Abstract
Free full text

Differential distribution of the adenovirus E1A proteins and colocalization of E1A with the 70-kilodalton cellular heat shock protein in infected cells.
Abstract
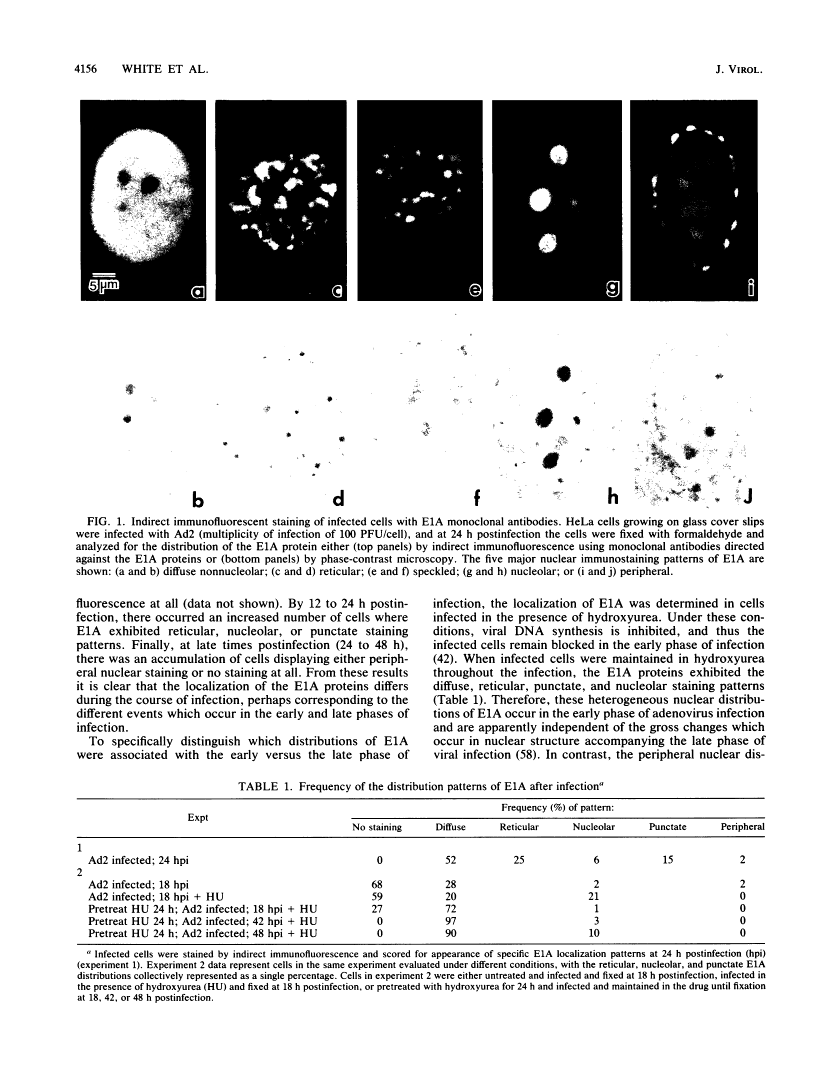
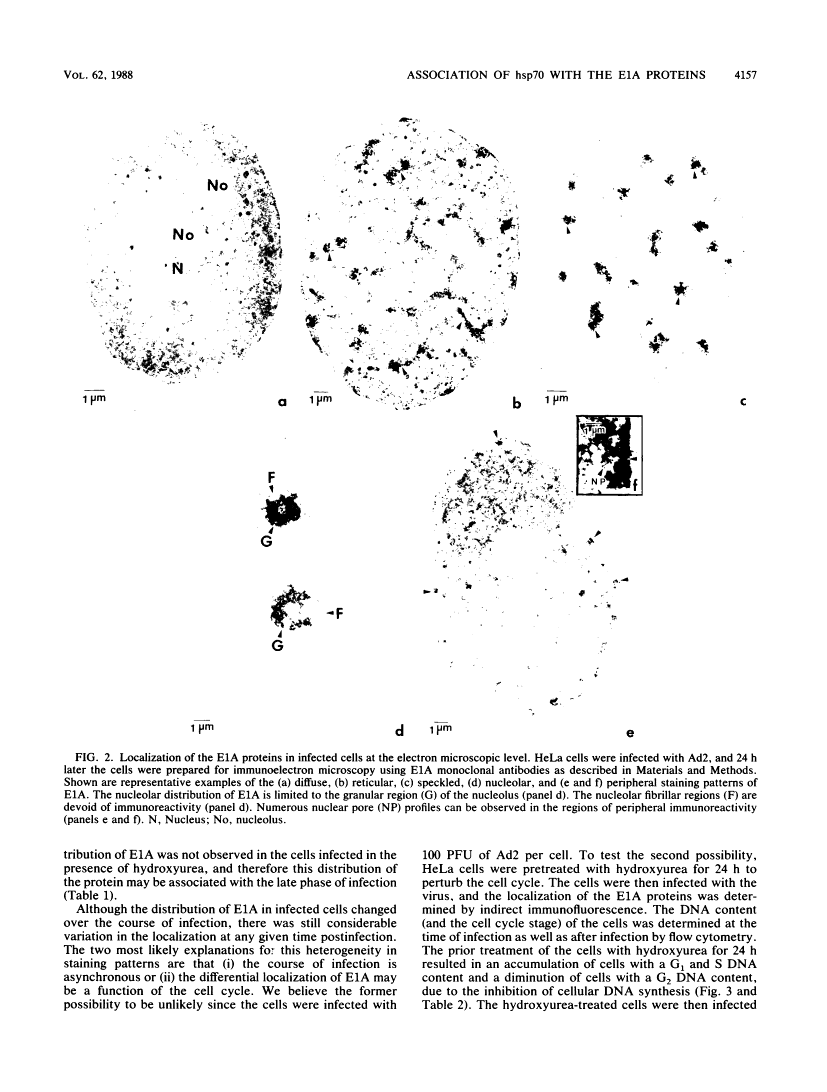
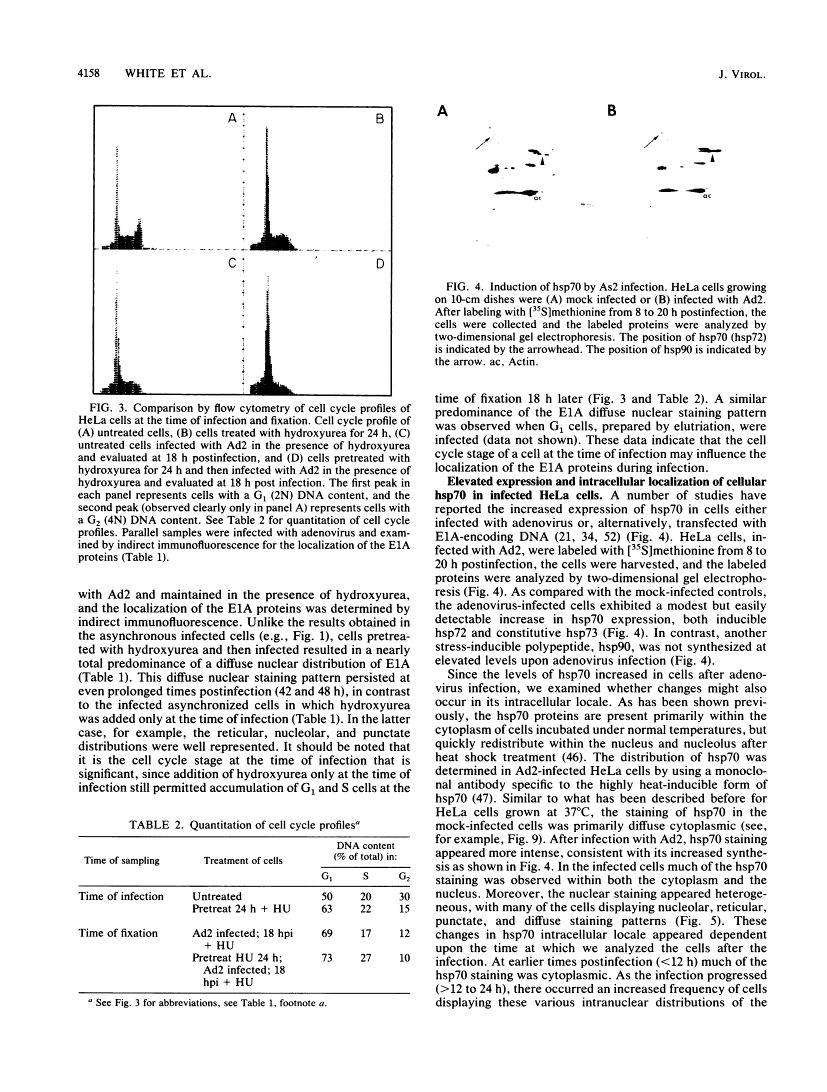
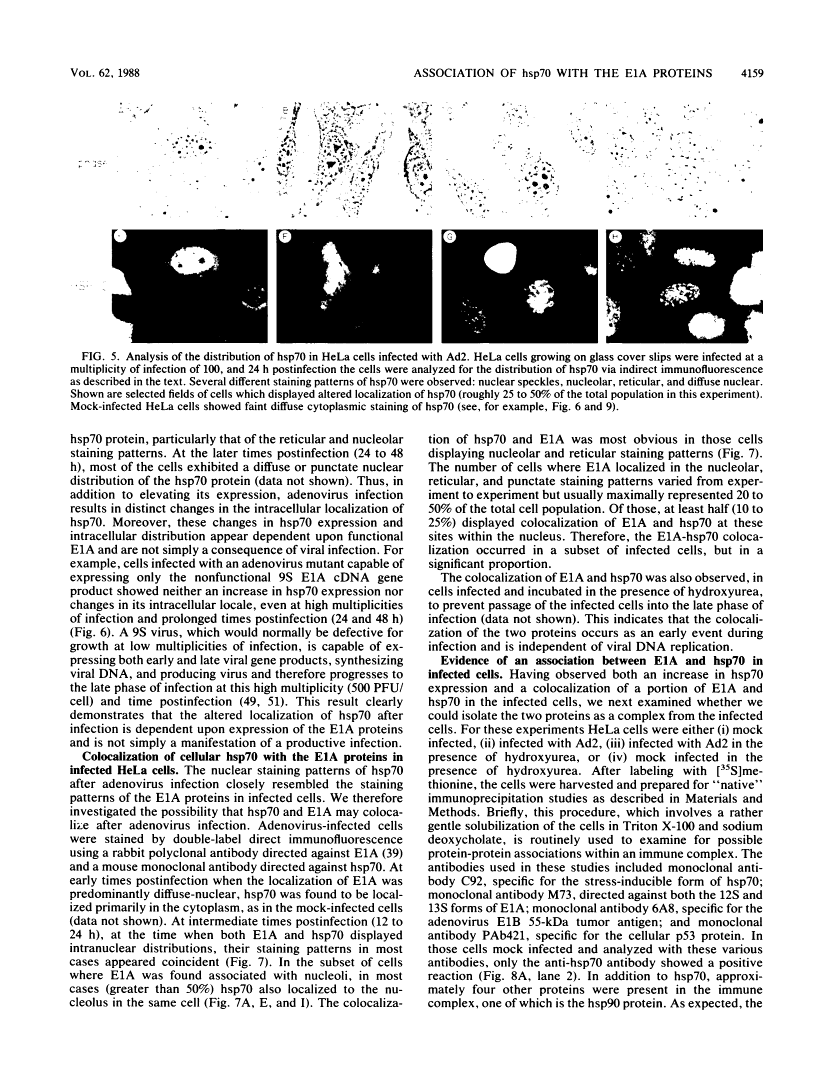
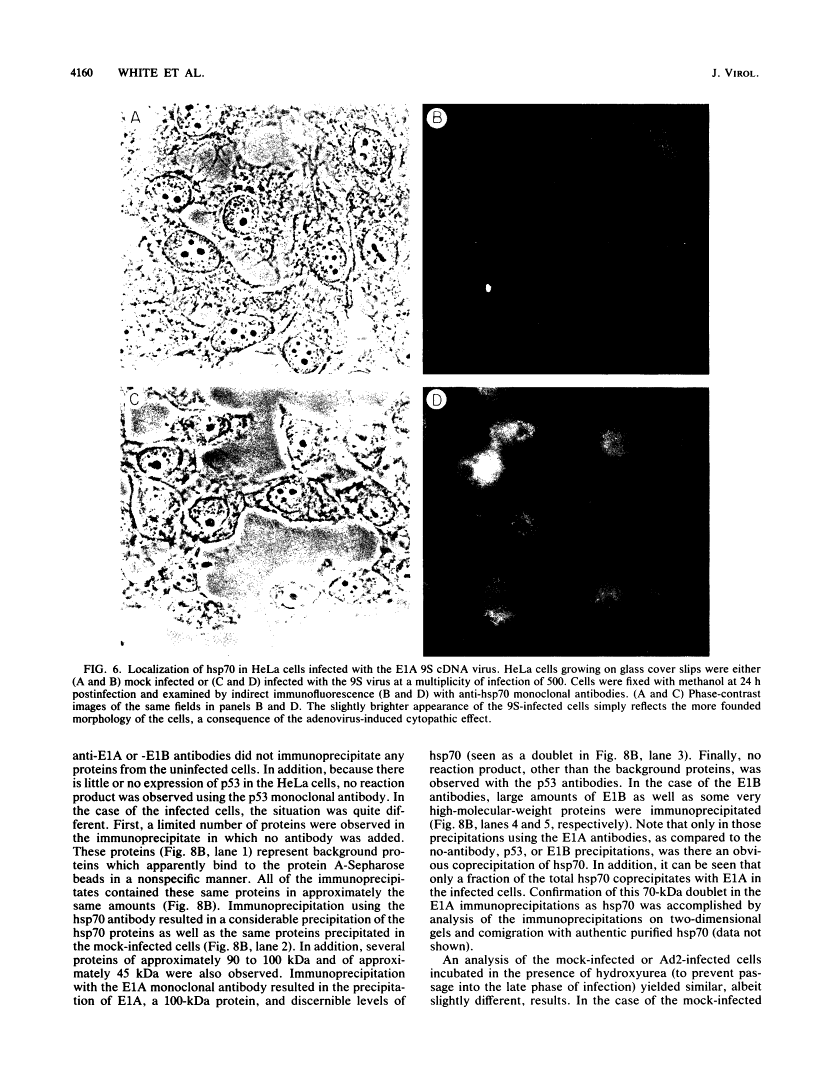
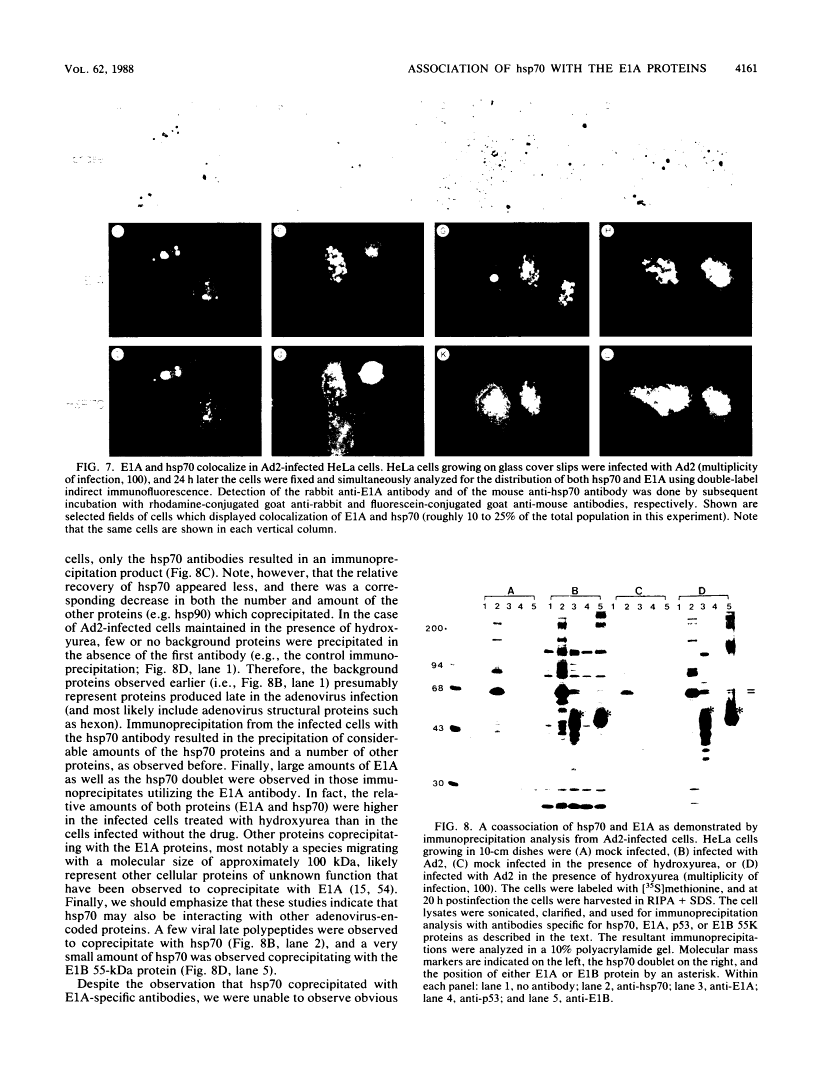
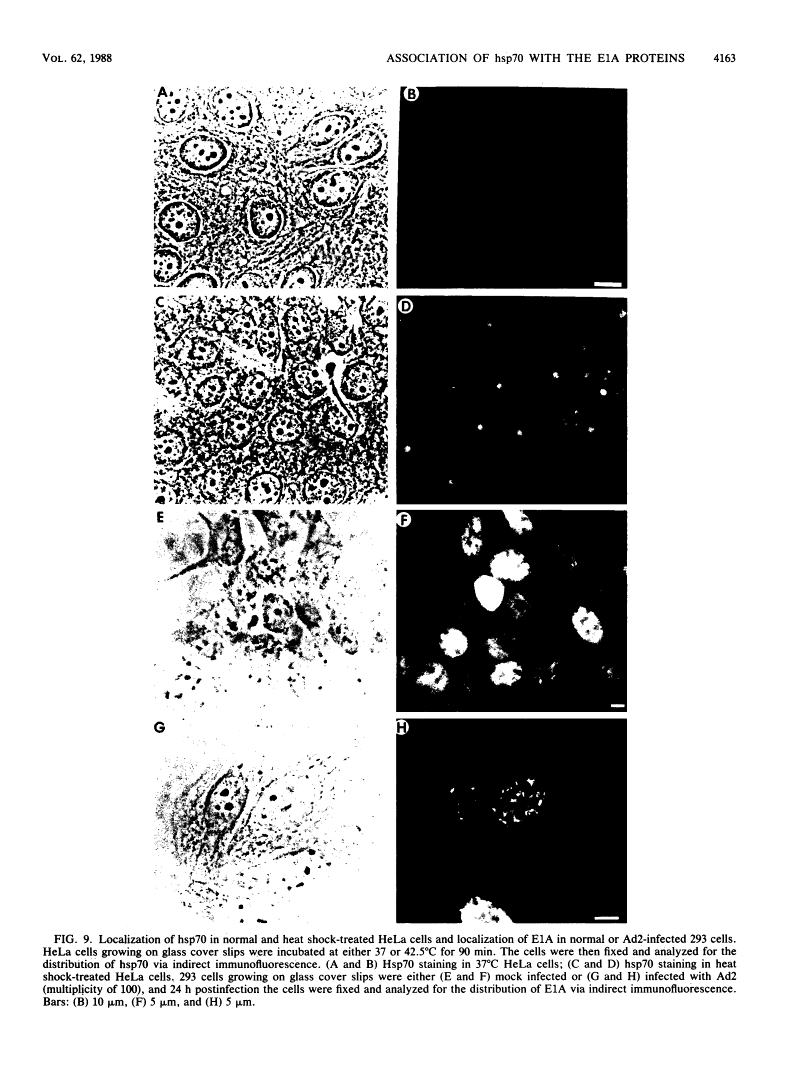
Five distinct localization patterns were observed for the adenovirus E1A proteins in the nuclei of infected HeLa cells: diffuse, reticular, nucleolar, punctate, and peripheral. The variable distribution of E1A was correlated with the time postinfection and the cell cycle stage of the host cell at the time of infection. All staining patterns, with the exception of peripheral E1A localization, were associated with the early phase of infection since only the diffuse, reticular, nucleolar, and punctate staining patterns were observed in the presence of hydroxyurea. Because the E1A proteins (12S and 13S) stimulate the expression of the cellular heat shock 70-kilodalton protein (hsp70), we examined the intracellular distribution of hsp70 in the adenovirus-infected cells. Whereas hsp70 was predominantly cytoplasmic in the cells before infection, after adenovirus infection most of the protein was now found within the nucleus. Specifically, hsp70 was found within the nucleoli as well as exhibiting reticular, diffuse, and punctate nuclear staining patterns, analogous to those observed for the E1A proteins. Double-label indirect immunofluorescence of E1A and hsp70 in infected cells demonstrated a colocalization of these proteins in the nucleus. Translocation of hsp70 to the nucleus was dependent upon both adenovirus infection and expression of the E1A proteins. The localization of hsp70 was unaltered by infection with an E1A 9S cDNA virus which does not synthesize a functional E1A gene product. Moreover, the discrete nuclear localization patterns of E1A and the colocalization of E1A with hsp70 were not observed in adenovirus-transformed 293 cells which constitutively express E1A and E1B. E1A displayed exclusively diffuse nuclear staining in 293 cells; however, localization of E1A into the discrete nuclear patterns occurred after adenovirus infection of 293 cells. Immunoprecipitation of labeled infected-cell extracts with a monoclonal antibody directed against the E1A proteins resulted in precipitation of small amounts of hsp70 along with E1A. These data indicate that the adenovirus E1A proteins colocalize with, and possibly form a physical complex with, cellular hsp70 in infected cells. The relevance of this association, with respect to the function of these proteins during infection and the association of other oncoproteins with hsp70, is discussed.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (4.9M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk AJ. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. [Abstract] [Google Scholar]
- Branton PE, Bayley ST, Graham FL. Transformation by human adenoviruses. Biochim Biophys Acta. 1985;780(1):67–94. [Abstract] [Google Scholar]
- Bardwell JC, Craig EA. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. [Europe PMC free article] [Abstract] [Google Scholar]
- Chatterjee PK, Bruner M, Flint SJ, Harter ML. DNA-binding properties of an adenovirus 289R E1A protein. EMBO J. 1988 Mar;7(3):835–841. [Europe PMC free article] [Abstract] [Google Scholar]
- Clarke CF, Cheng K, Frey AB, Stein R, Hinds PW, Levine AJ. Purification of complexes of nuclear oncogene p53 with rat and Escherichia coli heat shock proteins: in vitro dissociation of hsc70 and dnaK from murine p53 by ATP. Mol Cell Biol. 1988 Mar;8(3):1206–1215. [Europe PMC free article] [Abstract] [Google Scholar]
- Dodson M, Echols H, Wickner S, Alfano C, Mensa-Wilmot K, Gomes B, LeBowitz J, Roberts JD, McMacken R. Specialized nucleoprotein structures at the origin of replication of bacteriophage lambda: localized unwinding of duplex DNA by a six-protein reaction. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7638–7642. [Europe PMC free article] [Abstract] [Google Scholar]
- Feldman LT, Nevins JR. Localization of the adenovirus E1Aa protein, a positive-acting transcriptional factor, in infected cells infected cells. Mol Cell Biol. 1983 May;3(5):829–838. [Europe PMC free article] [Abstract] [Google Scholar]
- Ferguson B, Jones N, Richter J, Rosenberg M. Adenovirus E1a gene product expressed at high levels in Escherichia coli is functional. Science. 1984 Jun 22;224(4655):1343–1346. [Abstract] [Google Scholar]
- Flint SJ. Cellular transformation by adenoviruses. Pharmacol Ther. 1984;26(1):59–88. [Abstract] [Google Scholar]
- Gaynor RB, Tsukamoto A, Montell C, Berk AJ. Enhanced expression of adenovirus transforming proteins. J Virol. 1982 Oct;44(1):276–285. [Europe PMC free article] [Abstract] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. [Abstract] [Google Scholar]
- Haley KP, Overhauser J, Babiss LE, Ginsberg HS, Jones NC. Transformation properties of type 5 adenovirus mutants that differentially express the E1A gene products. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5734–5738. [Europe PMC free article] [Abstract] [Google Scholar]
- Harlow E, Crawford LV, Pim DC, Williamson NM. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. [Europe PMC free article] [Abstract] [Google Scholar]
- Harlow E, Franza BR, Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. [Europe PMC free article] [Abstract] [Google Scholar]
- Harlow E, Whyte P, Franza BR, Jr, Schley C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol Cell Biol. 1986 May;6(5):1579–1589. [Europe PMC free article] [Abstract] [Google Scholar]
- Hinds PW, Finlay CA, Frey AB, Levine AJ. Immunological evidence for the association of p53 with a heat shock protein, hsc70, in p53-plus-ras-transformed cell lines. Mol Cell Biol. 1987 Aug;7(8):2863–2869. [Europe PMC free article] [Abstract] [Google Scholar]
- Houweling A, van den Elsen PJ, van der Eb AJ. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. [Abstract] [Google Scholar]
- Imperiale MJ, Kao HT, Feldman LT, Nevins JR, Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. [Europe PMC free article] [Abstract] [Google Scholar]
- Itikawa H, Ryu J. Isolation and characterization of a temperature-sensitive dnaK mutant of Escherichia coli B. J Bacteriol. 1979 May;138(2):339–344. [Europe PMC free article] [Abstract] [Google Scholar]
- Kaczmarek L, Ferguson B, Rosenberg M, Baserga R. Induction of cellular DNA synthesis by purified adenovirus E1A proteins. Virology. 1986 Jul 15;152(1):1–10. [Abstract] [Google Scholar]
- Kao HT, Nevins JR. Transcriptional activation and subsequent control of the human heat shock gene during adenovirus infection. Mol Cell Biol. 1983 Nov;3(11):2058–2065. [Europe PMC free article] [Abstract] [Google Scholar]
- Kao HT, Capasso O, Heintz N, Nevins JR. Cell cycle control of the human HSP70 gene: implications for the role of a cellular E1A-like function. Mol Cell Biol. 1985 Apr;5(4):628–633. [Europe PMC free article] [Abstract] [Google Scholar]
- Khandjian EW, Türler H. Simian virus 40 and polyoma virus induce synthesis of heat shock proteins in permissive cells. Mol Cell Biol. 1983 Jan;3(1):1–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Kingston RE, Baldwin AS, Jr, Sharp PA. Regulation of heat shock protein 70 gene expression by c-myc. Nature. 1984 Nov 15;312(5991):280–282. [Abstract] [Google Scholar]
- Kingston RE, Cowie A, Morimoto RI, Gwinn KA. Binding of polyomavirus large T antigen to the human hsp70 promoter is not required for trans activation. Mol Cell Biol. 1986 Sep;6(9):3180–3190. [Europe PMC free article] [Abstract] [Google Scholar]
- Krippl B, Ferguson B, Jones N, Rosenberg M, Westphal H. Mapping of functional domains in adenovirus E1A proteins. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7480–7484. [Europe PMC free article] [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Leong K, Berk AJ. Adenovirus early region 1A protein increases the number of template molecules transcribed in cell-free extracts. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5844–5848. [Europe PMC free article] [Abstract] [Google Scholar]
- Lonberg-Holm K, Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. [Europe PMC free article] [Abstract] [Google Scholar]
- Lucher LA, Loewenstein PM, Green M. Phosphorylation in vitro of Escherichia coli-produced 235R and 266R tumor antigens encoded by human adenovirus type 12 early transformation region 1A. J Virol. 1985 Oct;56(1):183–193. [Europe PMC free article] [Abstract] [Google Scholar]
- Milarski KL, Morimoto RI. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9517–9521. [Europe PMC free article] [Abstract] [Google Scholar]
- Moran E, Grodzicker T, Roberts RJ, Mathews MB, Zerler B. Lytic and transforming functions of individual products of the adenovirus E1A gene. J Virol. 1986 Mar;57(3):765–775. [Europe PMC free article] [Abstract] [Google Scholar]
- Moran E, Mathews MB. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. [Abstract] [Google Scholar]
- Nevins JR. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. [Abstract] [Google Scholar]
- Pinhasi-Kimhi O, Michalovitz D, Ben-Zeev A, Oren M. Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature. 1986 Mar 13;320(6058):182–184. [Abstract] [Google Scholar]
- Ruley HE. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. [Abstract] [Google Scholar]
- Schmitt RC, Fahnestock ML, Lewis JB. Differential nuclear localization of the major adenovirus type 2 E1a proteins. J Virol. 1987 Feb;61(2):247–255. [Europe PMC free article] [Abstract] [Google Scholar]
- Simon MC, Kitchener K, Kao HT, Hickey E, Weber L, Voellmy R, Heintz N, Nevins JR. Selective induction of human heat shock gene transcription by the adenovirus E1A gene products, including the 12S E1A product. Mol Cell Biol. 1987 Aug;7(8):2884–2890. [Europe PMC free article] [Abstract] [Google Scholar]
- Spindler KR, Rosser DS, Berk AJ. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. [Europe PMC free article] [Abstract] [Google Scholar]
- Stabel S, Argos P, Philipson L. The release of growth arrest by microinjection of adenovirus E1A DNA. EMBO J. 1985 Sep;4(9):2329–2336. [Europe PMC free article] [Abstract] [Google Scholar]
- Stürzbecher HW, Chumakov P, Welch WJ, Jenkins JR. Mutant p53 proteins bind hsp 72/73 cellular heat shock-related proteins in SV40-transformed monkey cells. Oncogene. 1987 May;1(2):201–211. [Abstract] [Google Scholar]
- Thomas GP, Mathews MB. DNA replication and the early to late transition in adenovirus infection. Cell. 1980 Nov;22(2 Pt 2):523–533. [Abstract] [Google Scholar]
- Tsukamoto AS, Ponticelli A, Berk AJ, Gaynor RB. Genetic mapping of a major site of phosphorylation in adenovirus type 2 E1A proteins. J Virol. 1986 Jul;59(1):14–22. [Europe PMC free article] [Abstract] [Google Scholar]
- Walter G, Carbone A, Welch WJ. Medium tumor antigen of polyomavirus transformation-defective mutant NG59 is associated with 73-kilodalton heat shock protein. J Virol. 1987 Feb;61(2):405–410. [Europe PMC free article] [Abstract] [Google Scholar]
- Welch WJ, Feramisco JR. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984 Apr 10;259(7):4501–4513. [Abstract] [Google Scholar]
- Welch WJ, Mizzen LA. Characterization of the thermotolerant cell. II. Effects on the intracellular distribution of heat-shock protein 70, intermediate filaments, and small nuclear ribonucleoprotein complexes. J Cell Biol. 1988 Apr;106(4):1117–1130. [Europe PMC free article] [Abstract] [Google Scholar]
- Welch WJ, Suhan JP. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986 Nov;103(5):2035–2052. [Europe PMC free article] [Abstract] [Google Scholar]
- White E, Blose SH, Stillman BW. Nuclear envelope localization of an adenovirus tumor antigen maintains the integrity of cellular DNA. Mol Cell Biol. 1984 Dec;4(12):2865–2875. [Europe PMC free article] [Abstract] [Google Scholar]
- White E, Denton A, Stillman B. Role of the adenovirus E1B 19,000-dalton tumor antigen in regulating early gene expression. J Virol. 1988 Sep;62(9):3445–3454. [Europe PMC free article] [Abstract] [Google Scholar]
- White E, Grodzicker T, Stillman BW. Mutations in the gene encoding the adenovirus early region 1B 19,000-molecular-weight tumor antigen cause the degradation of chromosomal DNA. J Virol. 1984 Nov;52(2):410–419. [Europe PMC free article] [Abstract] [Google Scholar]
- White E, Stillman B. Expression of adenovirus E1B mutant phenotypes is dependent on the host cell and on synthesis of E1A proteins. J Virol. 1987 Feb;61(2):426–435. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu BJ, Hurst HC, Jones NC, Morimoto RI. The E1A 13S product of adenovirus 5 activates transcription of the cellular human HSP70 gene. Mol Cell Biol. 1986 Aug;6(8):2994–2999. [Europe PMC free article] [Abstract] [Google Scholar]
- Yamamoto T, McIntyre J, Sell SM, Georgopoulos C, Skowyra D, Zylicz M. Enzymology of the pre-priming steps in lambda dv DNA replication in vitro. J Biol Chem. 1987 Jun 15;262(17):7996–7999. [Abstract] [Google Scholar]
- Yee SP, Branton PE. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology. 1985 Nov;147(1):142–153. [Abstract] [Google Scholar]
- Yee SP, Rowe DT, Tremblay ML, McDermott M, Branton PE. Identification of human adenovirus early region 1 products by using antisera against synthetic peptides corresponding to the predicted carboxy termini. J Virol. 1983 Jun;46(3):1003–1013. [Europe PMC free article] [Abstract] [Google Scholar]
- Yoshinaga S, Dean N, Han M, Berk AJ. Adenovirus stimulation of transcription by RNA polymerase III: evidence for an E1A-dependent increase in transcription factor IIIC concentration. EMBO J. 1986 Feb;5(2):343–354. [Europe PMC free article] [Abstract] [Google Scholar]
- Zerler B, Moran B, Maruyama K, Moomaw J, Grodzicker T, Ruley HE. Adenovirus E1A coding sequences that enable ras and pmt oncogenes to transform cultured primary cells. Mol Cell Biol. 1986 Mar;6(3):887–899. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhai ZH, Nickerson JA, Krochmalnic G, Penman S. Alterations in nuclear matrix structure after adenovirus infection. J Virol. 1987 Apr;61(4):1007–1018. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.62.11.4153-4166.1988
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc253847?pdf=render
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/62/11/4153
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/62/11/4153
Citations & impact
Impact metrics
Article citations
Stress proteins: the biological functions in virus infection, present and challenges for target-based antiviral drug development.
Signal Transduct Target Ther, 5(1):125, 13 Jul 2020
Cited by: 63 articles | PMID: 32661235 | PMCID: PMC7356129
Review Free full text in Europe PMC
Promyelocytic leukemia protein isoform II inhibits infection by human adenovirus type 5 through effects on HSP70 and the interferon response.
J Gen Virol, 97(8):1955-1967, 23 May 2016
Cited by: 9 articles | PMID: 27217299 | PMCID: PMC5764125
Metabolism and metabolic burden by α1-antitrypsin production in human AGE1.HN cells.
Metab Eng, 16:103-114, 01 Feb 2013
Cited by: 10 articles | PMID: 23376655
Interaction of Marek's disease virus oncoprotein Meq with heat-shock protein 70 in lymphoid tumour cells.
J Gen Virol, 90(pt 9):2201-2208, 03 Jun 2009
Cited by: 17 articles | PMID: 19494050
pRB-E2F1 complexes are resistant to adenovirus E1A-mediated disruption.
J Virol, 82(9):4511-4520, 27 Feb 2008
Cited by: 17 articles | PMID: 18305049 | PMCID: PMC2293062
Go to all (40) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Selective induction of human heat shock gene transcription by the adenovirus E1A gene products, including the 12S E1A product.
Mol Cell Biol, 7(8):2884-2890, 01 Aug 1987
Cited by: 76 articles | PMID: 2959854 | PMCID: PMC367907
Role of the adenovirus E1B 19,000-dalton tumor antigen in regulating early gene expression.
J Virol, 62(9):3445-3454, 01 Sep 1988
Cited by: 36 articles | PMID: 2969984 | PMCID: PMC253469
Differential nuclear localization of the major adenovirus type 2 E1a proteins.
J Virol, 61(2):247-255, 01 Feb 1987
Cited by: 12 articles | PMID: 2949087 | PMCID: PMC253943
Lytic and transforming functions of individual products of the adenovirus E1A gene.
J Virol, 57(3):765-775, 01 Mar 1986
Cited by: 93 articles | PMID: 2936898 | PMCID: PMC252804
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: 1 P30 CA45508
Grant ID: CA13106
NIGMS NIH HHS (1)
Grant ID: GM33551