar]97.
Stănescu D., Sanna A., Veriter C., et al. Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax. 1996;51(3):267–271. 10.1136/thx.51.3.267. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]98.
Ofulue A. F., Ko M. Effects of depletion of neutrophils or macrophages on development of cigarette smoke-induced emphysema. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1999;277, 1, Part 1:L97–L105. [Abstract] [Google Scholar]99.
Churg A., Zay K., Shay S., et al. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. American Journal of Respiratory Cell and Molecular Biology. 2002;27(3):368–374. 10.1165/rcmb.4791. [Abstract] [CrossRef] [Google Scholar]100.
Schriver E. E., Davidson J. M., Sutcliffe M. C., Swindell B. B., Bernard G. R. Comparison of elastin peptide concentrations in body fluids from healthy volunteers, smokers, and patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease. 1992;145, 4, Part 1:762–766. 10.1164/ajrccm/145.4_Pt_1.762. [Abstract] [CrossRef] [Google Scholar]102.
Hubbard R. C., Fells G., Gadek J., Pacholok S., Humes J., Crystal R. G. Neutrophil accumulation in the lung in alpha 1-antitrypsin deficiency. Spontaneous release of leukotriene B4 by alveolar macrophages. Journal of Clinical Investigation. 1991;88(3):891–897. 10.1172/JCI115391. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]103.
Demedts I. K., Bracke K. R., van Pottelberge G., et al. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2007;175(10):998–1005. 10.1164/rccm.200608-1113OC. [Abstract] [CrossRef] [Google Scholar]104.
Botelho F. M., Nikota J. K., Bauer C. M. T., et al. Cigarette smoke-induced accumulation of lung dendritic cells is interleukin-1α-dependent in mice. Respiratory Research. 2012;13(1):p. 81. 10.1186/1465-9921-13-81. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]105.
Van Pottelberge G. R., Bracke K. R., Demedts I. K., et al. Selective accumulation of langerhans-type dendritic cells in small airways of patients with COPD. Respiratory Research. 2010;11(1):p. 35. 10.1186/1465-9921-11-35. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]106.
Rogers A. V., Adelroth E., Hattotuwa K., Dewar A., Jeffery P. K. Bronchial mucosal dendritic cells in smokers and ex-smokers with COPD: an electron microscopic study. Thorax. 2007;63(2):108–114. 10.1136/thx.2007.078253. [Abstract] [CrossRef] [Google Scholar]107.
Liao S. X., Ding T., Rao X.-M., et al. Cigarette smoke affects dendritic cell maturation in the small airways of patients with chronic obstructive pulmonary disease. Molecular Medicine Reports. 2015;11(1):219–225. 10.3892/mmr.2014.2759. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]108.
Givi M. E., Akbari P., Boon L., et al. Dendritic cells inversely regulate airway inflammation in cigarette smoke-exposed mice. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2016;310(1):L95–L102. 10.1152/ajplung.00251.2014. [Abstract] [CrossRef] [Google Scholar]109.
Arellano-Orden E., Calero-Acuña C., Moreno-Mata N., et al. Cigarette smoke decreases the maturation of lung myeloid dendritic cells. PLoS One. 2016;11(4, article e0152737) 10.1371/journal.pone.0152737. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]110.
Zanini A., Spanevello A., Baraldo S., et al. Decreased maturation of dendritic cells in the central airways of COPD patients is associated with VEGF, TGF-β and vascularity. Respiration. 2014;87(3):234–242. 10.1159/000356749. [Abstract] [CrossRef] [Google Scholar]112.
Tsoumakidou M., Tousa S., Semitekolou M., et al. Tolerogenic signaling by pulmonary CD1c+ dendritic cells induces regulatory T cells in patients with chronic obstructive pulmonary disease by IL-27/IL-10/inducible costimulator ligand. Journal of Allergy and Clinical Immunology. 2014;134(4):944–954.e8. 10.1016/j.jaci.2014.05.045. [Abstract] [CrossRef] [Google Scholar]113.
Grumelli S., Corry D. B., Song L. Z., et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Medicine. 2004;1(1, article e8) 10.1371/journal.pmed.0010008. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]114.
Pridgeon C., Bugeon L., Donnelly L., et al. Regulation of IL-17 in chronic inflammation in the human lung. Clinical Science. 2011;120(12):515–524. 10.1042/CS20100417. [Abstract] [CrossRef] [Google Scholar]115.
Ponce-Gallegos M., Ramírez-Venegas A., Falfán-Valencia R. Th17 profile in COPD exacerbations. International Journal of Chronic Obstructive Pulmonary Disease. 2017;12:1857–1865. 10.2147/COPD.S136592. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]116.
Saetta M., Mariani M., Panina-Bordignon P., et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2002;165(10):1404–1409. 10.1164/rccm.2107139. [Abstract] [CrossRef] [Google Scholar]117.
Urbanowicz R. A., Lamb J. R., Todd I., Corne J. M., Fairclough L. C. Enhanced effector function of cytotoxic cells in the induced sputum of COPD patients. Respiratory Research. 2010;11(1):p. 76. 10.1186/1465-9921-11-76. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]118.
Vernooy J. H. J., Möller G. M., van Suylen R. J., et al. Increased granzyme A expression in type II pneumocytes of patients with severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2007;175(5):464–472. 10.1164/rccm.200602-169OC. [Abstract] [CrossRef] [Google Scholar]119.
Chrysofakis G., Tzanakis N., Kyriakoy D., et al. Perforin expression and cytotoxic activity of sputum CD8+ lymphocytes in patients with COPD. Chest. 2004;125(1):71–76. 10.1378/chest.125.1.71. [Abstract] [CrossRef] [Google Scholar]120.
Majo J., Ghezzo H., Cosio M. G. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. European Respiratory Journal. 2001;17(5):946–953. 10.1183/09031936.01.17509460. [Abstract] [CrossRef] [Google Scholar]121.
D’Alessio F. R., Tsushima K., Aggarwal N. R., et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. Journal of Clinical Investigation. 2009;119(10):2898–2913. 10.1172/JCI36498. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]122.
Barceló B., Pons J., Ferrer J. M., Sauleda J., Fuster A., Agustí A. G. N. Phenotypic characterisation of T-lymphocytes in COPD: abnormal CD4+CD25+ regulatory T-lymphocyte response to tobacco smoking. European Respiratory Journal. 2008;31(3):555–562. 10.1183/09031936.00010407. [Abstract] [CrossRef] [Google Scholar]123.
Di Stefano A., Caramori G., Barczyk A., et al. Innate immunity but not NLRP3 inflammasome activation correlates with severity of stable COPD. Thorax. 2014;69(6):516–524. 10.1136/thoraxjnl-2012-203062. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]124.
van der Strate B. W. A., Postma D. S., Brandsma C. A., et al. Cigarette smoke-induced emphysema: A role for the B cell? American Journal of Respiratory and Critical Care Medicine. 2006;173(7):751–758. 10.1164/rccm.200504-594OC. [Abstract] [CrossRef] [Google Scholar]125.
Polverino F., Baraldo S., Bazzan E., et al. A novel insight into adaptive immunity in chronic obstructive pulmonary disease: B cell activating factor belonging to the tumor necrosis factor family. American Journal of Respiratory and Critical Care Medicine. 2010;182(8):1011–1019. 10.1164/rccm.200911-1700OC. [Abstract] [CrossRef] [Google Scholar]126.
Berenson C. S., Kruzel R. L., Eberhardt E., et al. Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations. Thorax. 2014;69(9):811–818. 10.1136/thoraxjnl-2013-203669. [Abstract] [CrossRef] [Google Scholar]127.
Brusselle G. G., Demoor T., Bracke K. R., Brandsma C.-A., Timens W. Lymphoid follicles in (very) severe COPD: beneficial or harmful? European Respiratory Journal. 2009;34(1):219–230. 10.1183/09031936.00150208. [Abstract] [CrossRef] [Google Scholar]128.
Seemungal T. A. R., Donaldson G. C., Paul E. A., Bestall J. C., Jeffries D. J., Wedzicha J. A. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1998;157(5):1418–1422. 10.1164/ajrccm.157.5.9709032. [Abstract] [CrossRef] [Google Scholar]129.
Garcha D. S., Thurston S. J., Patel A. R. C., et al. Changes in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPD. Thorax. 2012;67(12):1075–1080. 10.1136/thoraxjnl-2012-201924. [Abstract] [CrossRef] [Google Scholar]130.
Sethi S., Evans N., Grant B. J. B., Murphy T. F. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. New England Journal of Medicine. 2002;347(7):465–471. 10.1056/NEJMoa012561. [Abstract] [CrossRef] [Google Scholar]131.
Pela R., Marchesani F., Agostinelli C., et al. Airways microbial flora in COPD patients in stable clinical conditions and during exacerbations: a bronchoscopic investigation. Monaldi Archives for Chest Disease. 1998;53(3):262–267. [Abstract] [Google Scholar]132.
Soler N., Torres A., Ewig S., et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. American Journal of Respiratory and Critical Care Medicine. 1998;157(5):1498–1505. 10.1164/ajrccm.157.5.9711044. [Abstract] [CrossRef] [Google Scholar]133.
Singh M., Lee S. H., Porter P., et al. Human rhinovirus proteinase 2A induces TH1 and TH2 immunity in patients with chronic obstructive pulmonary disease. Journal of Allergy and Clinical Immunology. 2010;125(6):1369–1378.e2. 10.1016/j.jaci.2010.02.035. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]134.
Beckham J. D., Cadena A., Lin J., et al. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. Journal of Infection. 2005;50(4):322–330. 10.1016/j.jinf.2004.07.011. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]135.
Sethi S., Mallia P., Johnston S. L. New paradigms in the pathogenesis of chronic obstructive pulmonary disease II. Proceedings of the American Thoracic Society. 2009;6(6):532–534. 10.1513/pats.200905-025DS. [Abstract] [CrossRef] [Google Scholar]136.
Kang M.-J., Lee C. G., Lee J. Y., et al. Cigarette smoke selectively enhances viral PAMP– and virus-induced pulmonary innate immune and remodeling responses in mice. Journal of Clinical Investigation. 2008;118(8):2771–2784. 10.1172/JCI32709. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]137.
Selby C., Drost E., Lannan S., Wraith P. K., Macnee W. Neutrophil retention in the lungs of patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease. 1991;143(6):1359–1364. 10.1164/ajrccm/143.6.1359. [Abstract] [CrossRef] [Google Scholar]138.
Sethi S., Wrona C., Grant B. J. B., Murphy T. F. Strain-specific immune response to Haemophilus influenzae in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2004;169(4):448–453. 10.1164/rccm.200308-1181OC. [Abstract] [CrossRef] [Google Scholar]139.
Sethi S., Maloney J., Grove L., Wrona C., Berenson C. S. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2006;173(9):991–998. 10.1164/rccm.200509-1525OC. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]140.
Fujimoto K., Yasuo M., Urushibata K., Hanaoka M., Koizumi T., Kubo K. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. European Respiratory Journal. 2005;25(4):640–646. 10.1183/09031936.05.00047504. [Abstract] [CrossRef] [Google Scholar]141.
Meshi B., Vitalis T. Z., Ionescu D., et al. Emphysematous lung destruction by cigarette smoke. The effects of latent adenoviral infection on the lung inflammatory response. American Journal of Respiratory Cell and Molecular Biology. 2002;26(1):52–57. 10.1165/ajrcmb.26.1.4253. [Abstract] [CrossRef] [Google Scholar]142.
di Stefano A., Capelli A., Lusuardi M., et al. Severity of airflow limitation is associated with severity of airway inflammation in smokers. American Journal of Respiratory and Critical Care Medicine. 1998;158(4):1277–1285. 10.1164/ajrccm.158.4.9802078. [Abstract] [CrossRef] [Google Scholar]143.
Heguy A., O’Connor T. P., Luettich K., et al. Gene expression profiling of human alveolar macrophages of phenotypically normal smokers and nonsmokers reveals a previously unrecognized subset of genes modulated by cigarette smoking. Journal of Molecular Medicine. 2006;84(4):318–328. 10.1007/s00109-005-0008-2. [Abstract] [CrossRef] [Google Scholar]145.
Chen H., Cowan M. J., Hasday J. D., Vogel S. N., Medvedev A. E. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-κB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. The Journal of Immunology. 2007;179(9):6097–6106. 10.4049/jimmunol.179.9.6097. [Abstract] [CrossRef] [Google Scholar]147.
Bozinovski S., Vlahos R., Zhang Y., et al. Carbonylation caused by cigarette smoke extract is associated with defective macrophage immunity. American Journal of Respiratory Cell and Molecular Biology. 2011;45(2):229–236. 10.1165/rcmb.2010-0272OC. [Abstract] [CrossRef] [Google Scholar]148.
Metcalfe H. J., Lea S., Hughes D., Khalaf R., Abbott-Banner K., Singh D. Effects of cigarette smoke on Toll-like receptor (TLR) activation of chronic obstructive pulmonary disease (COPD) macrophages. Clinical & Experimental Immunology. 2014;176(3):461–472. 10.1111/cei.12289. [Abstract] [CrossRef] [Google Scholar]150.
Hodge S., Matthews G., Mukaro V., et al. Cigarette smoke-induced changes to alveolar macrophage phenotype and function are improved by treatment with procysteine. American Journal of Respiratory Cell and Molecular Biology. 2011;44(5):673–681. 10.1165/rcmb.2009-0459OC. [Abstract] [CrossRef] [Google Scholar]151.
Doyle I., Ratcliffe M., Walding A., et al. Differential gene expression analysis in human monocyte-derived macrophages: impact of cigarette smoke on host defence. Molecular Immunology. 2010;47(5):1058–1065. 10.1016/j.molimm.2009.11.008. [Abstract] [CrossRef] [Google Scholar]152.
Gaschler G. J., Zavitz C. C. J., Bauer C. M. T., et al. Cigarette smoke exposure attenuates cytokine production by mouse alveolar macrophages. American Journal of Respiratory Cell and Molecular Biology. 2008;38(2):218–226. 10.1165/rcmb.2007-0053OC. [Abstract] [CrossRef] [Google Scholar]153.
de Boer W. I., Sont J. K., van Schadewijk A., Stolk J., van Krieken J. H., Hiemstra P. S. Monocyte chemoattractant protein 1, interleukin 8, and chronic airways inflammation in COPD. The Journal of Pathology. 2000;190(5):619–626. 10.1002/(SICI)1096-9896(200004)190:5<619::AID-PATH555>3.0.CO;2-6. [Abstract] [CrossRef] [Google Scholar]154.
Shaykhiev R., Krause A., Salit J., et al. Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. The Journal of Immunology. 2009;183(4):2867–2883. 10.4049/jimmunol.0900473. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]155.
Birrell M. A., Wong S., Catley M. C., Belvisi M. G. Impact of tobacco-smoke on key signaling pathways in the innate immune response in lung macrophages. Journal of Cellular Physiology. 2008;214(1):27–37. 10.1002/jcp.21158. [Abstract] [CrossRef] [Google Scholar]156.
Taha R., Olivenstein R., Utsumi T., et al. Prostaglandin H synthase 2 expression in airway cells from patients with asthma and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2000;161(2):636–640. 10.1164/ajrccm.161.2.9811063. [Abstract] [CrossRef] [Google Scholar]157.
Karimi K., Sarir H., Mortaz E., et al. Toll-like receptor-4 mediates cigarette smoke-induced cytokine production by human macrophages. Respiratory Research. 2006;7(1):p. 66. 10.1186/1465-9921-7-66. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]158.
Todt J. C., Freeman C. M., Brown J. P., et al. Smoking decreases the response of human lung macrophages to double-stranded RNA by reducing TLR3 expression. Respiratory Research. 2013;14(1):p. 33. 10.1186/1465-9921-14-33. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]159.
Yu J.-H., Long L., Luo Z.-X., Li L.-M., You J.-R. Anti-inflammatory role of microRNA let-7c in LPS treated alveolar macrophages by targeting STAT3. Asian Pacific Journal of Tropical Medicine. 2016;9(1):72–75. 10.1016/j.apjtm.2015.12.015. [Abstract] [CrossRef] [Google Scholar]160.
Tan C., Xuan L., Cao S., Yu G., Hou Q., Wang H. Decreased histone deacetylase 2 (HDAC2) in peripheral blood monocytes (PBMCs) of COPD patients. PLoS One. 2016;11(1, article e0147380) 10.1371/journal.pone.0147380. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]161.
Ito K., Ito M., Elliott W. M., et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. New England Journal of Medicine. 2005;352(19):1967–1976. 10.1056/NEJMoa041892. [Abstract] [CrossRef] [Google Scholar]162.
Yang S.-R., Chida A. S., Bauter M. R., et al. Cigarette smoke induces proinflammatory cytokine release by activation of NF-κB and posttranslational modifications of histone deacetylase in macrophages. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2006;291(1):L46–L57. 10.1152/ajplung.00241.2005. [Abstract] [CrossRef] [Google Scholar]164.
Pryor W. A., Stone K. Oxidants in cigarette smoke radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Annals of the New York Academy of Sciences. 1993;686:12–27. 10.1111/j.1749-6632.1993.tb39148.x. [Abstract] [CrossRef] [Google Scholar]165.
Sarir H., Mortaz E., Karimi K., et al. Cigarette smoke regulates the expression of TLR4 and IL-8 production by human macrophages. Journal of Inflammation. 2009;6(1):p. 12. 10.1186/1476-9255-6-12. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]166.
Müller T., Gebel S. The cellular stress response induced by aqueous extracts of cigarette smoke is critically dependent on the intracellular glutathione concentration. Carcinogenesis. 1998;19(5):797–801. 10.1093/carcin/19.5.797. [Abstract] [CrossRef] [Google Scholar]167.
Maestrelli P., Páska C., Saetta M., et al. Decreased haem oxygenase-1 and increased inducible nitric oxide synthase in the lung of severe COPD patients. European Respiratory Journal. 2003;21(6):971–6. 10.1183/09031936.03.00098203. [Abstract] [CrossRef] [Google Scholar]168.
Ito K., Lim S., Caramori G., Chung K. F., Barnes P. J., Adcock I. M. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. The FASEB Journal. 2001;15(6):1110–1112. 10.1096/fj.00-0432fje. [Abstract] [CrossRef] [Google Scholar]169.
Trocme C., Deffert C., Cachat J., et al. Macrophage-specific NOX2 contributes to the development of lung emphysema through modulation of SIRT1/MMP-9 pathways. The Journal of Pathology. 2015;235(1):65–78. 10.1002/path.4423. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]170.
Aoshiba K., Tamaoki J., Nagai A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2001;281(6):L1392–L1401. [Abstract] [Google Scholar]171.
Cloonan S. M., Mumby S., Adcock I. M., Choi A. M. K., Chung K. F., Quinlan G. J. The “iron”-y of iron overload and iron deficiency in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2017;196(9):1103–1112. 10.1164/rccm.201702-0311PP. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]172.
Mohan S., Ho T., Kjarsgaard M., et al. Hemosiderin in sputum macrophages may predict infective exacerbations of chronic obstructive pulmonary disease: a retrospective observational study. BMC Pulmonary Medicine. 2017;17(1):p. 60. 10.1186/s12890-017-0408-4. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]173.
Philippot Q., Deslée G., Adair-Kirk T. L., et al. Increased iron sequestration in alveolar macrophages in chronic obtructive pulmonary disease. PLoS One. 2014;9(5, article e96285) 10.1371/journal.pone.0096285. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]174.
Wesselius L. J., Nelson M. E., Skikne B. S. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. American Journal of Respiratory and Critical Care Medicine. 1994;150(3):690–695. 10.1164/ajrccm.150.3.8087339. [Abstract] [CrossRef] [Google Scholar]175.
Plautz M. W., Bailey K., Wesselius L. J. Influence of cigarette smoking on crocidolite-induced ferritin release by human alveolar macrophages. Journal of Laboratory and Clinical Medicine. 2000;136(6):449–456. 10.1067/mlc.2000.110905. [Abstract] [CrossRef] [Google Scholar]176.
Thimmulappa R. K., Gang X., Kim J.-H., Sussan T. E., Witztum J. L., Biswal S. Oxidized phospholipids impair pulmonary antibacterial defenses: evidence in mice exposed to cigarette smoke. Biochemical and Biophysical Research Communications. 2012;426(2):253–259. 10.1016/j.bbrc.2012.08.076. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]177.
Beckett E. L., Stevens R. L., Jarnicki A. G., et al. A new short-term mouse model of chronic obstructive pulmonary disease identifies a role for mast cell tryptase in pathogenesis. Journal of Allergy and Clinical Immunology. 2013;131(3):752–762.e7. 10.1016/j.jaci.2012.11.053. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]178.
Li H., Yang T., Ning Q., et al. Cigarette smoke extract–treated mast cells promote alveolar macrophage infiltration and polarization in experimental chronic obstructive pulmonary disease. Inhalation Toxicology. 2015;27(14):822–831. 10.3109/08958378.2015.1116644. [Abstract] [CrossRef] [Google Scholar]179.
Foronjy R., Nkyimbeng T., Wallace A., et al. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2008;294(6):L1149–L1157. 10.1152/ajplung.00481.2007. [Abstract] [CrossRef] [Google Scholar]180.
Punturieri A., Filippov S., Allen E., et al. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K–deficient human macrophages. The Journal of Experimental Medicine. 2000;192(6):789–800. 10.1084/jem.192.6.789. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]181.
Russell R. E. K., Thorley A., Culpitt S. V., et al. Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteases. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2002;283(4):L867–L873. 10.1152/ajplung.00020.2002. [Abstract] [CrossRef] [Google Scholar]182.
Hautamaki R. D., Kobayashi D. K., Senior R. M., Shapiro S. D. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277(5334):2002–2004. 10.1126/science.277.5334.2002. [Abstract] [CrossRef] [Google Scholar]183.
Houghton A. M., Quintero P. A., Perkins D. L., et al. Elastin fragments drive disease progression in a murine model of emphysema. Journal of Clinical Investigation. 2006;116(3):753–759. 10.1172/JCI25617. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]184.
Shapiro S. D. The macrophage in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1999;160(Supplement 1):S29–S32. 10.1164/ajrccm.160.supplement_1.9. [Abstract] [CrossRef] [Google Scholar]185.
Gadek J., Fells G., Crystal R. Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science. 1979;206(4424):1315–1316. 10.1126/science.316188. [Abstract] [CrossRef] [Google Scholar]186.
Reddy V. Y., Zhang Q. Y., Weiss S. J. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(9):3849–3853. 10.1073/pnas.92.9.3849. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]187.
Shi G. P., Munger J. S., Meara J. P., Rich D. H., Chapman H. A. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. The Journal of Biological Chemistry. 1992;267(11):7258–7262. [Abstract] [Google Scholar]188.
Wallace A. M., Sandford A. J., English J. C., et al. Matrix metalloproteinase expression by human alveolar macrophages in relation to emphysema. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2008;5(1):13–23. 10.1080/15412550701817789. [Abstract] [CrossRef] [Google Scholar]189.
Finlay G. A., O'Driscoll L. R., Russell K. J., et al. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. American Journal of Respiratory and Critical Care Medicine. 1997;156(1):240–247. 10.1164/ajrccm.156.1.9612018. [Abstract] [CrossRef] [Google Scholar]190.
Wallace A. M., Loy L. B., Abboud R. T., et al. Expression of matrix metalloproteinase-1 in alveolar macrophages, type II pneumocytes, and airways in smokers: relationship to lung function and emphysema. Lung. 2014;192(4):467–472. 10.1007/s00408-014-9585-6. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]191.
Segura-Valdez L., Pardo A., Gaxiola M., Uhal B. D., Becerril C., Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000;117(3):684–694. 10.1378/chest.117.3.684. [Abstract] [CrossRef] [Google Scholar]192.
Woodruff P. G., Koth L. L., Yang Y. H., et al. A distinctive alveolar macrophage activation state induced by cigarette smoking. American Journal of Respiratory and Critical Care Medicine. 2005;172(11):1383–1392. 10.1164/rccm.200505-686OC. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]193.
Molet S., Belleguic C., Lena H., et al. Increase in macrophage elastase (MMP-12) in lungs from patients with chronic obstructive pulmonary disease. Inflammation Research. 2005;54(1):31–36. 10.1007/s00011-004-1319-4. [Abstract] [CrossRef] [Google Scholar]194.
Imai K., Dalal S. S., Chen E. S., et al. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. American Journal of Respiratory and Critical Care Medicine. 2001;163(3):786–791. 10.1164/ajrccm.163.3.2001073. [Abstract] [CrossRef] [Google Scholar]195.
Lim S., Roche N., Oliver B. G., Mattos W., Barnes P. J., Chung K. F. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. American Journal of Respiratory and Critical Care Medicine. 2000;162(4):1355–1360. 10.1164/ajrccm.162.4.9910097. [Abstract] [CrossRef] [Google Scholar]196.
Krotova K., Marek G. W., Wang R. L., et al. Alpha-1 antitrypsin-deficient macrophages have increased matriptase-mediated proteolytic activity. American Journal of Respiratory Cell and Molecular Biology. 2017;57(2):238–247. 10.1165/rcmb.2016-0366OC. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]197.
Churg A., Wang R. D., Tai H., et al. Macrophage metalloelastase mediates acute cigarette smoke–induced inflammation via tumor necrosis factor-α release. American Journal of Respiratory and Critical Care Medicine. 2003;167(8):1083–1089. 10.1164/rccm.200212-1396OC. [Abstract] [CrossRef] [Google Scholar]198.
Cho S. J., Weiden M. D., Lee C. G. Chitotriosidase in the pathogenesis of inflammation, interstitial lung diseases and COPD. Allergy, Asthma & Immunology Research. 2015;7(1):14–21. 10.4168/aair.2015.7.1.14. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]199.
Létuvé S., Kozhich A., Humbles A., et al. Lung chitinolytic activity and chitotriosidase are elevated in chronic obstructive pulmonary disease and contribute to lung inflammation. The American Journal of Pathology. 2010;176(2):638–649. 10.2353/ajpath.2010.090455. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]200.
Létuvé S., Kozhich A., Arouche N., et al. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. The Journal of Immunology. 2008;181(7):5167–5173. 10.4049/jimmunol.181.7.5167. [Abstract] [CrossRef] [Google Scholar]201.
Lundborg M., Dahlén S. E., Johard U., et al. Aggregates of ultrafine particles impair phagocytosis of microorganisms by human alveolar macrophages. Environmental Research. 2006;100(2):197–204. 10.1016/j.envres.2005.08.007. [Abstract] [CrossRef] [Google Scholar]202.
Lundborg M., Johard U., Låstbom L., Gerde P., Camner P. Human alveolar macrophage phagocytic function is impaired by aggregates of ultrafine carbon particles. Environmental Research. 2001;86(3):244–253. 10.1006/enrs.2001.4269. [Abstract] [CrossRef] [Google Scholar]203.
Martí-Lliteras P., Regueiro V., Morey P., et al. Nontypeable Haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infection and Immunity. 2009;77(10):4232–4242. 10.1128/IAI.00305-09. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]204.
Berenson C. S., Garlipp M. A., Grove L. J., Maloney J., Sethi S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. The Journal of Infectious Diseases. 2006;194(10):1375–1384. 10.1086/508428. [Abstract] [CrossRef] [Google Scholar]205.
Taylor A. E., Finney-Hayward T. K., Quint J. K., et al. Defective macrophage phagocytosis of bacteria in COPD. European Respiratory Journal. 2010;35(5):1039–1047. 10.1183/09031936.00036709. [Abstract] [CrossRef] [Google Scholar]206.
Berenson C. S., Kruzel R. L., Wrona C. T., Mammen M. J., Sethi S. Impaired innate COPD alveolar macrophage responses and Toll-like receptor-9 polymorphisms. PLoS One. 2015;10(9, article e0134209) 10.1371/journal.pone.0134209. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]207.
Berenson C. S., Kruzel R. L., Eberhardt E., Sethi S. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. The Journal of Infectious Diseases. 2013;208(12):2036–2045. 10.1093/infdis/jit400. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]208.
Vecchiarelli A., Dottorini M., Puliti M., Todisco T., Cenci E., Bistoni F. Defective candidacidal activity of alveolar macrophages and peripheral blood monocytes from patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease. 1991;143, 5, Part 1:1049–1054. 10.1164/ajrccm/143.5_Pt_1.1049. [Abstract] [CrossRef] [Google Scholar]209.
Ferrara F., D’Adda D., Falchi M., Dall’Asta L. The macrophagic activity of patients affected by pneumonia or chronic obstructive pulmonary disease. International Journal of Tissue Reactions. 1996;18(4-6):109–114. [Abstract] [Google Scholar]210.
Phipps J. C., Aronoff D. M., Curtis J. L., Goel D., O’Brien E., Mancuso P. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infection and Immunity. 2010;78(3):1214–1220. 10.1128/IAI.00963-09. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]211.
Higashimoto Y., Fukuchi Y., Ishida K., et al. Effect of chronic tobacco smoke exposure on the function of alveolar macrophages in mice. Respiration. 1994;61(1):23–27. 10.1159/000196298. [Abstract] [CrossRef] [Google Scholar]212.
Prieto A., Reyes E., Bernstein E. D., et al. Defective natural killer and phagocytic activities in chronic obstructive pulmonary disease are restored by glycophosphopeptical (inmunoferón) American Journal of Respiratory and Critical Care Medicine. 2001;163(7):1578–1583. 10.1164/ajrccm.163.7.2002015. [Abstract] [CrossRef] [Google Scholar]213.
Müns G., Rubinstein I., Bergmann K. C. Phagocytosis and oxidative burst of blood phagocytes in chronic obstructive airway disease. Scandinavian Journal of Infectious Diseases. 1995;27(4):369–373. 10.3109/00365549509032733. [Abstract] [CrossRef] [Google Scholar]214.
Vandivier R. W., Henson P. M., Douglas I. S. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129(6):1673–1682. 10.1378/chest.129.6.1673. [Abstract] [CrossRef] [Google Scholar]215.
Kirkham P. A., Spooner G., Rahman I., Rossi A. G. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochemical and Biophysical Research Communications. 2004;318(1):32–37. 10.1016/j.bbrc.2004.04.003. [Abstract] [CrossRef] [Google Scholar]216.
Minematsu N., Blumental-Perry A., Shapiro S. D. Cigarette smoke inhibits engulfment of apoptotic cells by macrophages through inhibition of actin rearrangement. American Journal of Respiratory Cell and Molecular Biology. 2011;44(4):474–482. 10.1165/rcmb.2009-0463OC. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]217.
Eltboli O., Bafadhel M., Hollins F., et al. COPD exacerbation severity and frequency is associated with impaired macrophage efferocytosis of eosinophils. BMC Pulmonary Medicine. 2014;14(1):p. 112. 10.1186/1471-2466-14-112. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]218.
Hodge S., Hodge G., Scicchitano R., Reynolds P. N., Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunology & Cell Biology. 2003;81(4):289–296. 10.1046/j.1440-1711.2003.t01-1-01170.x. [Abstract] [CrossRef] [Google Scholar]219.
Hodge S., Hodge G., Ahern J., Jersmann H., Holmes M., Reynolds P. N. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. American Journal of Respiratory Cell and Molecular Biology. 2007;37(6):748–755. 10.1165/rcmb.2007-0025OC. [Abstract] [CrossRef] [Google Scholar]220.
Kazeros A., Harvey B.-G., Carolan B. J., Vanni H., Krause A., Crystal R. G. Overexpression of apoptotic cell removal receptor MERTK in alveolar macrophages of cigarette smokers. American Journal of Respiratory Cell and Molecular Biology. 2008;39(6):747–757. 10.1165/rcmb.2007-0306OC. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]221.
Serban K. A., Petrusca D. N., Mikosz A., et al. Alpha-1 antitrypsin supplementation improves alveolar macrophages efferocytosis and phagocytosis following cigarette smoke exposure. PLoS One. 2017;12(4, article e0176073) 10.1371/journal.pone.0176073. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]222.
Tran H. B., Barnawi J., Ween M., et al. Cigarette smoke inhibits efferocytosis via deregulation of sphingosine kinase signaling: reversal with exogenous S1P and the S1P analogue FTY720. Journal of Leukocyte Biology. 2016;100(1):195–202. 10.1189/jlb.3A1015-471R. [Abstract] [CrossRef] [Google Scholar]223.
Barnawi J., Jersmann H., Haberberger R., Hodge S., Meech R. Reduced DNA methylation of sphingosine-1 phosphate receptor 5 in alveolar macrophages in COPD: a potential link to failed efferocytosis. Respirology. 2017;22(2):315–321. 10.1111/resp.12949. [Abstract] [CrossRef] [Google Scholar]224.
Barnawi J., Tran H., Jersmann H., et al. Potential link between the sphingosine-1-phosphate (S1P) system and defective alveolar macrophage phagocytic function in chronic obstructive pulmonary disease (COPD) PLoS One. 2015;10(10, article e0122771) 10.1371/journal.pone.0122771. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]225.
Petrusca D. N., Gu Y., Adamowicz J. J., et al. Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. Journal of Biological Chemistry. 2010;285(51):40322–40332. 10.1074/jbc.M110.137604. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]226.
Baqir M., Chen C. Z., Martin R. J., et al. Cigarette smoke decreases MARCO expression in macrophages: implication in Mycoplasma pneumoniae infection. Respiratory Medicine. 2008;102(11):1604–1610. 10.1016/j.rmed.2008.05.002. [Abstract] [CrossRef] [Google Scholar]228.
Bewley M. A., Belchamber K. B. R., Chana K. K., et al. Differential effects of p38, MAPK, PI3K or rho kinase inhibitors on bacterial phagocytosis and efferocytosis by macrophages in COPD. PLoS One. 2016;11(9, article e0163139) 10.1371/journal.pone.0163139. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]229.
Richens T. R., Linderman D. J., Horstmann S. A., et al. Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. American Journal of Respiratory and Critical Care Medicine. 2009;179(11):1011–1021. 10.1164/rccm.200807-1148OC. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]230.
Bewley M. A., Preston J. A., Mohasin M., et al. Impaired mitochondrial microbicidal responses in chronic obstructive pulmonary disease macrophages. American Journal of Respiratory and Critical Care Medicine. 2017;196(7):845–855. 10.1164/rccm.201608-1714OC. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]232.
Ginhoux F., Schultze J. L., Murray P. J., Ochando J., Biswas S. K. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nature Immunology. 2015;17(1):34–40. 10.1038/ni.3324. [Abstract] [CrossRef] [Google Scholar]233.
Pons A. R., Noguera A., Blanquer D., Sauleda J., Pons J., Agustí A. G. Phenotypic characterisation of alveolar macrophages and peripheral blood monocytes in COPD. European Respiratory Journal. 2005;25(4):647–652. 10.1183/09031936.05.00062304. [Abstract] [CrossRef] [Google Scholar]234.
Löfdahl J. M., Wahlström J., Sköld C. M. Different inflammatory cell pattern and macrophage phenotype in chronic obstructive pulmonary disease patients, smokers and non-smokers. Clinical & Experimental Immunology. 2006;145(3):428–437. 10.1111/j.1365-2249.2006.03154.x. [Abstract] [CrossRef] [Google Scholar]235.
Kaku Y., Imaoka H., Morimatsu Y., et al. Overexpression of CD163, CD204 and CD206 on alveolar macrophages in the lungs of patients with severe chronic obstructive pulmonary disease. PLoS One. 2014;9(1, article e87400) 10.1371/journal.pone.0087400. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]236.
Droemann D., Goldmann T., Tiedje T., Zabel P., Dalhoff K., Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respiratory Research. 2005;6(1):p. 68. 10.1186/1465-9921-6-68. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]237.
Wu Y., Xu H., Li L., Yuan W., Zhang D., Huang W. Susceptibility to Aspergillus infections in rats with chronic obstructive pulmonary disease via deficiency function of alveolar macrophages and impaired activation of TLR2. Inflammation. 2016;39(4):1310–1318. 10.1007/s10753-016-0363-x. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]238.
Koarai A., Yanagisawa S., Sugiura H., et al. Cigarette smoke augments the expression and responses of Toll-like receptor 3 in human macrophages. Respirology. 2012;17(6):1018–1025. 10.1111/j.1440-1843.2012.02198.x. [Abstract] [CrossRef] [Google Scholar]239.
Kunz L. I. Z., Lapperre T. S., Snoeck-Stroband J. B., et al. Smoking status and anti-inflammatory macrophages in bronchoalveolar lavage and induced sputum in COPD. Respiratory Research. 2011;12(1):p. 34. 10.1186/1465-9921-12-34. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]240.
Gutierrez P., Closa D., Piñer R., Bulbena O., Menéndez R., Torres A. Macrophage activation in exacerbated COPD with and without community-acquired pneumonia. European Respiratory Journal. 2010;36(2):285–291. 10.1183/09031936.00118909. [Abstract] [CrossRef] [Google Scholar]241.
McComb J. G., Ranganathan M., Liu X. H., et al. CX3CL1 up-regulation is associated with recruitment of CX3CR1+ mononuclear phagocytes and T lymphocytes in the lungs during cigarette smoke-induced emphysema. The American Journal of Pathology. 2008;173(4):949–961. 10.2353/ajpath.2008.071034. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]242.
Landsman L., Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. The Journal of Immunology. 2007;179(6):3488–3494. 10.4049/jimmunol.179.6.3488. [Abstract] [CrossRef] [Google Scholar]243.
Landsman L., Varol C., Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. The Journal of Immunology. 2007;178(4):2000–2007. 10.4049/jimmunol.178.4.2000. [Abstract] [CrossRef] [Google Scholar]244.
Xiong Z., Leme A. S., Ray P., Shapiro S. D., Lee J. S. CX3CR1+ lung mononuclear phagocytes spatially confined to the interstitium produce TNF-α and IL-6 and promote cigarette smoke-induced emphysema. The Journal of Immunology. 2011;186(5):3206–3214. 10.4049/jimmunol.1003221. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]245.
Frankenberger M., Menzel M., Betz R., et al. Characterization of a population of small macrophages in induced sputum of patients with chronic obstructive pulmonary disease and healthy volunteers. Clinical & Experimental Immunology. 2004;138(3):507–516. 10.1111/j.1365-2249.2004.02637.x. [Abstract] [CrossRef] [Google Scholar]246.
Dhaliwal K., Scholefield E., Ferenbach D., et al. Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. American Journal of Respiratory and Critical Care Medicine. 2012;186(6):514–524. 10.1164/rccm.201112-2132OC. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]247.
Poliska S., Csanky E., Szanto A., et al. Chronic obstructive pulmonary disease-specific gene expression signatures of alveolar macrophages as well as peripheral blood monocytes overlap and correlate with lung function. Respiration. 2011;81(6):499–510. 10.1159/000324297. [Abstract] [CrossRef] [Google Scholar]248.
Traves S. L., Smith S. J., Barnes P. J., Donnelly L. E. Specific CXC but not CC chemokines cause elevated monocyte migration in COPD: a role for CXCR2. Journal of Leukocyte Biology. 2004;76(2):441–450. 10.1189/jlb.1003495. [Abstract] [CrossRef] [Google Scholar]249.
Costa C., Traves S. L., Tudhope S. J., et al. Enhanced monocyte migration to CXCR3 and CCR5 chemokines in COPD. European Respiratory Journal. 2016;47(4):1093–1102. 10.1183/13993003.01642-2015. [Abstract] [CrossRef] [Google Scholar]250.
Tschernig T., Rabung A., Voss M., Meier C., Bals R., Beisswenger C. Chronic inhalation of cigarette smoke reduces phagocytosis in peripheral blood leukocytes. BMC Research Notes. 2015;8(1):p. 705. 10.1186/s13104-015-1706-7. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]251.
Pérez-Rial S., del Puerto-Nevado L., Terrón-Expósito R., Girón-Martínez Á., González-Mangado N., Peces-Barba G. Role of recently migrated monocytes in cigarette smoke-induced lung inflammation in different strain of mice. PLoS One. 2013;8(9, article e72975) 10.1371/journal.pone.0072975. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]253.
Nicholson G. C., Tennant R. C., Carpenter D. C., et al. A novel flow cytometric assay of human whole blood neutrophil and monocyte CD11b levels: upregulation by chemokines is related to receptor expression, comparison with neutrophil shape change, and effects of a chemokine receptor (CXCR2) antagonist. Pulmonary Pharmacology & Therapeutics. 2007;20(1):52–59. 10.1016/j.pupt.2005.11.009. [Abstract] [CrossRef] [Google Scholar]254.
Dang X., Qu X., Wang W., et al. Bioinformatic analysis of microRNA and mRNA regulation in peripheral blood mononuclear cells of patients with chronic obstructive pulmonary disease. Respiratory Research. 2017;18(1):p. 4. 10.1186/s12931-016-0486-5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]256.
López-Campos J. L., Tan W., Soriano J. B. Global burden of COPD. Respirology. 2016;21(1):14–23. 10.1111/resp.12660. [Abstract] [CrossRef] [Google Scholar]257.
van oud Alblas A. B., van Furth R. Origin, kinetics, and characteristics of pulmonary macrophages in the normal steady state. Journal of Experimental Medicine. 1979;149(6):1504–1518. 10.1084/jem.149.6.1504. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]258.
Stefano A. d., Capelli A., Lusuardi M., et al. Severity of airflow limitation is associated with severity of airway inflammation in smokers. American Journal of Respiratory and Critical Care Medicine. 1998;158(4):1277–1285. 10.1164/ajrccm.158.4.9802078. [Abstract] [CrossRef] [Google Scholar]259.
Saetta M., di Stefano A., Maestrelli P., et al. Activated T-lymphocytes and macrophages in bronchial mucosa of subjects with chronic bronchitis. American Review of Respiratory Disease. 1993;147(2):301–306. 10.1164/ajrccm/147.2.301. [Abstract] [CrossRef] [Google Scholar]260.
Finkelstein R., Fraser R. S., Ghezzo H., Cosio M. G. Alveolar inflammation and its relation to emphysema in smokers. American Journal of Respiratory and Critical Care Medicine. 1995;152(5):1666–1672. 10.1164/ajrccm.152.5.7582312. [Abstract] [CrossRef] [Google Scholar]261.
Grashoff W. F., Sont J. K., Sterk P. J., et al. Chronic obstructive pulmonary disease: role of bronchiolar mast cells and macrophages. The American Journal of Pathology. 1997;151(6):1785–1790. [Europe PMC free article] [Abstract] [Google Scholar]262.
Tomita K., Caramori G., Lim S., et al. Increased p21CIP1/WAF1 and B cell lymphoma leukemia-xL expression and reduced apoptosis in alveolar macrophages from smokers. American Journal of Respiratory and Critical Care Medicine. 2002;166(5):724–731. 10.1164/rccm.2104010. [Abstract] [CrossRef] [Google Scholar]263.
Beyer M., Händler K., Günther P., et al. Navigating disease phenotypes–a multidimensional single-cell resolution compass leads the way. Current Opinion in Systems Biology. 2017;3:147–153. 10.1016/j.coisb.2017.05.004. [CrossRef] [Google Scholar]
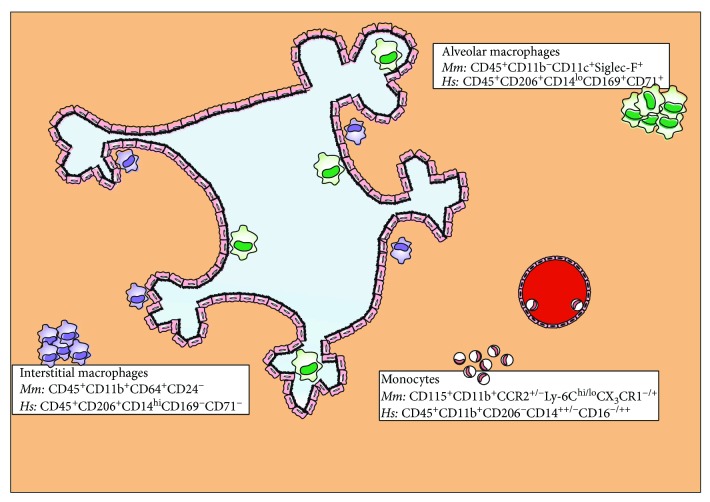
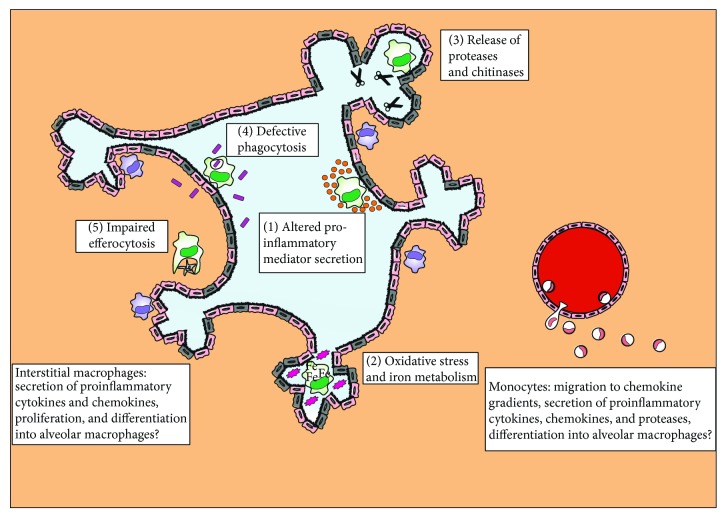
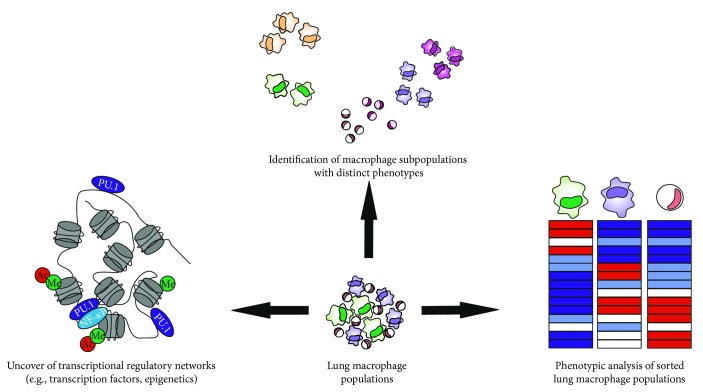

 1
1




