Abstract
Free full text

Loss of CXCR4 in Myeloid Cells Enhances Antitumor Immunity and Reduces Melanoma Growth through NK Cell and FASL Mechanisms
Associated Data
Abstract
The chemokine receptor, CXCR4, is involved in cancer growth, invasion, and metastasis. Several promising CXCR4 antagonists have been shown to halt tumor metastasis in preclinical studies, and clinical trials evaluating the effectiveness of these agents in cancer patients are ongoing. However, the impact of targeting CXCR4 specifically on immune cells is not clear. Here we demonstrate that genetic deletion of CXCR4 in myeloid cells (CXCR4MyeΔ/Δ) enhances the antitumor immune response, resulting in significantly reduced melanoma tumor growth. Moreover, CXCR4MyeΔ/Δ mice exhibited slowed tumor progression compared to CXCR4WT mice in an inducible melanocyte BrafV600E/Pten−/− mouse model. The percentage of Fas ligand (FasL)-expressing myeloid cells was reduced in CXCR4MyeΔ/Δ mice as compared to myeloid cells from CXCR4WT mice. In contrast, there was an increased percentage of NK cells expressing FasL in tumors growing in CXCR4MyeΔ/Δ mice. NK cells from CXCR4MyeΔ/Δ mice also exhibited increased tumor cell killing capacity in vivo, based on clearance of NK-sensitive Yac-1 cells. NK cell–mediated killing of Yac-1 cells occurred in a FasL-dependent manner, which was partially dependent upon the presence of CXCR4MyeΔ/Δ neutrophils. Furthermore, enhanced NK cell activity in CXCR4MyeΔ/Δ mice was also associated with increased production of IL18 by specific leukocyte sub-populations. These data suggest that CXCR4-mediated signals from myeloid cells suppress NK cell–mediated tumor surveillance, and thereby enhance tumor growth. Systemic delivery of a peptide antagonist of CXCR4 to tumor bearing CXCR4WT mice resulted in enhanced NK-cell activation and reduced tumor growth, supporting potential clinical implications for CXCR4 antagonism in some cancers.
Introduction
Chemokine receptor 4 (CXCR4) is a 7-transmembrane G protein-coupled receptor that interacts with its endogenous ligand CXCL12, also known as stromal cell-derived factor-1 (SDF-1), regulates many key physiological processes (1). However, CXCR4 is also highly expressed in more than 23 human cancers, where it has been reported to be expressed by tumor cells as well as stromal cells, enabling it to promote tumorigenesis, progression, metastasis and influence relapse, and prognosis (2). CXCR4 antagonism has been shown to disrupt tumor–stromal interactions, reduce tumor growth and metastatic burden and even overcome cancer cell resistance to cytotoxic drugs (3). CXCL12-based peptides and CXCR4-based small-molecule antagonists (4, 5) are in phase I/II clinical trials in patients with advanced solid tumors. The CXCR4/CXCL12 axis is not only a therapeutic target on tumor cells, but also is involved in inflammation and immunity in the tumor microenvironment (6). However, the impact of systemic targeting of CXCR4 on the immune cells has not been clearly elucidated.
In this study, we used genetic knockout of CXCR4 in myeloid cells to demonstrate that disruption of CXCR4/CXCL12 signaling in these cells inhibits the outgrowth of circulating B16 melanoma cells in the lung and inhibits tumor growth in an inducible BrafV600E/Pten null melanoma mouse model. We illustrate that loss of expression of CXCR4 in myeloid cells results in enhanced expression of cytokine IL18 that activates NK cells and enhances antitumor immunity. The CXCR4 peptide antagonist, LY2510924, also enhances antitumor activity in part by activating NK cells. Together our data provide new insight into the mechanism by which CXCR4 antagonism inhibits tumor growth.
Materials and Methods
Cell lines and Establishment of Tumor Models:
PyMT breast cancer cells were provided by the Hal Moses laboratory. This cell line was established from a spontaneous PyMT tumor growing in C57BL/6 mice. The cells have been previously characterized(7–9). The YAC1 cells were obtained within the last year from ATTC. The B16F0 cells were also obtained from ATTC, expanded, aliquoted and frozen in liquid nitrogen. Aliquots were used in the experiments here. The PYMT cells were obtained from Harold L Moses at passage 3, expanded, frozen back and passage 4–5 cells were used for experiments here. All cell cultures were mycoplasma free. Cultures are tested monthly for mycoplasma using a sensitive PCR technique (e-MycoTM Plus, LiliF Diagnostics). Any mycoplasma positive cultures are discarded. We did not genetically re-authenticate the cell lines, but we verified the cytological and histological authenticity of the cells in culture and in mouse models.
PyMT breast cancer cells (1×106 ) derived from C57BL/6 mice were intravenously injected into C57BL/6 CXCR4myeΔ/Δ or littermate control CXCR4WT mice. B16F0 melanoma cells were obtained from ATCC. B16F0 melanoma cells (1×106) were intravenously injected into CXCR4myeΔ/Δ mice (11mice/group) or littermate CXCR4WT mice (9 mice/group).
An inducible melanoma mouse model was established by breeding mice such that alleles of brafCA, Tyr::CreER and ptenlox4−5/Lox4−5 are all present in each mouse. Subsequently Cre-mediated conversion of brafV600 to brafV600E and the deletion of exons 4 and 5 of pten were induced with topical administration of 4-hydroxytamoxifen (4-HT)(10). These mice received a transplant of bone marrow cells (1×106) from CXCR4myeΔ/Δ or CXCR4WT mice and were subsequently treated with 5 mM 4-HT to induce melanoma formation, followed by bone marrow reconstitution.
Myeloid CXCR4 knockout models
All animal experiments were approved by the Vanderbilt University Institutional Animal Care and Use Committee. To delete Cxcr4 in myeloid cells in C57BL/6 mice, the gene encoding Cre-recombinase under the control of the murine LyzM gene regulatory region (11) was introduced into CXCR4f/f mice obtained from Jackson Laboratories. The resulting mice were then bred to mice harboring the loxP-flanked tdTomato (mT) following the EGFP (mG) cassette, which was inserted into the Gt(ROSA)26Sor locus (Jackson Laboratories). These mT/mG mice served as a Cre-reporter strain and after Cre-mediated recombination myeloid cells that are CXCR4-null are green. Mice with CXCR4-null myeloid cells are designated as CXCR4myeΔ/Δ mice. Littermates LysMCre::mT/mG mice without CXCR4f/f alleles were used as CXCR4WT controls.
Bone marrow transplant and inducible/spontaneous melanoma models
Recipient C57BL/6 mice carrying BrafV600E_/Ptenf/f/Tyr-Cre alleles (obtained from Jackson Laboratories) were given 100 mg/L neomycin,10 mg/L polymyxin B in pH2 water 1 week before transplant then continuously for 6 weeks after transplantation. Mice received one dose of 10-Gy irradiation (Cesium Gamma irradiator). Four hours later, mice were injected via tail vein with bone marrow cells (1×106) from C57BL/6 donor mice (CXCR4myeΔ/Δ mice or myeloid CXCR4WT mice). The reconstitution of bone marrow in recipient mice was examined by monitoring peripheral fluorescent green-positive myeloid and tomato-positive lymphocytes using flow cytometry. The melanomas were induced with topical administration of 4-hydroxytamoxifen at 5 mM daily for three days. One and a half months after bone marrow transplantation, tumor growth was evaluated and statistically analyzed by two-way ANOVA.
Establishment of NK sensitive target Yac-1–reporter cell line
Yac-1 cells were obtained directly from ATCC at the time of this study and maintained in RPMI 1640 medium supplemented with 5% fetal bovine serum, 2 mM glutamine, 10mM HEPES (pH 7.4), and antibiotics (100 units of penicillin/mL and 100 μg of streptomycin/mL). A lentiviral vector, pRBow-FLuc-BFP, contains dual expression cassette with a single MCV promoter driving expression of both a luminescent and a blue fluorescent protein (BFP). Yac-1 cells were infected with viral particles in complete RPMI medium supplemented with 8 μg/mL of polybrene (Sigma). Twenty-four hours after infection, the cells expressing the construct were selected with blasticidin (12 μg/mL) for 10 days. Cells expressing and Fluc were identified by flow cytometry and chemiluminescence, respectively. The selected cells were used for ex vivo and in vivo imaging NK cell cytotoxicity assay.
In vivo imaging of NK cell cytotoxicity
To determine NK cell cytotoxicity, YAC-1-luciferase (Luc) cells (8×106 cells in 1 mL HBSS) were injected into mice via the tail vein. After being anesthetized with isoflurane, mice were then injected intraperitoneally with 3 mg D-luciferin in 200 μl PBS for each imaging session. Whole body images were taken 10 minutes after D-luciferin injection. The mice were repeatedly imaged in the same position for 2 minutes at 1, 4, and 24 hours after injection of YAC-1–Luc cells using an IVIS-200 imaging system (Xenogen Imaging Technologies) to acquire the photons of light emitted from the mice. Regional luciferase signals were quantified using Living Image 4.1 software (Xenogen).
NK cytotoxicity assay ex vivo
NK effector cells were derived from murine spleen via negatively isolation approach using EasySep™ mouse NK Cell Isolation Kit (Stemcell Technologies) and the NK purity was over 80%. YAC-1–Luc target cells were washed once in RPMI media and plated at a concentration of 10,000 cells per 0.1 mL in 96-well microplates. Target cells were co-cultured with increasing numbers of purified NK cells, in a final volume of 0.2 mL at 5% CO2, 37°C. After a 4-hour incubation, co-cultured cells were washed three times with PBS. The cells were lysed using the Luciferase Assay System with Reporter Lysis Buffer (Promega) and the intracellular luciferase activity was determined by reading 10 seconds of chemiluminescence per the manufacturer’s protocol (Promega). Plating target cells in media without effector cells served as a control.
Depletion of immune cells in vivo
For functional abrogation of macrophages, mice were treated daily via oral gavage with 200 μg of colony-stimulating factor-1 receptor (CSF-1R) (Novartis Pharmaceuticals Corporation) or 20% captisol as vehicle control for 10 days (12). For depletion of neutrophils, mice were peritoneally injected with 250 μg of Ly6G monoclonal antibody (mAb) (Clone 1A8, BioXCell) or the same amount IgG2a isotype control mAb (Clone 2A3, BioXCell) daily for three days and maintained at 100 μg/mouse every other day (13). (For depletion of CD8+ T cells, mice were peritoneally injected with 200 μg of CD8a mAb (Clone YTS 169.34, BioXCell) or the same amount IgG2a isotype control mAb (Clone 2A3, BioXCell) daily for three days and maintained with 100 μg/mouse every other day(14). For specifically depleting NK cells, mice were intravenously injected 300 μg rabbit antibody to asialo-GM1 (Wako Chemicals), or a volume of rabbit serum containing an equivalent amount of control IgG, two days before tumor implantation and maintained with an injection of 300 μg anti-asialo-GM1 or IgG control twice a week (15)
Flow cytometry analysis and antibodies
For flow cytometry analyses, tissues were minced on a programmable dissociator and digested with an enzyme solution of collagenase 1 (1500 CDU), Dispase II (1mg/ml) and DNase 1 (0.1 mg/ml). A detailed antibodies used (listed in supplementary data), staining, and flow cytometry analyses protocols is according to our previous published methodology (16). For intravenous antibody staining and flow cytometry, animals were injected intravenously with 2 μg allophycocyanin-conjugated anti-mouse CD45 mAb. Mouse lungs were cut into 1–2mm slices and digested in buffer containing 2 mg/ml collagenase and 0.1 mg/ml DNase I (8). Digested lungs were passed through a 70 μm strainer to obtain a single cell suspension. Mouse spleens were pressed through 40 μm strainer using syringe plunger to obtain a single cell suspension. Cells were incubated with Ghost Dye TM Violet 510, an amine reactive viability dye used to discriminate live/dead cells, and washed with FACS buffer (PBS containing 2% v/v FBS). After blocking Fc receptors with anti-mouse CD16/CD32 mAb in FACS buffer for 15 min, cells were incubated with mAbs to mouse CD45R (B220)-FITC, CD8α-FITC, NK1.1-PE, CD3ε-BV421 in addition to BV421-conjugated CD1d tetramers loaded with GalCer (CD1d-tet) for additional 1 hour on ice as described (14, 17). Cells were washed twice in FACS buffer and data acquired with FACSCanto II (Becton Dickenson). FACS data, comparing leukocytes from CXCR4myeΔ/Δ to CXCR4WT (.fcs files) were analyzed using FlowJo software (Version 10.1). For cell counting, absolute numbers of lymphocytes were counted using AccuCheck counting beads (ThermoFisher). Frequency of NK cells, type I NKT cells and type II NKT cells within total lymphocyte gate were obtained and used to calculate absolute numbers of each of these population.
Cytokine Quantitation
IL18 concentrations in the lung tumor tissue and serum were determined by ELISA (Cat. 7625, R&D Systems) 16 days after B16F0 melanoma cells were intravenously implanted into CXCR4WT and CXCR4MyeΔ/Δ mice.
Statistical analysis
Data are summarized in figures using the mean ± SD. Treatment effects in standard two-group experiments were compared using the Wilcoxon rank-sum test. The experimental differences between two groups by variables, such as difference between CXCR4WT and CXCR4myeΔ/Δ mice across effector ratios, were assessed in the context of a two-way analysis of variance (ANOVA). Pairwise differences between two groups were compared using model based mean comparisons. The Benjamini and Hochberg (BH) correction was used to adjust p value for multiple comparison as noted (denoted by asterisks) in text and figure legends to control the within experiment false discovery rate to less than 5%. Also, * for p<0.05, ** for p<0.01, and *** for p<0.001.
Results
Deletion of CXCR4 in myeloid cells results in an antitumor phenotype.
Murine C57BL/6 mice with deletion of CXCR4 in myeloid cells were generated by tissue-specific LysM-Cre-mediated recombination. The resulting CXCR4myeΔ/Δ mice exhibited only barely detectable expression of both Cxcr4 transcript and CXCR4 protein (Supplementary Fig. S1A-C). To determine how CXCR4 expression in myeloid cells impacts immunity in an experimental melanoma metastasis model, syngeneic B16F0 melanoma cells were intravenously injected into CXCR4myeΔ/Δ mice littermate CXCR4WT mice. Two weeks later, lung weight in CXCR4myeΔ/Δ tumor bearing mice was 52% lower than in the CXCR4WT tumor bearing mice (398 ± 192 mg vs 762±70 mg, p<0.001, Fig. 1A and 1B). In a separate experiment, two weeks after PyMT breast cancer cells were intravenously injected into CXCR4myeΔ/Δ or littermate control CXCR4WT mice, the lung tumor weight in CXCR4WT mice was over 70% greater than in CXCR4myeΔ/Δ mice compared to CXCR4WT mice( p<0.01, n=6, Supplementary Fig S1D-E) ) Thus, disruption of the CXCR4/CXCL12 signaling axis in myeloid cells altered the tumor microenvironment and effectively suppressed outgrowth of both melanoma or breast cancer cells in the lung.
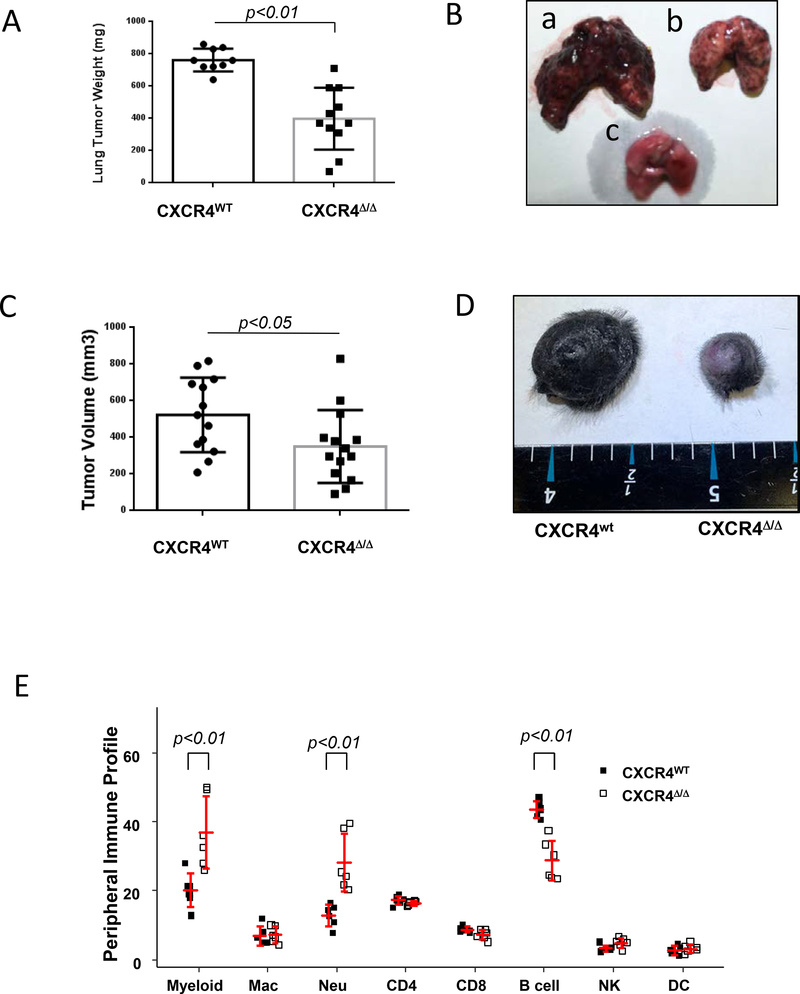
A, experimental lung metastasis. Syngeneic melanoma cells (B16F0, 1×106) were intravenously injected into CXCR4MyeΔ/Δ mice (n=11) or littermate myeloid CXCR4WT mice (n=9). Two weeks after injection, the lungs were weighed. The lung tumor burden was calculated as the weight of tumor-bearing lung minus that of tumor-free lung and analyzed by Wilcoxon rank-rum test. B, representative photo of the tumor-bearing lung was from CXCR4WT mouse (a); or from CXCR4MyeΔ/Δ mouse (b) or from tumor-free mouse (c). C, Inducible spontaneous melanoma. The BrafCA::Ptenf/f::Tyr-Cre C57BL/6 mice were irradiated and then transplanted with bone marrow cells from CXCR4MyeΔ/Δ mice or CXCR4WT mice. Recipient mice were topically treated with 4-hydroxytamoxifen to induce tumor formation and 1.5 mo after bone marrow transplantation, tumor volume was determined and analyzed by Wilcoxon rank-sum test. D, representative photo for the skin of tumor-bearing mouse with bone marrow transplants from CXCR4WT or CXCR4MyeΔ/Δ mouse. E, profile of immune cell populations from peripheral blood. Peripheral blood was collected from CXCR4MyeΔ/Δ mice (n=6) or CXCR4WT mice (n=6). CD45+ leukocytes were prepared and stained with fluorochrome-conjugated antibodies specific for immune cell surface markers as indicated: Myeloid cells (CD11b+); Macrophages (CD11b+/ F4/80+Gr1-); Neutrophils (CD11b+/Gr1+). The stained cells were subjected to flow cytometry analysis. Percentage of subtypes of immune cells in the total CD45+ cell population was graphed and statistically analyzed by the two-way ANOVA with model based mean comparisons and BH p value correction.
To further examine whether disruption of CXCR4/CXCL12 signaling in myeloid cells influences tumorigenesis of melanoma, a 4-hydroxytamoxifen (4-HT) inducible braf/pten mouse melanoma model with bone marrow transplant from CXCR4MyeΔ/Δ and CXCR4WT donor mice was used. One and one-half months after bone marrow transplantation, 100% melanoma incidence occurred in both groups receiving the bone marrow cells from CXCR4MyeΔ/Δ and CXCR4WT donor mice. However, the tumor weight in mice receiving myeloid CXCR4MyeΔ/Δ bone marrow was 67% lower than that of recipients of CXCR4WT bone marrow (p<0.05) (Fig. 1C and 1D). Data shown here demonstrate that disruption of CXCR4/CXCL12 signaling in myeloid cells suppresses B-RafV600E/Pten−/−-driven melanocyte transformation and melanoma formation.
To examine whether disruption of CXCR4/CXCL12 signaling altered the immune cell population in blood, immune cells from peripheral blood were stained with specific antibodies and analyzed by flow cytometry. Cells in which the tissue-specific LysM-Cre mediated deletion of myeloid CXCR4 were identified based on GFP expression. This analysis showed that the peripheral myeloid cell population was increased by 0.85 fold in CXCR4MyeΔ/Δ mice in comparison to that of CXCR4WT mice (Fig. 1E, p<0.01, n=6). Neutrophils, but not macrophages, were significantly increased by 1.33-fold in the CXCR4MyeΔ/Δ mice (p<0.001). In addition, B cells were decreased by 35% (p<0.001) in mice with CXCR4 deletion in myeloid cells. These data suggest a functional link between the immune cell alterations and anti-tumor phenotype in the CXCR4myeΔ/Δ mouse. However, we failed to observe a difference in the total CD3+ T cell infiltration into the tumor in the inducible melanoma tumors grown in mice that were transplanted with bone marrow cells from CXCR4myeΔ/Δ or CXCR4WT mice (p=0.74, n=10) (Supplementary Fig. S1 I-J).
A cell cycle analysis was performed in neutrophils derived from bone marrow and peripheral blood of non-tumor bearing CXCR4MyeΔ/Δ or CXCR4WT mice. An increased percentage of cells in S phase was observed in the bone marrow neutrophils from CXCR4MyeΔ/Δ mice by 0.54-fold in comparison to CXCR4WT mice (p<0.01, n=6) (Supplementary Fig. S1Fa). In addition, the percent peripheral neutrophils in sub-G0 phase of cell cycle was significantly reduced by 97% in CXCR4MyeΔ/Δ mice in comparison to that from CXCR4WT mice (p<0.01, n=6) (Supplementary Fig. S1Fb). Thus, blockage of CXCR4 signaling in neutrophils is associated with a 1.33-fold increase in numbers of neutrophils in the peripheral blood of CXCR4MyeΔ/Δ mice and a 0.54-fold increase in the bone marrow neutrophils in S phase. To further investigate the role of CXCL12 on bone marrow neutrophils, these cells isolated from wild type C57BL/6 mice were exposed to increasing concentrations of CXCL12 (1 to 100 ng/ml) in vitro over three days. Results showed an induction of proliferation on day 1 in response to ≥6.2 ng/ml (Supplementary Fig. S1G), followed by a slight decline in Ki67+ cells and a 2-fold increase in Annexin V staining on day two that peaked with 12.5 ng/ml CXCR4 (Supplementary Fig. S1H), indicating that CXCL12 may play a bifunctional role in vitro. However, CXCL12 in the bone marrow niche has been reported to hold neutrophils in the marrow and when CXCR4 is antagonized there is a release of neutrophils into the peripheral blood (31). Thus, it is highly possible that premature release from the bone marrow is the major mechanism of increased neutrophils in peripheral blood.
NK cells, but not NKT cells, facilitate antitumor effect of CXCR4 loss in myeloid cells
To identify whether the innate or acquired immune response plays a role in the antitumor phenotype, CXCR4MyeΔ/Δ mice were treated with antibodies against asialo-GM1 or CD8. This treatment efficiently depleted NK cells by 93% (Supplementary Fig. S2A) and CD8+ T cells by 98% (Supplementary Fig. S2B) in the respective mice, when compared to mice treated with an isotype matched non-specific antibody. Syngeneic B16F0 melanoma cells were intravenously implanted into these mice. After two weeks of tumor growth in the lung, we observed that NK cell depletion (Fig. 2A), but not CD8+ T cell depletion (Supplementary Fig. S2C), reversed the suppression of tumor growth in CXCR4myeΔ/Δ mice. Hence, NK cells appear to be required for the enhanced antitumor immunity in the CXCR4MyeΔ/Δ tumor-bearing mice.
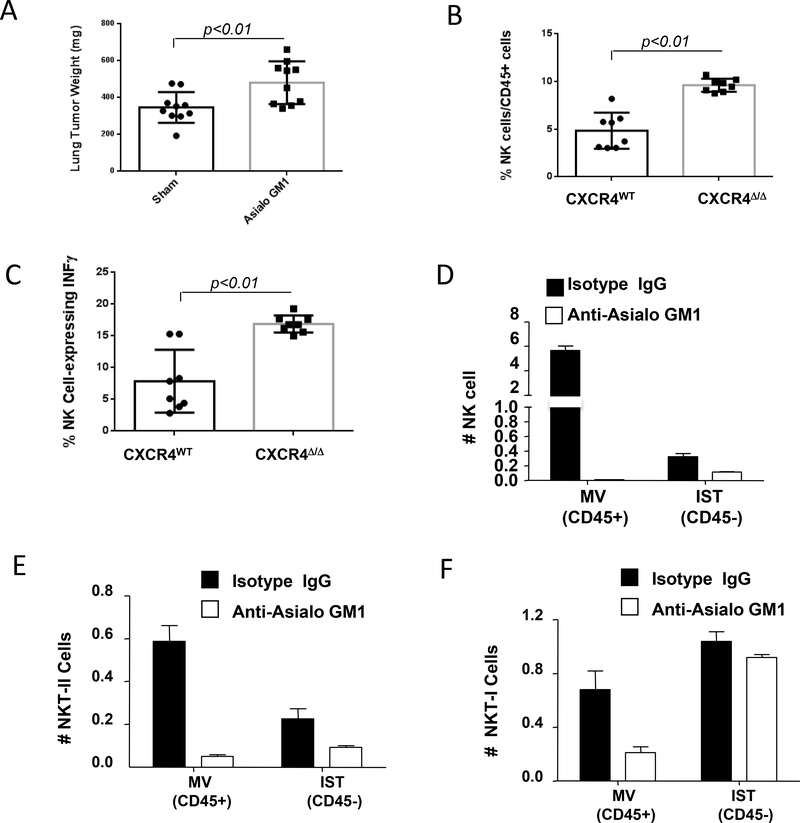
A, depletion of NK cells attenuated antitumor immunity of CXCR4MyeΔ/Δ mice. CXCR4MyeΔ/Δ mice (10/group) were treated for two days with asialo-GM1 antibody or normal rabbit serum containing an equivalent amount of IgG. Over 93% NK cells were depleted in asialo-GM1 antibody treated mice. Mice were intravenously implanted with 1.2×105 B16F0 melanoma cells. Two weeks after tumor cell implantation, the lung weight was determined and compared to that of mice not injected with B16F0 cells (Wilcoxon rank-sum test). B, tumor NK cell infiltration. 2×105 B16F0 melanoma cells were intravenously injected into CXCR4MyeΔ/Δ mice and CXCR4WT mice (8/group). Sixteen days after injection of tumor cells, the lung tumor infiltrating NK cells were analyzed by flow cytometry (Wilcoxon rank-sum test). C, Intratumoral quantitation of IFNγ-expressing NK cells was determined by FACS (Wilcoxon rank-sum test). D, depletion of interstitial and vascular NK cells. C57BL/6 mice (5/group) were treated with asialo-GM1 antibody or isotype IgG. Two days after treatment, mice were implanted intravenously with B16F0 melanoma. 5 minutes before euthanasia, mice were intravenously injected with 2 μg allophycocyanin-conjugated anti-mouse CD45 mAb. The interstitial (IST, CD45-) and marginated vascular (MV, CD45+) populations in lung tumors were analyzed by flow cytometry. E, simultaneously, the interstitial and marginated vascular populations Type II NKT (E) or type I NKT (F) in lung tumors were determined by flow cytometry. For A. B. and C, group comparisons were made using Wilcoxon rank-sum test.
To examine the NK cell response in the absence of CD8+ T cells, NK cell surface markers of activation (CD69, CD107a, and NKG2D), inactivation (NKG2A), and intramembrane Fas ligand (FasL) (CD178) were analyzed on NK cells isolated from tumors growing in mice depleted of CD8+ T cells by anti-CD8 IgG, as compared to treatment with isotype control IgG. There was no significant alteration in NK surface markers in CXCR4myeΔ/Δ mice after CD8+ T lymphocyte depletion (Supplementary Fig. S2D). Therefore, we conclude that CD8+ T cells are not required for the enhanced activation of NK cells observed in CXCR4myeΔΔ mice.
NK cells that express IFNγ were reported to have greater antitumor activity (18). To further study the mechanisms of NK cells anti-tumor immunity mediated by myeloid CXCR4, we examined the expression of IFNγ in NK cells infiltrating the melanoma tissues in the lung of CXCR4MyeΔ/Δ mice and CXCR4WT mice after i.v. injection of melanoma tumor cells. The results indicate that 2-fold more NK cells infiltrated into the metastatic tumor site of CXCR4MyeΔ/Δ mice in comparison to that of CXCR4WT mice after tail vein injection with B16F0 melanoma cells (Fig. 2B, p<0.01, n=8). The percentage of IFNγ-expressing NK cells in the lung was 2.1-fold higher in the tumor-bearing CXCR4MyeΔ/Δ mice than in CXCR4WT mice (Fig. 2C, p<0.01, n=8).
To evaluate whether NKT cells might contribute to the total natural killer antitumor immunity in the CXCR4mye−/− mice, NKT cells were monitored in vascular and tumor tissues of B16F0 melanoma-bearing mice after treatment with anti-asialo-GM1 to deplete NK cells (Fig. 2D). Type II NKT with properties of immune-suppression, but not Type I NKT cells with properties of enhanced anti-tumor immunity, were depleted in lung tumors after treatment with asialo-GM1 antibody (Fig. 2E&F). Since an increase in tumor growth was observed when asialo-GM1 antibody was used to deplete both type II NKT cells and NK cells, leaving the antitumor activity of the type I NKT cells intact, our data suggest that NK cells are primarily responsible for the antitumor response modulated by loss of CXCR4 in myeloid cells (Supplementary Fig. S2A).
Myeloid CXCR4 promotes innate immunity through the Fas/FasL signaling pathway
To ascertain whether the myeloid CXCR4 signaling pathway is required for mediation of tumor-specific NK cell cytotoxicity, Yac-1 cells were genetically engineered to express firefly luciferase (Fluc) as a reporter permitting the imaging and quantification of Fluc expression in vivo over a time course of 1, 4 and 24h after injection of luciferin. Subsequently, NK-sensitive Fluc-expressing target Yac-1 cells (8×106) were injected via tail vein into CXCR4MyeΔ/Δ or littermate CXCR4WT mice (8 mice/group). We observed that target Yac-1-Fluc cells remaining in the CXCR4MyeΔ/Δ mice were reduced by 60% 4 hours post-injection of luciferin (, p=0.02) and 97% 24 hours post-injection ( p<0.01) compared with that of CXCR4WT mice (Fig. 3A-B), but not at the 1-hour post-injection time point (p=0.67). To confirm this finding, NK cells were isolated by negative selection from the spleens of CXCR4MyeΔ/Δ or littermate CXCR4WT mice. The purified NK cells were co-cultured with Yac-1-Fluc cells at different ratios as indicated in vitro for 4 hours, and subsequent NK cytotoxicity was evaluated. In comparison to the NK cells from CXCR4WT mice, the cytotoxicity of NK cells from CXCR4MyeΔ/Δ mice demonstrated enhanced killing over a range of effector to target (E/T) ratios (Fig. 3C).
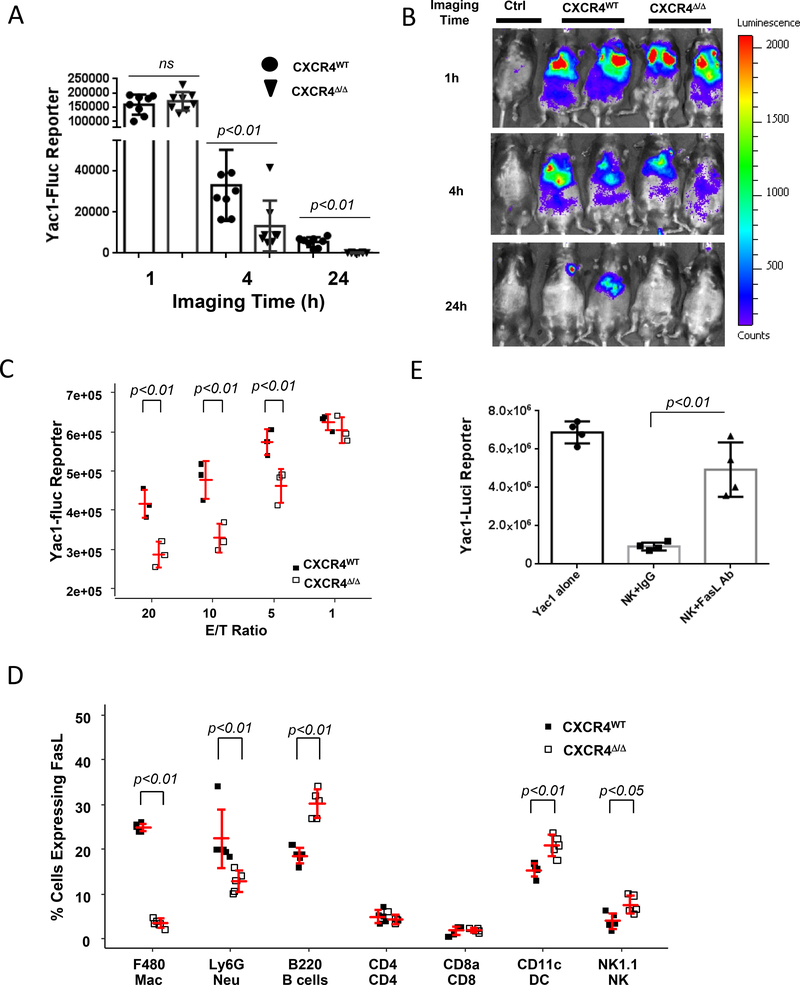
A, NK-cell cytotoxicity in vivo. 1×108 Yac1-Fluc cells were intravenously injected into CXCR4MyeΔ/Δ or CXCR4WT mice. Images were taken at 1, 4 or 24 hours post Yac1 cells injection. Animals without Yac1 cell injection served as negative imaging background control (Ctrl). Data were expressed as log mean ± SD (n=8, Wilcoxon rank-sum test with BH p value correction). B, representative photos of imaged mice. C, NK-cell cytotoxicity in vitro. Murine NK cells were negatively isolated from the spleen of the CXCR4MyeΔ/Δ or CXCR4WT mice. The NK cells were cocultured with Yac1-Fluc reporter cells for 4h. Luciferase activity was determined for the un-lysed Yac1 cells to reflect the reverse NK-cell killing activity. Data are expressed as mean ± SD (n=3) and statistically analyzed by two-way Analysis of Variance (ANOVA) with least-squares means using (BH) p value adjustment for multiple comparisons. D, mFasL (CD178) expression on immune cells. Peripheral immune cells from CXCR4MyeΔ/Δ mice (n=5) or CXCR4WT mice (n=5), were stained with CD178-APC and F4/80-BV421, Ly6G-APC/Cy7, CD8-Alexa Flour700, CD4-pacific blue, B220-Alexa Flour700, Nk1.1-APC/Cy7 and CD11c-AF700. The cells were sorted and analyzed by flow cytometry for CD178 expression on the various cell populations. Two-way ANOVA with model based mean comparisons and BH p value correction. E. neutralization of FasL affects NK -cell cytotoxicity ex vivo. NK cells were negatively selected from the spleens of CXCR4MyeΔ/Δ mice, and co-cultured with Yac1-luci reporter cells at a 20:1 ratio for 5h. Anti-FasL antibody (10μg/ml) or isotype IgG was applied. The luciferase activity was determined in the surviving target cells (n=5). P values were determined by Wilcoxon rank-sum test to evaluate differences between NK+IgG and NK+FasL mAb.
To examine the cytokine profiles in the mice, serum was collected from CXCR4MyeΔ/Δ or CXCR4WT mice and subjected to cytokine array analysis detecting 62 cytokines (Supplementary Table S1). Results in Fig. 3A show that FasL was 7.9 ± 0.5-fold lower in CXCR4MyeΔ/Δ mice compared to CXCR4WT mice, whereas other cytokines were unchanged or altered only by 2-fold. Membrane-bound FasL, but not soluble FasL, is essential for Fas-mediated cytotoxic activity (19). This prompted us to examine the Fas/FasL expression on immune cells. Transmembrane FasL (CD178) expression by peripheral leukocytes was determined by flow cytometry in CXCR4WT mice and CXCR4myeΔ/Δ mice. We observed a 7.1-fold lower FasL-expressing macrophages in CXCR4MyeΔ/Δ mice (p<0.001) in CXCR4myeΔ/Δ compared to CXCR4WT mice. There were also 9.2-fold reduction in FasL-expressing neutrophils (p<0.001) but a 1.6-fold increase in FasL-expressing B cells (, p<0.001) and DCs (p<0.01) in CXCR4MyeΔ/Δ mice. FasL expression in NK cells was significantly increased by 2-fold (p<0.05), whereas FasL expression remained unchanged in CD4+ and CD8+ T lymphocytes in CXCR4MyeΔ/Δ mice (Fig. 3D). Nevertheless, there were no significant differences in the expression of Fas receptor on immune cells from CXCR4MyeΔ/Δ mice as compared to CXCR4WT mice, except for F4/80+ macrophages, B220+ B cells, and CD11c+ dendritic cells, where expression of the FAS receptor (CD95) was altered by ~10% in CXCR4MyeΔ/Δ mice as compared to CXCR4WT (Supplementary Fig. S3B).
To examine whether FasL mediated signals affect NK cell cytotoxicity, NK cells were prepared from spleens of CXCR4MyeΔ/Δ mice. Antibody blocking FasL (10 μg/ml) or isotype-matched control antibody (10 μg/ml) was added into co-culture of NK cells and Yac-1-Fluc cells to neutralize soluble FasL in the medium and transmembrane FasL on NK cell surface. FasL neutralization on NK cells inhibited cytotoxicity against target Yac-1 cells (p<0.01, n=5, Fig. 3E). This finding suggests that FasL directly mediates NK cell cytotoxicity in the YAC-1 tumor model.
NK cells also kill target cells by releasing antitumor cytotoxic molecules such as perforin and granzymes. Surface expression of CD107a is a functional marker of NK-cell degranulation. To investigate whether NK-cell degranulation was also a mechanism for the enhanced FasL-mediated NK-cell antitumor cytotoxicity in vivo in CXCR4myeΔΔ mice, Yac-1 cells (8×106) were intravenously injected into CXCR4WT or CXCR4MyeΔ/Δ mice (5 mice/group). Peripheral leukocytes were sampled over a time course of 0 hours, 1 hour, 4 hours and 24 hours after Yac-1 cell injection. The induced expression of CD107a on the NK cells was analyzed by flow cytometry. We found that NK cells exhibited this degranulation marker especially at the 4-hour time point, but the percentage of CD107+ NK cells did not vary between CXCR4WT or CXCR4MyeΔ/Δ mice (Supplementary Fig. S3C). Moreover, our finding that NK cell number in peripheral blood was altered over the short time course after injection of YAC1 cells in vivo (Supplementary Fig. S3D) suggests that the circulating NK cells in CXCR4MyeΔ/Δ mice are depleted as they move into the lung to attack the YAC1 cells. Since a greater percentage of NK cells from CXCR4MyeΔ/Δ mice express FASL (Fig. 3D), it is presumed that these cells are available for YAC1 killing through both degranulation and the Fas/FasL death pathway.
Neutrophils are essential for enhanced NK-cell activity in vivo.
To identify which myeloid cells (neutrophil or macrophage) initiated NK-cell cytotoxicity in vivo, over 95% neutrophils were eliminated from mice by injection of specific Ly6G mAb into CXCR4MyeΔ/Δ mice (Supplementary Fig. S4A). This resulted in 46.2% decline in NK cell cytotoxicity towards target Yac-1 cells by at 4 hours (p=0.02, n=6), and 83% at 24 hours (p<0.01, n=6) compared with mice treated with IgG2a isotype mAb (Fig. 4A and B). Next, we sought to investigate the molecular determinants of NK-cell activation by neutrophils. To accomplish this, murine neutrophils were depleted with 250 μg of Ly6G mAb for three days followed by intravenous injection with Yac-1 cells for the effector-target cell interaction in lungs. After a 24-hour interaction, lung leukocytes were isolated, and NK cells were identified by flow cytometric analysis. CD3-NK1.1+ cells expressing CD69, NKG2A, NKG2D, CD27 and intracellular IFNγ and Ki67 were quantified. We observed that in comparison to the neutrophil sufficient CXCR4MyeΔ/Δ mice, neutrophil-deficient CXCR4MyeΔ/Δ mice showed significant reduction of NK cells expressing CD69, IFNγ and Ki67 (p<0.05, n=6, Fig. 4C). However, there was no reduction in NK cells expressing the differentiation markers CD27 or NKG2A. Thus, neutrophils are required for NK-cell proliferation and activation, but not for NK-cell maturation, based on CD27 and NKG2A expression.
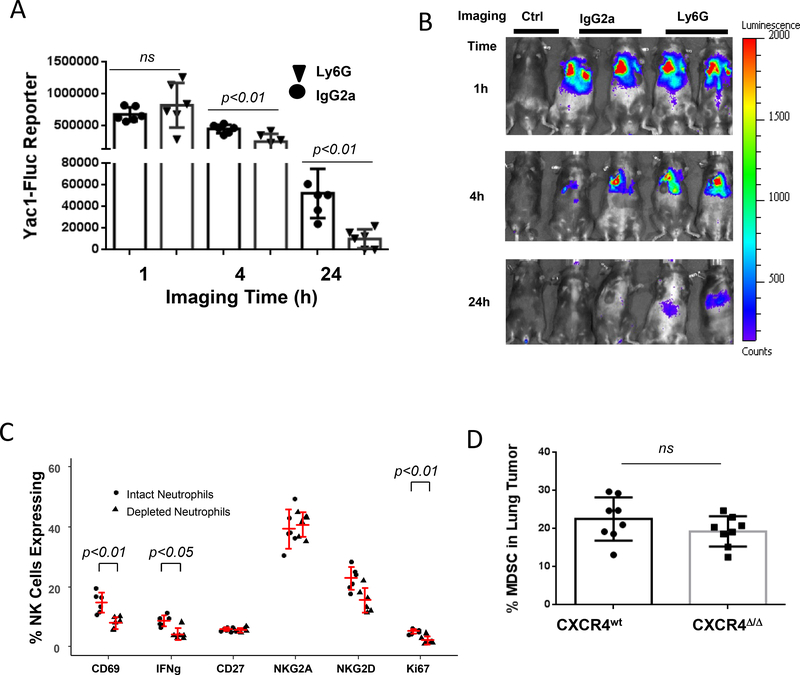
A, NK cell cytotoxicity toward YAC1 cells in neutrophil depleted mice. The neutrophils in CXCR4MyeΔ/Δ mice were depleted with 250 μg/ Ly6G mAb as described in Methods. Subsequently 1×108 Yac1-Fluc cells were intravenously injected into the mice. Imaging was performed at indicated time points post Yac1 cells injection. Mice without Yac1 cell injection served as the negative imaging background control (Ctrl).Data analysis was with Wilcoxon rank-sum test with BH p-value correction. B, representative photos of imaging of mice. C, depletion of neutrophils abrogated NK activation. Mice were injected in the peritoneum with 250 μg of Ly6G mAb or IgG isotype control daily for three days, then 1×108 Yac1 cells were intravenously injected. 24 hours after cell injection, the lung leukocytes were isolated and NK cells were sorted using Percy/cy5.5 conjugated CD3 and APC-conjugated NK1.1+ cells by FACS. Subsequent cell surface expression of CD69-APC, NKG2A-APC, NKG2D-APC, CD27-pacific blue, and intracellular IFNγ-Alex Fluor700 and Ki67-pacific blue were analyzed. Data were analyzed by two-way ANOVA with model based mean comparisons and BH p-value correction. D, MDSC population in metastatic tumor. 2×105 B16F0 melanoma cells were intravenously injected into CXCR4MyeΔ/Δ mice and CXCR4WT mice (8/group). After 16 days of injection, the lung tumor infiltrated MDSC cells were analyzed by flow cytometry. The Wilcoxon rank-sum test was used for data analysis.
We next sought to determine whether macrophages contributed to the activation of NK cells. The polarization/differentiation of macrophages was blocked by daily oral gavage of 200 μg of BLZ945, a CSF-1R inhibitor, for 10 days (20). Results demonstrated that NK-cell cytotoxicity, as monitored by YAC-1 luciferase activity in vivo, was not influenced by treatment of mice with CSF-1R inhibitor (p>0.05, n=4, Fig. S4B). In addition, when mouse macrophages were physically depleted by delivery of clodronate (1 mg), similar results were achieved as when macrophages were functionally inactivated with BLZ945 (Supplementary Fig. S4C).
CXCR4 is expressed on myeloid-derived suppressor cells (MDSCs) and its inhibition reduces MDSC recruitment to tumors (21). Thus, loss of CXCR4 on myeloid cells could result in reduced recruitment of MDSCs to tumors, which could account for the reduction in tumor growth in CXCR4myeΔ/Δ mice. To investigate the role of MDSCs in metastatic lung melanoma of CXCR4MyeΔ/Δ mice, the percentages of tumor infiltrating CD11b+/Ly6G+/Ly6Clow myeloid cells were analyzed by flow cytometry. We failed to observe a difference in the size of tumor MDSC population between CXCR4MyeΔ/Δ mice and CXCR4WT mice (Fig. 4D p=0.17, n=8).
NK cell activation requires cytokine participation
IL18 can activate NK cells (22) and invariant natural killer T cells (iNKT) cells (23). Neutrophils are the principal source of IL18 (22). Expression of IL18 was quantified in neutrophils and macrophages from CXCR4WT and CXCR4MyeΔ/Δ mice. Twenty-four hours after mice were intravenously injected with 8×106 Yac-1 cells, intracellular IL18 of lung myeloid cells was determined. Neutrophils deficient in CXCR4 showed enhanced IL18 expression, in comparison to CXCR4WT neutrophils (Fig. 5A&B, 84.2±5.4% vs. 56.6±8.0%, p<0.01, n=6). To confirm neutrophil-derived IL18 may provide a potential source for NK activation in metastatic lung tumor, IL18 concentrations in the lung tumor tissue and serum were determined 16 days after B16F0 melanoma cells were intravenously implanted into CXCR4WT and CXCR4MyeΔ/Δ mice. IL18 concentration was significantly increased in either of tumor tissue or serum of CXCR4MyeΔ/Δ mice compared with CXCR4WT mice (Fig. 5C&D, tumor tissue 1.69-fold increase; serum 1.5-fold increase, p<0.01, n=8). Exogenous delivery of CXCL12 significantly reduced the production of IL18 by cultured bone marrow cells over two days by 1/7-fold (Supplementary Fig. S5C, p<0.01, n=8). Given the observed increase in annexin V staining after 2 days of CXCL12 treatment (Supplementary Fig. S1H), we cannot rule out the possibility that some of the reduction is based upon reduced viability of CXCL12-treated cells. Thus, neutrophils with CXCR4 deficiency promote NK-cell activation via enhanced IL18 production.
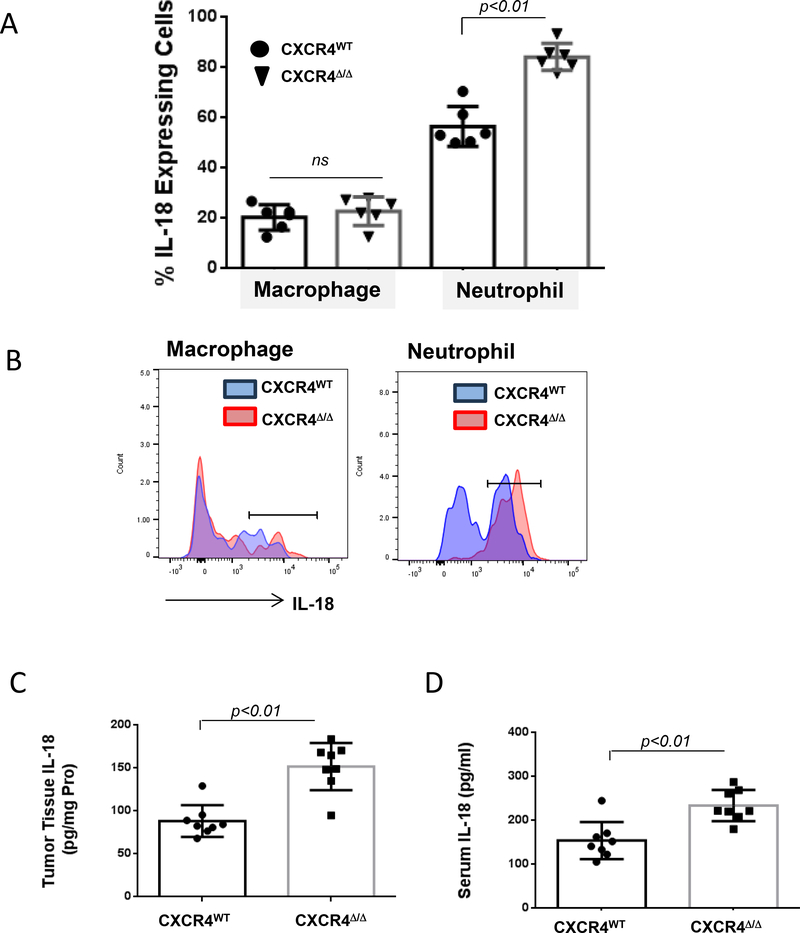
A, Yac1 cells (1×108) were intravenously injected into myeloid CXCR4Δ/Δ or CXCR4WT mice. Twenty-four hours after injection, intracellular IL18 expression in the lung myeloid cells was determined by FACS. Data were analyzed by the Wilcoxon rank-sum test with BH p-value correction (n=6). B, a representative graph is shown. C, IL18 expression. B16F0 melanoma cells (2×105) were intravenously injected into CXCR4MyeΔ/Δ or CXCR4WT mice (n=8). Sixteen days after injection, IL18 in lung tumors was determined with mouse IL18 ELISA. D, simultaneously IL18 level in serum was measured. For C and D, data were analyzed by Wilcoxon rank-sum test.
CXCR4 antagonist enhances NK cell antitumor activity independent of CD8+ T cells.
A potent and highly selective CXCR4 antagonist, the small cyclic peptide LY2510924, is currently in clinical trials for metastatic renal cancer, relapsed or refractory acute myeloid leukemia (AML), and other advanced refractory solid tumors (24). This targeted therapy is based on the mechanism that CXCR4 is overexpressed in a variety of human cancers where it directly stimulates tumor cell proliferation and metastasis, leading to poor overall survival. To investigate the potential effect of LY2510924 on metastatic tumor progression in vivo, immunocompetent C57BL/6 mice were intravenously injected with syngeneic malignant B16F0 melanoma cells, then treated with 3 mg/kg subcutaneous injection of LY2510924 twice a day (25). After two weeks of treatment, the tumor burden in the lungs was reduced 61% compared with PBS vehicle control treated animals (p<0.001, n=9) (Fig. 6A). Drug treatment did not affect mouse body weight, indicating reduced tumor burden was not due to toxicity. Mice treated with the CXCR4 antagonist exhibited alterations in the peripheral blood immune cell profile (Fig. 6B) which were similar to changes induced by CXCR4 loss (Fig. 1E). Both myeloid CXCR4 deletion and CXCR4 antagonism resulted in elevation of both neutrophil and NK cell populations (p<0.01, n=6). To examine antitumor immunity in the tumor microenvironment, tumor tissue was digested, and tumor-infiltrating leukocytes were stained with fluorescently labeled antibodies and analyzed by flow cytometry. We observed that the ratio of NK cells to total CD45+ cells within tumor was 1.6-fold higher in mice treated with LY2510924 compared to PBS treated controls (p<0.001, n=8, Fig. 6C). The activation marker CD69 on NK cells was 1.9-fold higher in NK cells from LY2510924 treated mice compared with PBS treated control mice (p<0.001, n=8, Fig. 6D). However, the expression of cell surface markers, i.e. CD25, CD62L and CD69, or intracellular markers, Foxp3, IL-4 and IFNγ, on tumor infiltrating CD3+/CD4+ T cells was not altered by LY2510924 treatment of tumor-bearing mice (Supplementary Fig. S6 A-B). In contrast, the tumor-infiltrating CD103+ CD8+ T cells were significantly decreased after LY2510924 treatment (p<0.001, n=8), whereas alterations in cell surface markers CD69, CD107b and PD-1 were not observed on the CD8+ T cells in these tumors (Supplementary Fig. S6C).
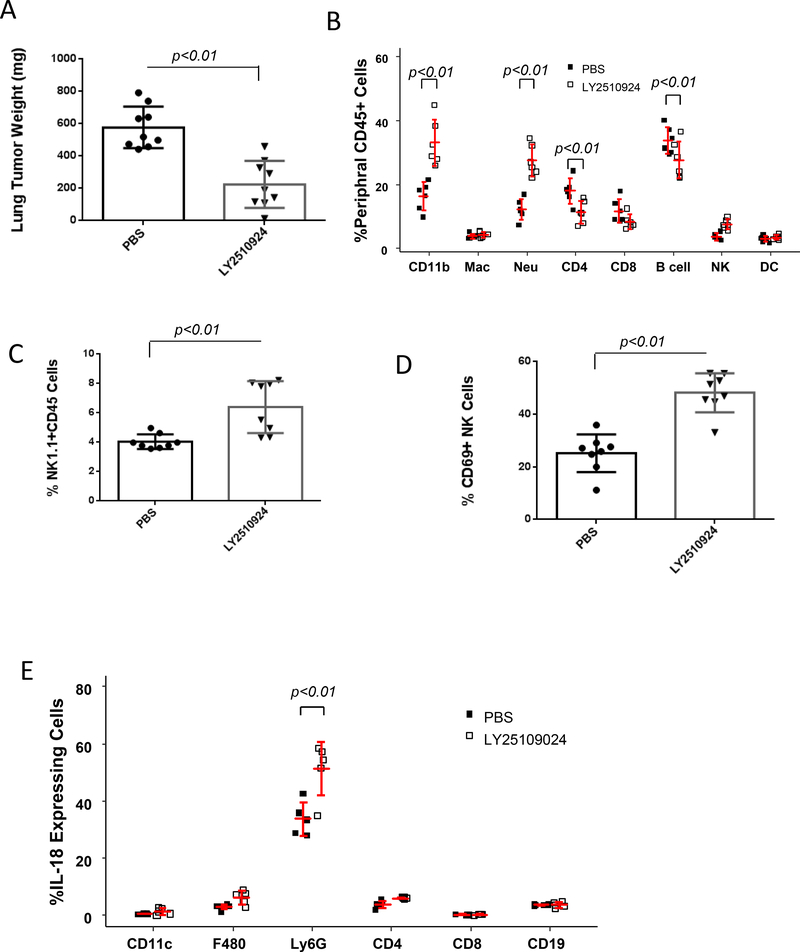
A, LY2510924 anti-tumor activity. C57BL/6 mice (9 mice/group) were intravenously injected with B16F0 melanoma cells (1×105) (14) and treated subcutaneously with 3 mg/kg of LY2510924, or PBS vehicle twice a day. After two weeks of treatment, mice were sacrificed and the weight of tumor in tumor-bearing lungs was determined by subtracting the weight of the tumor-free lung. Data were plotted and statistically analyzed by the Wilcoxon rank-sum test. B, peripheral immune cell profile. Blood was collected two weeks after PBS or LY2510924 treatment. Erythrocytes were excluded with lysis buffer and leukocytes were stained with specific antibodies for flow cytometry analysis using two-way ANOVA with model based mean comparisons and BH p-value correction. C and D, NK-cell distribution and activation. Leukocytes were isolated from the tumor-bearing lungs, stained with CD45-APC/Cy7, NK1.1-PE, and CD69-APC and analyzed by flow cytometry (Wilcoxon rank-sum test). E, Intracellular IL18 expression in leukocytes that infiltrated into lung tumors was analyzed by flow cytometry. Data were analyzed by two-way ANOVA with model based mean comparisons and BH p-value correction.
We also examined the effects of LY2510924 on the growth of orthotopic implants of B16F0 melanoma tumors (Supplementary Fig. S6D-F) and achieved similar results as observed with melanoma tumors in the lung (Fig. 6A-C). Since IL18 acts as an NK cell activator (26), intracellular IL18 production in the infiltrating cells was investigated. We observed that LY2510924 induced significant increase IL18 production in tumor-infiltrating myeloid cells as compared to PBS control, with its major source coming from neutrophils and a minor source from macrophages and CD4+ T cells (Supplementary Fig. S6G). Thus, the CXCR4 antagonist, LY2510924, confers antitumor activity, at least in part through induction of IL18 that can activate NK cells.
To determine whether a subset of melanoma patients may benefit from CXCR4 inhibition, we examined the survival of melanoma patients with high expression of the myeloid marker ITGAM, for which the mRNA expression z-score threshold was +1 (Supplementary Fig. S7. When survival data were analyzed in relation to a high or low CXCR4 expression using a TCGA data set of 469 skin cutaneous melanoma samples, analysis revealed that those melanoma patients with high levels of CXCR4 exhibited a significantly reduced progression free survival (PFS) compared to patients with a low expression of CXCR4. In contrast, when PFS in all the melanoma tumors (without selecting the high ITGAM tumors) was examined, CXCR4 expression did not affect the PFS (Supplementary Fig. S7A-B). These data suggest that melanoma clinical trials with a CXCR4 antagonist might consider selecting patients with tumors with a high frequency of infiltrating myeloid cells.
Discussion
The CXCR4/CXCL12 signaling pathway is involved in multiple stages of tumorigenesis. CXCL12 is highly expressed in the tumor metastatic sites such as the liver, lung, lymph nodes and bone marrow and is also secreted by fibroblasts in vitro and in vivo (27). CXCR4 is over-expressed by tumor cells, and this expression is consistent with increased recurrence and poor overall survival in multiple cancers including breast, lung, kidney, colon, ovarian, brain cancers, lymphoma and leukemia (27–29). The critical roles of CXCR4 in cancer have triggered the development of specific antagonists for clinical application. (30, 31).
Cytokines modulate innate immunity responses to cancers (22, 23, 26, 32). The importance of IL18 has been recognized in NK-cell activation and in direct induction of IFNγ production by NK cells (33). Thus, the crucial effector cytokine that controls NK-cell activation is IFNγ, the bulk of which is derived from NK cells. Jaeger et al suggested a role for neutrophils as non-redundant regulatory cells ensuring the terminal maturation of NK cells; neutrophil-induced NK-cell maturation may occur not only in the bone marrow but also at the periphery where neutrophils are able to interact with NK cells (34). Neutrophils provide a major source of IL18, which induces NK-cell activation. However, CXCR4/CXCL12 signaling is also essential for development of NK cells in MX-Cre-generated CXCR4-deficient mice (35), underscoring discriminatory power of CXCR4/CXCL12 deficiency in liver and lymphocytes. Our results show that elevations in IL18 in various leukocytes may trigger the enhanced NK cell–mediated tumor-cell killing observed in CXCR4myeΔ/Δ mice.
NK cells acquire effector function through a licensing process and exert an antitumor effect, which is also consistent with reports describing neutrophils as pivotal activators of NK cells and NKT cells (23, 36).This study sought to test the hypothesis that CXCR4 null neutrophils enhance NK cell antitumor immunity through a Fas/FasL signal pathway. FasL is an apoptosis-inducing member of the TNF cytokine family, and its receptor Fas plays an essential role for the shutdown of chronic immune responses (37). Tumor associated fibroblasts secrete CXCL12 (31), which potentially contributes to the induction of apoptosis in CD4+ and CD8+ T lymphocytes via CXCR4 receptor by upregulation of Fas/FasL signal pathway (38, 39). The effector mechanism of NK-cell cytotoxicity may also be through the perforin-mediated granule exocytosis pathway (40). However, since membrane-bound FasL was highly expressed on NK cells of CXCR4myeΔ/Δ mice, it is likely that the increased NK-cell activity toward the YAC-1 tumor cells in the CXCR4myeΔ/Δ mice is, at least in part, FASL-dependent (41). Our data suggest that the process of neutrophils licensing NK-cell antitumor cytotoxicity is controlled in part by CXCR4/CXCL12-Fas/FasL signal cascades.
Systemic blockade of CXCR4 with the CXCR4 antagonist, LY2510924, resulted in enhanced production of IL18 by neutrophils as well as enhanced antitumor immunity, providing insight as to how CXCR4 antagonists may be useful in the clinic for treatment of cancer. In agreement with our results, Alterio et al reported that interference of stromal CXCR4 signaling by using heterozygote CXCR4+/− lineage mice or the CXCR4 antagonist, AMD3100, inhibits lung metastasis (42). We conclude that the CXCR4/CXCL12 axis modulates innate immune responses through inhibition of neutrophil licensing of NK cell cytotoxicity, and loss of this CXCR4-mediated role results in enhanced NK-cell activation, which is neutrophil-dependent and correlates with FasL and IL18 expression (Fig. 7).
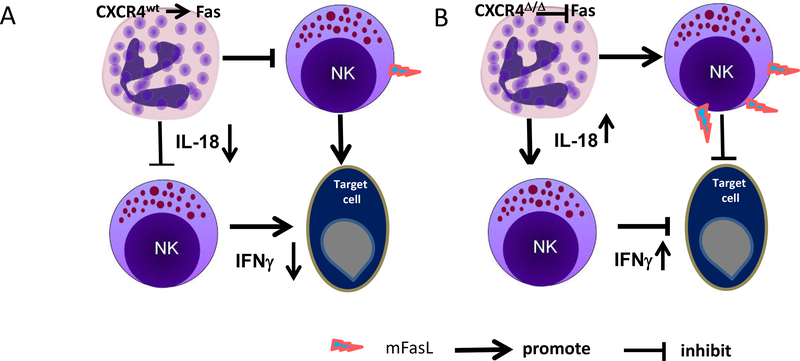
A, myeloid cells in CXCR4WT mice promote NK cell apoptosis through Fas signal pathway and inactivation of NK cells through restricted IL18 production, resulting in reduced NK cell antitumor immunity. B, knockout of CXCR4 in myeloid cells enhances neutrophil release of IL18, which boosts the percentage of IFNγ-expressing NK cells. These activated NK cells exhibit enhanced NK cell–mediated tumor cell killing. NK cells from mice with knock out of CXCR4 in myeloid cells exhibit increased Fas ligand -mediated killing of Fas-expressing tumor cells, conferring enhanced antitumor immunity.
NK cells contribute to murine antitumor immunity and are associated with clinical prognosis in human cancers (43, 44). Consistent with our observation that CXCR4 antagonism enhanced NK cell antitumor activity through a mechanism involving neutrophils, we showed that melanoma patients with high CXCR4 expression combined with infiltration of tumor-associated myeloid cells had poorer PFS than those with low CXCR4 expression. CXCR4 antagonists are entering clinical trials for metastatic melanoma and breast cancer (47, 48), and phase I clinical trials have shown that combining CXCR4 antagonist with pembrolizumab is safe (47). Based upon the data reported here, we anticipate that patients with tumors that have high myeloid infiltration and high CXCR4 expression will benefit from these antagonists, especially when combined with immune checkpoint inhibitors.
Acknowledgements:
This work was supported by awards from the Department of Veterans Affairs, TVHS through a SRCS Award and a MERIT Award to AR (101BX002301), by grants from the NIH: CA34590 (to AR), CA200681 (to SN), and CA P30–068485. It is also supported by a CDA Award to AV from the Harry Lloyd Foundation for Melanoma Research and by a Breast Cancer Research Foundation Award to AV (IIDRP-16–001). We are thankful to Andrew C. Johnson for outstanding technical support and to Mark Boothby for scientific discussions and advice.
References:
Full text links
Read article at publisher's site: https://doi.org/10.1158/2326-6066.cir-18-0045
Read article for free, from open access legal sources, via Unpaywall:
https://cancerimmunolres.aacrjournals.org/content/canimm/6/10/1186.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1158/2326-6066.cir-18-0045
Article citations
Harnessing the Power of NK Cell Receptor Engineering as a New Prospect in Cancer Immunotherapy.
Pharmaceutics, 16(9):1143, 29 Aug 2024
Cited by: 0 articles | PMID: 39339180 | PMCID: PMC11434712
Review Free full text in Europe PMC
Heterogeneous characterization of neutrophilic cells in head and neck cancers.
Head Neck, 46(10):2591-2599, 15 Apr 2024
Cited by: 0 articles | PMID: 38622975
Review
Exploiting innate immunity for cancer immunotherapy.
Mol Cancer, 22(1):187, 27 Nov 2023
Cited by: 29 articles | PMID: 38008741 | PMCID: PMC10680233
Review Free full text in Europe PMC
Maternal CXCR4 deletion results in placental defects and pregnancy loss mediated by immune dysregulation.
JCI Insight, 8(21):e172216, 08 Nov 2023
Cited by: 1 article | PMID: 37815869 | PMCID: PMC10721256
Understanding the functional relevance of oral neutrophils, phenotype and properties in OSCC.
Med Oncol, 40(5):134, 03 Apr 2023
Cited by: 3 articles | PMID: 37010645
Review
Go to all (37) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Defective FasL expression is associated with increased resistance to melanoma liver metastases and enhanced natural killer cell activity.
Melanoma Res, 29(4):401-412, 01 Aug 2019
Cited by: 1 article | PMID: 30932943 | PMCID: PMC6597306
Targeted Deletion of CXCR2 in Myeloid Cells Alters the Tumor Immune Environment to Improve Antitumor Immunity.
Cancer Immunol Res, 9(2):200-213, 11 Nov 2020
Cited by: 40 articles | PMID: 33177110 | PMCID: PMC7864868
Sensitization of B16 tumor cells with a CXCR4 antagonist increases the efficacy of immunotherapy for established lung metastases.
Mol Cancer Ther, 5(10):2592-2599, 01 Oct 2006
Cited by: 32 articles | PMID: 17041104 | PMCID: PMC2228334
The role of Fas ligand and transforming growth factor beta in tumor progression: molecular mechanisms of immune privilege via Fas-mediated apoptosis and potential targets for cancer therapy.
Cancer, 100(11):2281-2291, 01 Jun 2004
Cited by: 77 articles | PMID: 15160330
Review
Funding
Funders who supported this work.
BLRD VA (4)
Grant ID: IK6 BX005225
Grant ID: I01 BX002301
Grant ID: I01 BX001444
Grant ID: IK6 BX004595
Department of Veterans Affairs (1)
Grant ID: 101BX002301
NCI NIH HHS (5)
Grant ID: K12 CA090625
Grant ID: R01 CA116021
Grant ID: R01 CA034590
Grant ID: P30 CA068485
Grant ID: R01 CA200681
NHLBI NIH HHS (1)
Grant ID: R01 HL121139
NIH (2)
Grant ID: CA200681
Grant ID: CA34590
NIH HHS (1)
Grant ID: S10 OD021804





