Abstract
Free full text

Bacteriophage T4 DNA topoisomerase mediates illegitimate recombination in vitro.
Abstract
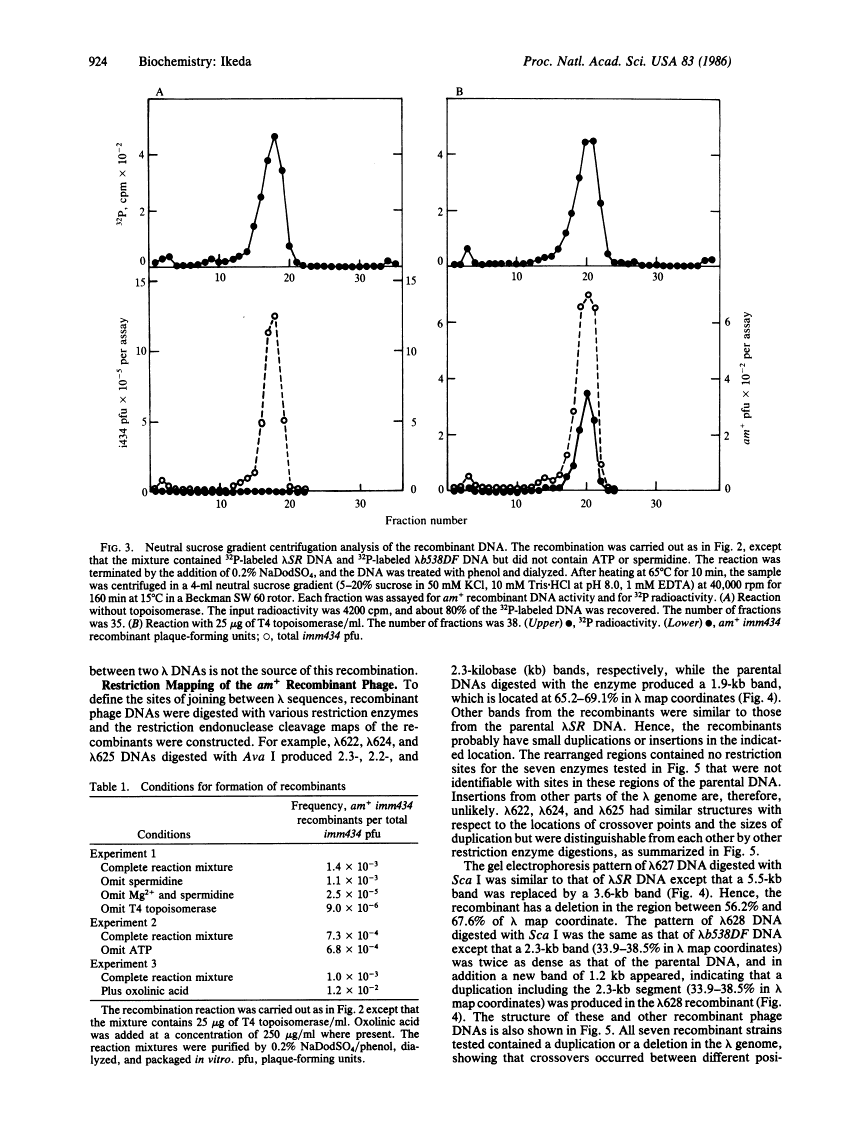
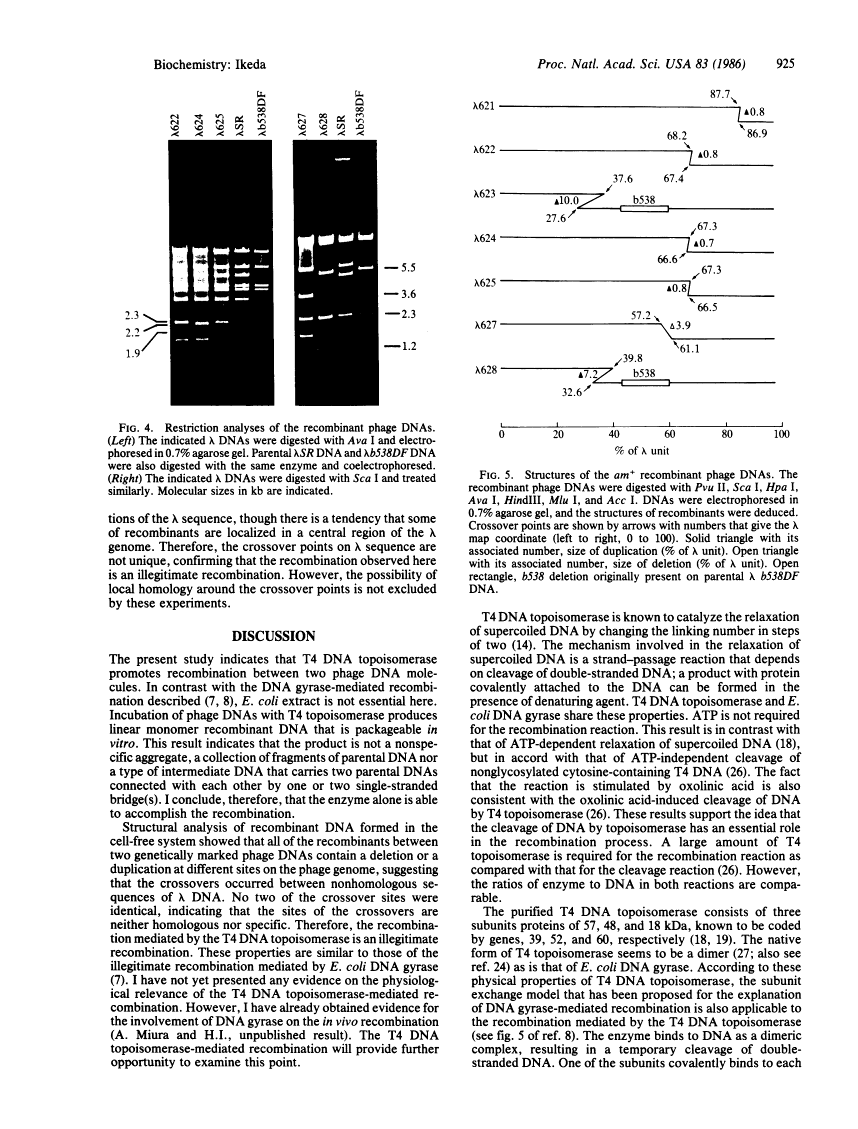
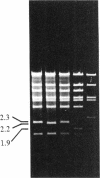
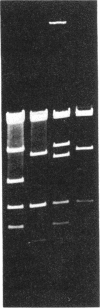
We have found that purified T4 DNA topoisomerase promotes recombination between two phage lambda DNA molecules in an in vitro system. In this cross, T4 DNA topoisomerase alone is able to catalyze the recombination and produce a linear monomer recombinant DNA that can be packaged in vitro. ATP is not required for this recombination, while oxolinic acid stimulates it. The recombinant DNA molecules contain duplications or deletions, and the crossovers take place between nonhomologous and nonspecific sequences of lambda DNA. Therefore, the recombination mediated by the T4 DNA topoisomerase is an illegitimate recombination that is similar to that mediated by Escherichia coli DNA gyrase. A model was proposed previously in which DNA gyrase molecules that are bound to DNA associate with each other and lead to the exchange of DNA strands through the exchange of DNA gyrase subunits. This model is also applicable to the recombination mediated by T4 DNA topoisomerase.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (992K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Weisberg RA, Adhya S. Illegitimate recombination in bacteria and bacteriophage. Annu Rev Genet. 1977;11:451–473. [Abstract] [Google Scholar]
- Albertini AM, Hofer M, Calos MP, Miller JH. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. [Abstract] [Google Scholar]
- Marvo SL, King SR, Jaskunas SR. Role of short regions of homology in intermolecular illegitimate recombination events. Proc Natl Acad Sci U S A. 1983 May;80(9):2452–2456. [Europe PMC free article] [Abstract] [Google Scholar]
- Franklin NC. Extraordinary recombinational events in Escherichia coli. Their independence of the rec+ function. Genetics. 1967 Apr;55(4):699–707. [Europe PMC free article] [Abstract] [Google Scholar]
- Inselburg J. Formation of deletion mutations in recombination-deficient mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1266–1267. [Europe PMC free article] [Abstract] [Google Scholar]
- Ikeda H, Moriya K, Matsumoto T. In vitro study of illegitimate recombination: involvement of DNA gyrase. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):399–408. [Abstract] [Google Scholar]
- Ikeda H, Shiozaki M. Nonhomologous recombination mediated by Escherichia coli DNA gyrase: possible involvement of DNA replication. Cold Spring Harb Symp Quant Biol. 1984;49:401–409. [Abstract] [Google Scholar]
- Ikeda H, Aoki K, Naito A. Illegitimate recombination mediated in vitro by DNA gyrase of Escherichia coli: structure of recombinant DNA molecules. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3724–3728. [Europe PMC free article] [Abstract] [Google Scholar]
- Naito A, Naito S, Ikeda H. Homology is not required for recombination mediated by DNA gyrase of Escherichia coli. Mol Gen Genet. 1984;193(2):238–243. [Abstract] [Google Scholar]
- Gellert M, Mizuuchi K, O'Dea MH, Nash HA. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. [Europe PMC free article] [Abstract] [Google Scholar]
- Mizuuchi K, Fisher LM, O'Dea MH, Gellert M. DNA gyrase action involves the introduction of transient double-strand breaks into DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1847–1851. [Europe PMC free article] [Abstract] [Google Scholar]
- Kreuzer KN, Cozzarelli NR. Formation and resolution of DNA catenanes by DNA gyrase. Cell. 1980 May;20(1):245–254. [Abstract] [Google Scholar]
- Gellert M, Mizuuchi K, O'Dea MH, Itoh T, Tomizawa JI. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. [Europe PMC free article] [Abstract] [Google Scholar]
- Sugino A, Peebles CL, Kreuzer KN, Cozzarelli NR. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. [Europe PMC free article] [Abstract] [Google Scholar]
- Ikeda H, Kawasaki I, Gellert M. Mechanism of illegitimate recombination: common sites for recombination and cleavage mediated by E. coli DNA gyrase. Mol Gen Genet. 1984;196(3):546–549. [Abstract] [Google Scholar]
- Liu LF, Liu CC, Alberts BM. T4 DNA topoisomerase: a new ATP-dependent enzyme essential for initiation of T4 bacteriophage DNA replication. Nature. 1979 Oct 11;281(5731):456–461. [Abstract] [Google Scholar]
- Stetler GL, King GJ, Huang WM. T4 DNA-delay proteins, required for specific DNA replication, form a complex that has ATP-dependent DNA topoisomerase activity. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3737–3741. [Europe PMC free article] [Abstract] [Google Scholar]
- Kobayashi I, Ikeda H. Formation of recombinant DNA of bacteriophage lambda by recA function of Escherichia coli without duplication, transcription, translation, and maturation. Mol Gen Genet. 1977 Jun 24;153(3):237–245. [Abstract] [Google Scholar]
- Sakakibara Y, Tomizawa JI. Replication of colicin E1 plasmid DNA in cell extracts. Proc Natl Acad Sci U S A. 1974 Mar;71(3):802–806. [Europe PMC free article] [Abstract] [Google Scholar]
- Wold MS, Mallory JB, Roberts JD, LeBowitz JH, McMacken R. Initiation of bacteriophage lambda DNA replication in vitro with purified lambda replication proteins. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6176–6180. [Europe PMC free article] [Abstract] [Google Scholar]
- Sternberg N, Tiemeier D, Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. [Abstract] [Google Scholar]
- Kreuzer KN, Jongeneel CV. Escherichia coli phage T4 topoisomerase. Methods Enzymol. 1983;100:144–160. [Abstract] [Google Scholar]
- Ikeda H, Matsumoto T. Transcription promotes recA-independent recombination mediated by DNA-dependent RNA polymerase in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4571–4575. [Europe PMC free article] [Abstract] [Google Scholar]
- Kreuzer KN, Alberts BM. Site-specific recognition of bacteriophage T4 DNA by T4 type II DNA topoisomerase and Escherichia coli DNA gyrase. J Biol Chem. 1984 Apr 25;259(8):5339–5346. [Abstract] [Google Scholar]
- Seasholtz AF, Greenberg GR. Identification of bacteriophage T4 gene 60 product and a role for this protein in DNA topoisomerase. J Biol Chem. 1983 Jan 25;258(2):1221–1226. [Abstract] [Google Scholar]
- Botchan M, Stringer J, Mitchison T, Sambrook J. Integration and excision of SV40 DNA from the chromosome of a transformed cell. Cell. 1980 May;20(1):143–152. [Abstract] [Google Scholar]
- Razzaque A, Mizusawa H, Seidman MM. Rearrangement and mutagenesis of a shuttle vector plasmid after passage in mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3010–3014. [Europe PMC free article] [Abstract] [Google Scholar]
- Razzaque A, Mizusawa H, Seidman MM. Rearrangement and mutagenesis of a shuttle vector plasmid after passage in mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3010–3014. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.83.4.922
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc322982?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Structural and functional insights into the T-even type bacteriophage topoisomerase II.
Nat Commun, 15(1):8719, 08 Oct 2024
Cited by: 0 articles | PMID: 39379365 | PMCID: PMC11461880
The ancestral role of ATP hydrolysis in type II topoisomerases: prevention of DNA double-strand breaks.
Nucleic Acids Res, 39(15):6327-6339, 27 Apr 2011
Cited by: 49 articles | PMID: 21525132 | PMCID: PMC3159449
Review Free full text in Europe PMC
Four different integrative recombination events involved in the mobilization of the gonococcal 5.2 kb beta-lactamase plasmid pSJ5.2 in Escherichia coli.
Plasmid, 60(3):200-211, 26 Sep 2008
Cited by: 1 article | PMID: 18778732 | PMCID: PMC2615557
Deletion hotspot in the argininosuccinate lyase gene: association with topoisomerase II and DNA polymerase alpha sites.
Hum Mutat, 27(11):1065-1071, 01 Nov 2006
Cited by: 6 articles | PMID: 16941645
Bacteriophage T4, a model system for understanding the mechanism of type II topoisomerase inhibitors.
Biochim Biophys Acta, 1400(1-3):339-347, 01 Oct 1998
Cited by: 23 articles | PMID: 9748648
Review
Go to all (30) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Illegitimate recombination mediated by calf thymus DNA topoisomerase II in vitro.
Proc Natl Acad Sci U S A, 85(7):2076-2080, 01 Apr 1988
Cited by: 82 articles | PMID: 2832845 | PMCID: PMC279931
Common sites for recombination and cleavage mediated by bacteriophage T4 DNA topoisomerase in vitro.
J Biol Chem, 264(22):12785-12790, 01 Aug 1989
Cited by: 16 articles | PMID: 2546934
Illegitimate recombination mediated in vitro by DNA gyrase of Escherichia coli: structure of recombinant DNA molecules.
Proc Natl Acad Sci U S A, 79(12):3724-3728, 01 Jun 1982
Cited by: 69 articles | PMID: 6285361 | PMCID: PMC346499